Impact of Visceral Leishmaniasis on Local Organ Metabolism in Hamsters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Infections
2.2. Sample Preparation for LC-MS/MS
2.3. Liquid Chromatography–Tandem Mass Spectrometry
2.4. LC-MS/MS Data Analysis
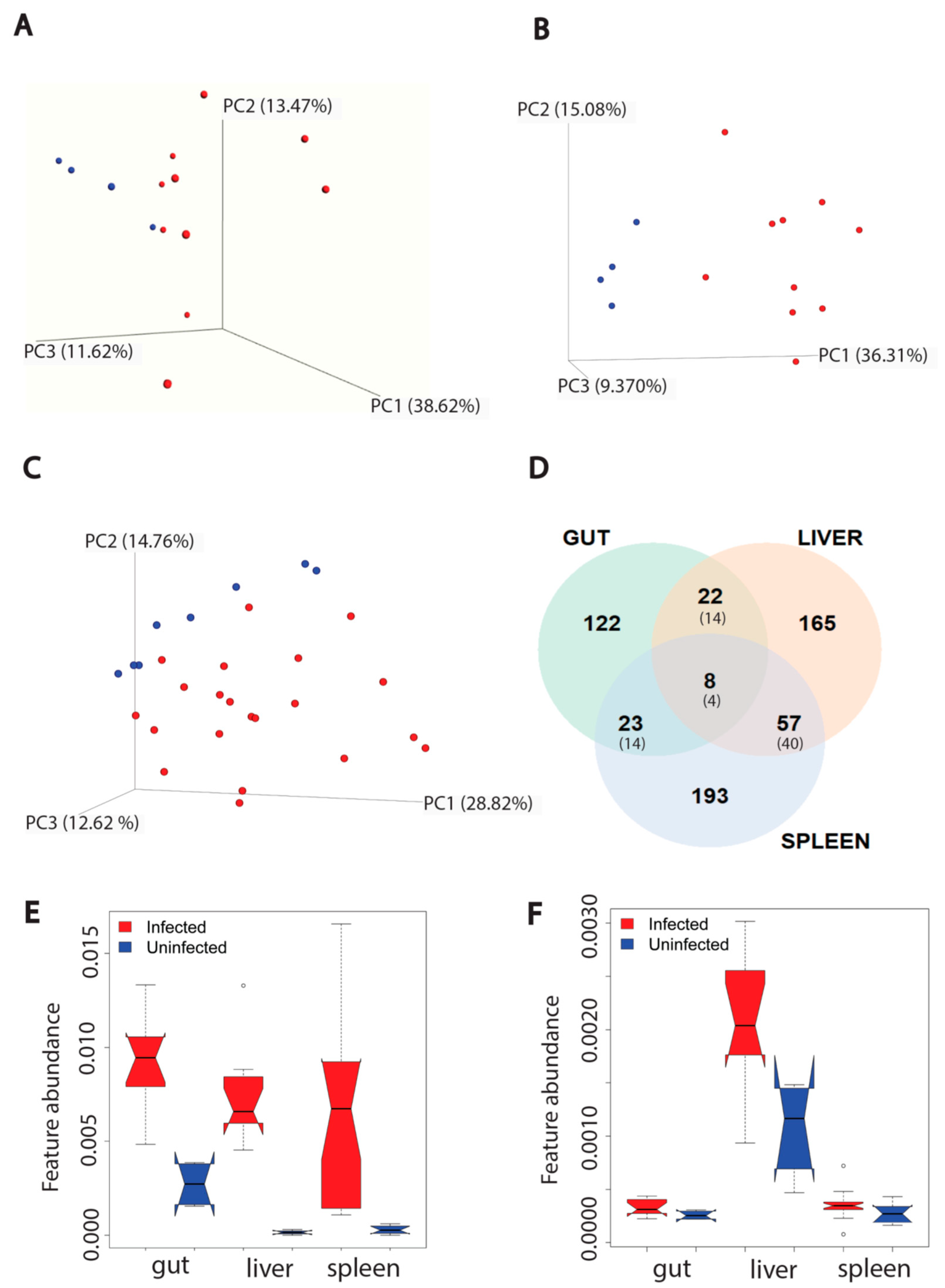
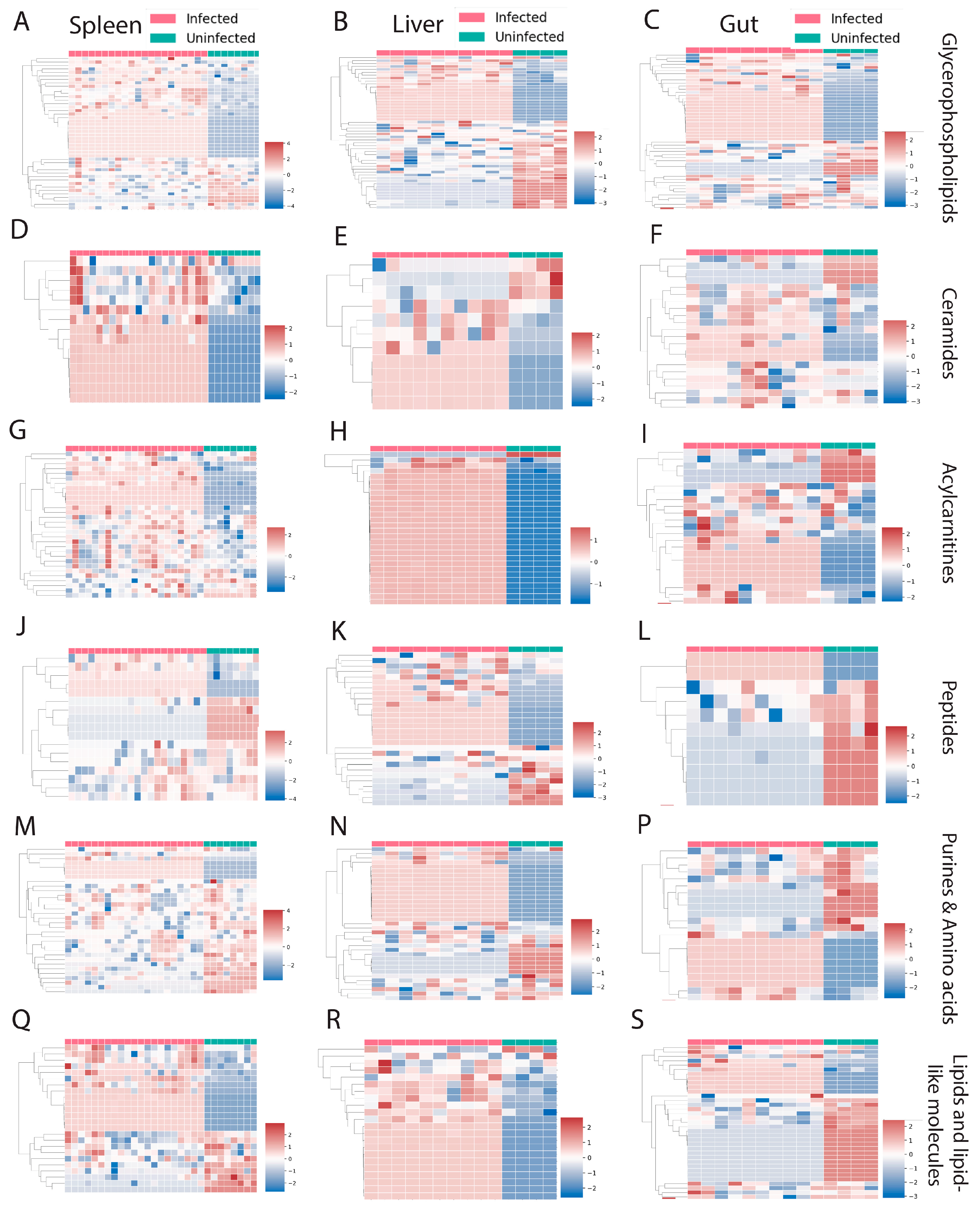
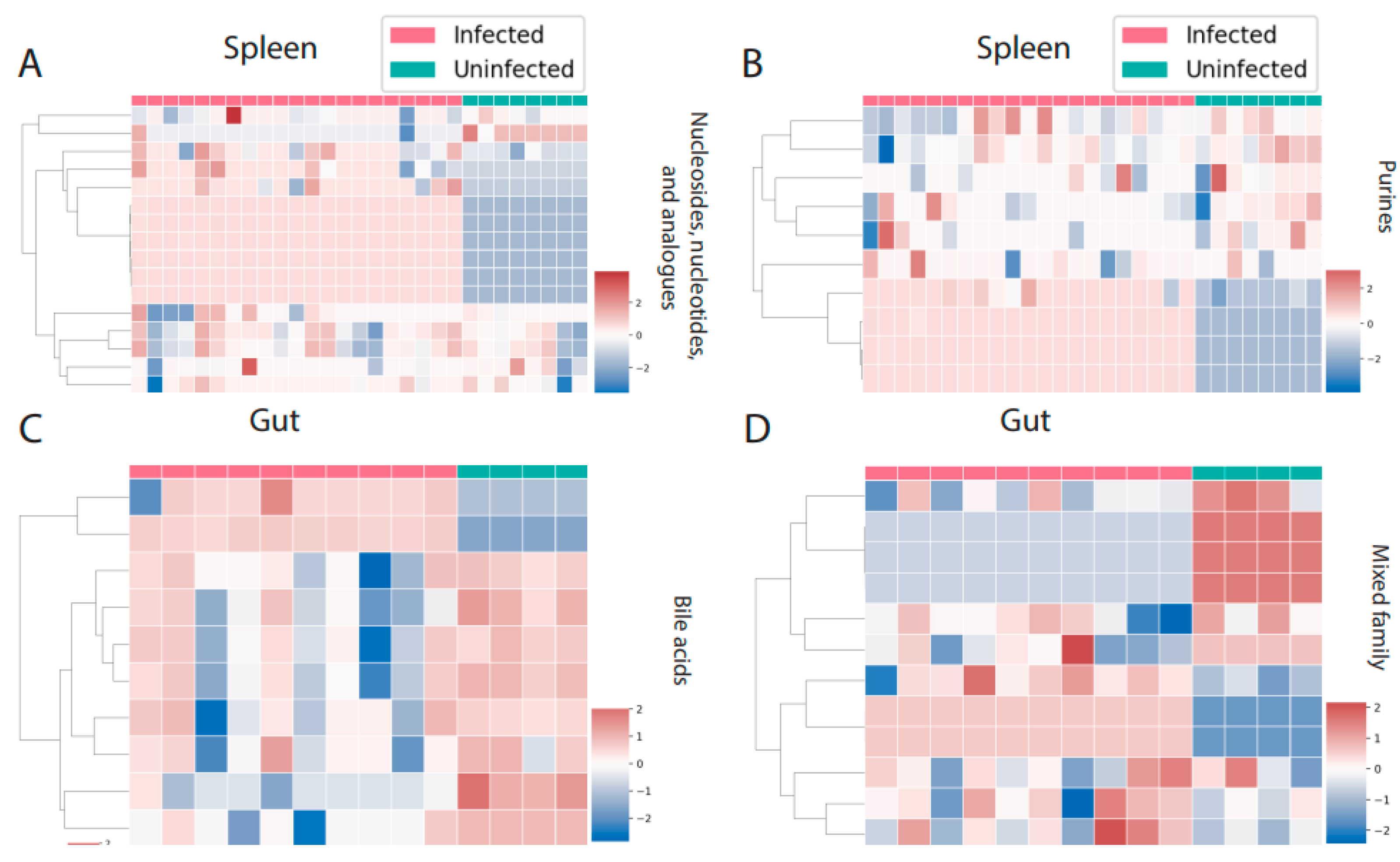
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; Team, W.H.O.L.C. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Downing, T.; Votýpka, J.; Kuhls, K.; Lukeš, J.; Cannet, A.; Ravel, C.; Marty, P.; Delaunay, P.; Kasbari, M.; et al. Leishmania Infections: Molecular Targets and Diagnosis. Mol. Asp. Med. 2017, 57, 1–29. [Google Scholar] [CrossRef]
- Franssen, S.U.; Durrant, C.; Stark, O.; Moser, B.; Downing, T.; Imamura, H.; Dujardin, J.-C.; Sanders, M.J.; Mauricio, I.; Miles, M.A.; et al. Global Genome Diversity of the Leishmania Donovani Complex. eLife 2020, 9, e51243. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Lockwood, D.N. Treatment of Visceral Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151–158. [Google Scholar] [CrossRef]
- Lindoso, J.A.L.; Moreira, C.H.V.; Cunha, M.A.; Queiroz, I.T. Visceral Leishmaniasis and HIV Coinfection: Current Perspectives. HIV AIDS 2018, 10, 193–201. [Google Scholar] [CrossRef]
- Hossain, E.; Khanam, S.; Dean, D.A.; Wu, C.; Lostracco-Johnson, S.; Thomas, D.; Kane, S.S.; Parab, A.R.; Flores, K.; Katemauswa, M.; et al. Mapping of Host-Parasite-Microbiome Interactions Reveals Metabolic Determinants of Tropism and Tolerance in Chagas Disease. Sci. Adv. 2020, 6, eaaz2015. [Google Scholar] [CrossRef]
- Westrop, G.D.; Williams, R.A.M.; Wang, L.; Zhang, T.; Watson, D.G.; Silva, A.M.; Coombs, G.H. Metabolomic Analyses of Leishmania Reveal Multiple Species Differences and Large Differences in Amino Acid Metabolism. PLoS ONE 2015, 10, e0136891. [Google Scholar] [CrossRef]
- Silva, A.M.; Cordeiro-da-Silva, A.; Coombs, G.H. Metabolic Variation during Development in Culture of Leishmania donovani Promastigotes. PLoS Negl. Trop. Dis. 2011, 5, e1451. [Google Scholar] [CrossRef]
- Berg, M.; Vanaerschot, M.; Jankevics, A.; Cuypers, B.; Maes, I.; Mukherjee, S.; Khanal, B.; Rijal, S.; Roy, S.; Opperdoes, F.; et al. Metabolic Adaptations of Leishmania Donovani in Relation to Differentiation, Drug Resistance, And Drug Pressure. Mol. Microbiol. 2013, 90, 428–442. [Google Scholar] [CrossRef]
- Das, S.; Saha, T.; Shaha, C. Tissue/Biofluid Specific Molecular Cartography of Leishmania donovani Infected BALB/c Mice: Deciphering Systemic Reprogramming. Front. Cell Infect. Microbiol. 2021, 11, 694470. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Kim, S.; Yin, X.; Zhang, X.; Kato, I. Integrating Two-Dimensional Gas and Liquid Chromatography-Mass Spectrometry for Untargeted Colorectal Cancer Metabolomics: A Proof-of-Principle Study. Metabolites 2020, 10, 343. [Google Scholar] [CrossRef]
- Prodhan, M.A.I.; Shi, B.; Song, M.; He, L.; Yuan, F.; Yin, X.; Bohman, P.; McClain, C.J.; Zhang, X. Integrating Comprehensive Two-Dimensional Gas Chromatography Mass Spectrometry and Parallel Two-Dimensional Liquid Chromatography Mass Spectrometry for Untargeted Metabolomics. Analyst 2019, 144, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC–MS And LC–MS for Untargeted Metabolomics Profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Rai, A.K. Hamster, A Close Model for Visceral Leishmaniasis: Opportunities and Challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, J.; Dong, K.; Chen, D.; Yuan, D.; Chen, J. Metabolic Characterization and Biomarkers Screening for Visceral Leishmaniasis in Golden Hamsters. Acta Trop. 2022, 225, 106222. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Paun, A.; Romano, A.; Langston, H.; Langner, C.A.; Moore, I.N.; Bock, K.W.; Francisco, A.F.; Brenchley, J.M.; Sacks, D.L. Fatal Progression of Experimental Visceral Leishmaniasis Is Associated with Intestinal Parasitism and Secondary Infection by Commensal Bacteria and Is Delayed by Antibiotic Prophylaxis. PLoS Pathog. 2020, 16, e1008456. [Google Scholar] [CrossRef]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A Cross-Platform Toolkit for Mass Spectrometry and Proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. Mzmine 2: Modular Framework for Processing, Visualizing, And Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. Emperor: A Tool for Visualizing High-Throughput Microbial Community Data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome. Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- McLuskey, K.; Wandy, J.; Vincent, I.; van der Hooft, J.J.J.; Rogers, S.; Burgess, K.; Daly, R. Ranking Metabolite Sets by Their Activity Levels. Metabolites 2021, 11, 103. [Google Scholar] [CrossRef]
- Ham, B.M.; Jacob, J.T.; Cole, R.B. MALDI-TOF MS of Phosphorylated Lipids in Biological Fluids Using Immobilized Metal Affinity Chromatography and a Solid Ionic Crystal Matrix. Anal. Chem. 2005, 77, 4439–4447. [Google Scholar] [CrossRef]
- Jarmusch, A.K.; Aron, A.T.; Petras, D.; Phelan, V.V.; Bittremieux, W.; Acharya, D.D.; Ahmed, M.M.A.; Bauermeister, A.; Bertin, M.J.; Boudreau, P.D.; et al. A Universal Language for Finding Mass Spectrometry Data Patterns. bioRxiv 2022. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Ato, M.; Kaye, P.M. Macrophages, Pathology and Parasite Persistence in Experimental Visceral Leishmaniasis. Trends Parasitol. 2004, 20, 524–530. [Google Scholar] [CrossRef]
- Mándi, Y.; Vécsei, L. The Kynurenine System and Immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a Promising Antimicrobial Agent? A Multi-Perspective Review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef] [PubMed]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Balcazar, D.E.; Vanrell, M.C.; Romano, P.S.; Pereira, C.A.; Goldbaum, F.A.; Bonomi, H.R.; Carrillo, C. The Superfamily Keeps Growing: Identification in Trypanosomatids of Ribj, the First Riboflavin Transporter Family in Protists. PLoS Negl. Trop. Dis. 2017, 11, e0005513. [Google Scholar] [CrossRef] [PubMed]
- Tonnetti, L.; Thorp, A.M.; Reddy, H.L.; Keil, S.D.; Doane, S.K.; Goodrich, R.P.; Leiby, D.A. Reduction of Leishmania Donovani Infectivity in Whole Blood Using Riboflavin and Ultraviolet Light. Transfusion 2015, 55, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Cardo, L.J.; Rentas, F.J.; Ketchum, L.; Salata, J.; Harman, R.; Melvin, W.; Weina, P.J.; Mendez, J.; Reddy, H.; Goodrich, R. Pathogen Inactivation of Leishmania Donovani Infantum in Plasma and Platelet Concentrates Using Riboflavin and Ultraviolet Light. Vox Sang. 2006, 90, 85–91. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.; Ebaid, H.; Abdelmottaleb Moussa, S.A.; Alhazza, I.M.; Hassan, I.; Alaamer, A.; Al Tamimi, J. Potential Effects of the Combination of Nicotinamide, Vitamin B2 And Vitamin C on Oxidative-Mediated Hepatotoxicity Induced by Thioacetamide. Lipids Health Dis. 2018, 17, 29. [Google Scholar] [CrossRef]
- Khaydukov, E.; Mironova, K.; Semchishen, V.; Generalova, A.; Nechaev, A.; Khochenkov, D.; Stepanova, E.; Lebedev, O.; Zvyagin, A.; Deyev, S. Riboflavin Photoactivation by Upconversion Nanoparticles for Cancer Treatment. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Howe, C.G.; Li, Z.; Zens, M.S.; Palys, T.; Chen, Y.; Channon, J.Y.; Karagas, M.R.; Farzan, S.F. Dietary B Vitamin Intake Is Associated with Lower Urinary Monomethyl Arsenic and Oxidative Stress Marker 15-F(2t)-Isoprostane among New Hampshire Adults. J. Nutr. 2017, 147, 2289–2296. [Google Scholar] [CrossRef]
- von Martels, J.Z.; Bourgonje, A.R.; Klaassen, M.A.; Alkhalifah, H.A.; Sadaghian Sadabad, M.; Vich Vila, A.; Gacesa, R.; Gabriëls, R.Y.; Steinert, R.E.; Jansen, B.H. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP Study]. J. Crohn’s Colitis 2020, 14, 595–607. [Google Scholar]
- Melby, P.C.; Chandrasekar, B.; Zhao, W.; Coe, J.E. The Hamster as a Model of Human Visceral Leishmaniasis: Progressive Disease and Impaired Generation of Nitric Oxide in the Face of a Prominent Th1-Like Cytokine Response. J. Immunol. 2001, 166, 1912. [Google Scholar] [CrossRef]
- Lawler, N.G.; Gray, N.; Kimhofer, T.; Boughton, B.; Gay, M.; Yang, R.; Morillon, A.-C.; Chin, S.-T.; Ryan, M.; Begum, S.; et al. Systemic Perturbations in Amine and Kynurenine Metabolism Associated with Acute SARS-CoV-2 Infection and Inflammatory Cytokine Responses. J. Proteome Res. 2021, 20, 2796–2811. [Google Scholar] [CrossRef] [PubMed]
- Sühs, K.-W.; Novoselova, N.; Kuhn, M.; Seegers, L.; Kaever, V.; Müller-Vahl, K.; Trebst, C.; Skripuletz, T.; Stangel, M.; Pessler, F. Kynurenine Is a Cerebrospinal Fluid Biomarker for Bacterial and Viral Central Nervous System Infections. J. Infect. Dis. 2019, 220, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Girones, N.; Carbajosa, S.; Guerrero, N.A.; Poveda, C.; Chillon-Marinas, C.; Fresno, M. Global Metabolomic Profiling of Acute Myocarditis Caused by Trypanosoma Cruzi Infection. PLoS Negl. Trop. Dis. 2014, 8, e3337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine Is an Endothelium-Derived Relaxing Factor Produced During Inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Kimura, A.; Nakahama, T.; Chinen, I.; Masuda, K.; Nohara, K.; Fujii-Kuriyama, Y.; Kishimoto, T. Aryl Hydrocarbon Receptor Negatively Regulates Dendritic Cell Immunogenicity Via a Kynurenine-Dependent Mechanism. Proc. Natl. Acad. Sci. USA 2010, 107, 19961–19966. [Google Scholar] [CrossRef]
- Zhang, K.; Beverley, S.M. Phospholipid and Sphingolipid Metabolism in Leishmania. Mol. Biochem. Parasitol. 2010, 170, 55–64. [Google Scholar] [CrossRef]
- Parab, A.R.; McCall, L.I. Tryp-ing Up Metabolism: Role of Metabolic Adaptations in Kinetoplastid Disease Pathogenesis. Infect. Immun. 2021, 89, e00644-20. [Google Scholar] [CrossRef]
- Azzouz, S.; Lawton, P. In Vitro Effects of Purine and Pyrimidine Analogues on Leishmania Donovani and Leishmania Infantum Promastigotes and Intracellular Amastigotes. Acta Parasitol. 2017, 62, 582–588. [Google Scholar] [CrossRef]
- Lin, C.; Jaén Batista, D.d.G.; Mazzeti, A.L.; Donola Girão, R.; de Oliveira, G.M.; Karalic, I.; Hulpia, F.; Soeiro, M.d.N.C.; Maes, L.; Caljon, G.; et al. N6-Modification Of 7-Deazapurine Nucleoside Analogues as Anti-Trypanosoma Cruzi and Anti-Leishmania Agents: Structure-Activity Relationship Exploration And In Vivo Evaluation. Eur. J. Med. Chem. 2022, 231, 114165. [Google Scholar] [CrossRef]
- Nascimento, L.F.M.; Miranda, D.F.H.; Moura, L.D.; Pinho, F.A.; Werneck, G.L.; Khouri, R.; Reed, S.G.; Duthie, M.S.; Barral, A.; Barral-Netto, M.; et al. Allopurinol Therapy Provides Long Term Clinical Improvement, But Additional Immunotherapy Is Required for Sustained Parasite Clearance, In L. Infantum-Infected Dogs. Vaccine X 2019, 4, 100048. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Akpunarlieva, S.; Barrett, M.; Burchmore, R. A Defined Medium for Leishmania Culture Allows Definition of Essential Amino Acids. Exp. Parasitol. 2018, 185, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Parab, A.R.; Thomas, D.; Lostracco-Johnson, S.; Siqueira-Neto, J.L.; McKerrow, J.H.; Dorrestein, P.C.; McCall, L.-I. Dysregulation of Glycerophosphocholines in the Cutaneous Lesion Caused by Leishmania major in Experimental Murine Models. Pathogens 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Lal, C.S.; Pandey, K.; Das, V.N.R.; Das, P.; Roychoudhury, K.; Roy, S. Human Visceral Leishmaniasis: Decrease in Serum normalization was done in R versionLoad. Ann. Trop. Med. Parasitol. 2011, 105, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Liberopoulos, E.N.; Apostolou, F.; Gazi, I.F.; Kostara, C.; Bairaktari, E.T.; Tselepis, A.D.; Elisaf, M. Visceral Leishmaniasis Is Associated with Marked Changes in Serum Lipid Profile. Eur. J. Clin. Investig. 2014, 44, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.S.; Verma, R.B.; Verma, N.; Siddiqui, N.A.; Rabidas, V.N.; Pandey, K.; Singh, D.; Kumar, S.; Paswan, R.K.; Kumari, A.; et al. Hypertriglyceridemia: A Possible Diagnostic Marker of Disease Severity in Visceral Leishmaniasis. Infection 2016, 44, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Semini, G.; Paape, D.; Paterou, A.; Schroeder, J.; Barrios-Llerena, M.; Aebischer, T. Changes to Cholesterol Trafficking in Macrophages by Leishmania Parasites Infection. Microbiologyopen 2017, 6, e00469. [Google Scholar] [CrossRef]
- Ghosh, J.; Das, S.; Guha, R.; Ghosh, D.; Naskar, K.; Das, A.; Roy, S. Hyperlipidemia Offers Protection Against Leishmania Donovani Infection: Role of Membrane Cholesterol. J. Lipid. Res. 2012, 53, 2560–2572. [Google Scholar] [CrossRef]
- Rallis, T.; Day, M.J.; Saridomichelakis, M.N.; Adamama-Moraitou, K.K.; Papazoglou, L.; Fytianou, A.; Koutinas, A.F. Chronic Hepatitis Associated with Canine Leishmaniosis (Leishmania Infantum): A Clinicopathological Study of 26 Cases. J. Comp. Pathol. 2005, 132, 145–152. [Google Scholar] [CrossRef]
- Silva, L.C.; Castro, R.S.; Figueiredo, M.M.; Michalick, M.S.; Tafuri, W.L.; Tafuri, W.L. Canine Visceral Leishmaniasis as a Systemic Fibrotic Disease. Int. J. Exp. Pathol. 2013, 94, 133–143. [Google Scholar] [CrossRef]
- Rancoule, C.; Espenel, S.; Trone, J.C.; Langrand-Escure, J.; Vallard, A.; Rehailia-Blanchard, A.; El Meddeb Hamrouni, A.; Xia, Y.; Guy, J.B.; Ben-Mrad, M.; et al. Lysophosphatidic Acid (LPA) as a Pro-Fibrotic and Pro-Oncogenic Factor: A Pivotal Target to Improve the Radiotherapy Therapeutic Index. Oncotarget 2017, 8, 43543–43554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, S.H.; Chan, M.L.; Marathe, G.K.; Parveen, F.; Chen, C.H.; Ke, L.Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Albers, H.M.H.G.; Dong, A.; van Meeteren, L.A.; Egan, D.A.; Sunkara, M.; van Tilburg, E.W.; Schuurman, K.; van Tellingen, O.; Morris, A.J.; Smyth, S.S.; et al. Boronic Acid-Based Inhibitor of Autotaxin Reveals Rapid Turnover of LPA in the Circulation. Proc. Natl. Acad. Sci. USA 2010, 107, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- Ivens, A.C.; Peacock, C.S.; Worthey, E.A.; Murphy, L.; Aggarwal, G.; Berriman, M.; Sisk, E.; Rajandream, M.-A.; Adlem, E.; Aert, R.; et al. The Genome of the Kinetoplastid Parasite, Leishmania Major. Science 2005, 309, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. Comparative Genomic Analysis of Three Leishmania Species That Cause Diverse Human Disease. Nat. Genet. 2007, 39, 839–847. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesani, M.; Gosmanov, C.; Paun, A.; Lewis, M.D.; McCall, L.-I. Impact of Visceral Leishmaniasis on Local Organ Metabolism in Hamsters. Metabolites 2022, 12, 802. https://doi.org/10.3390/metabo12090802
Lesani M, Gosmanov C, Paun A, Lewis MD, McCall L-I. Impact of Visceral Leishmaniasis on Local Organ Metabolism in Hamsters. Metabolites. 2022; 12(9):802. https://doi.org/10.3390/metabo12090802
Chicago/Turabian StyleLesani, Mahbobeh, Camil Gosmanov, Andrea Paun, Michael D. Lewis, and Laura-Isobel McCall. 2022. "Impact of Visceral Leishmaniasis on Local Organ Metabolism in Hamsters" Metabolites 12, no. 9: 802. https://doi.org/10.3390/metabo12090802
APA StyleLesani, M., Gosmanov, C., Paun, A., Lewis, M. D., & McCall, L.-I. (2022). Impact of Visceral Leishmaniasis on Local Organ Metabolism in Hamsters. Metabolites, 12(9), 802. https://doi.org/10.3390/metabo12090802






