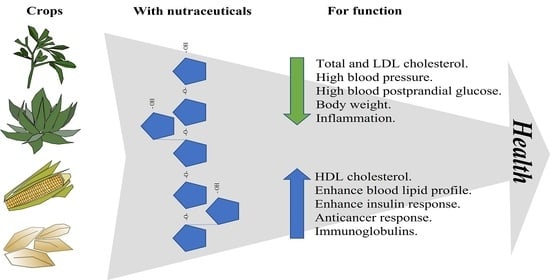
Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms
Abstract
:1. Main Crops of Hidalgo, Mexico, and Their Use in the Gastronomy and Health
2. Nutrients and Bioactive Compounds in Important Crops
2.1. Alfalfa (Medicago sativa L.)
2.2. Maguey (Agave spp.)
2.3. Maize (Zea mays L.)
2.4. Forage Oats (Avena sativa L.)
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Oladipo, A.; Enwemiwe, V.; Ejeromedoghene, O.; Adebayo, A.; Ogunyemi, O.; Fu, F. Production and functionalities of specialized metabolites from different organic sources. Metabolites 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Wishart, D.S.; Wani, S.H.; Hameed, M.K.; Mubin, M.; Saleem, F. Advances in metabolomics-driven diagnostic breeding and crop improvement. Metabolites 2022, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- SIAP/SADER. Hidalgo Infografia Agroalimentaria 2019. Available online: https://agroproductores.com/wp-content/uploads/2020/09/Hidalgo-Infografia-Agroalimentaria-2019.pdf (accessed on 2 July 2022).
- SIAP/SADER. Anuario Estadístico de la Producción Agrícola. 2022. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 3 July 2022).
- La Cocina Hidalguense|Mexico Desconocido. (n.d.). 2007. Available online: https://www.mexicodesconocido.com.mx/la-cocina-hidalguense.html (accessed on 15 June 2021).
- SIAP/SADER. Monografías/SIAP. 2021. Available online: https://www.gob.mx/siap/articulos/monografias-32658 (accessed on 2 July 2022).
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309, 125786. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierro, J.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martin, D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Castellini, C.; Falcinelli, B.; Sileoni, V.; Marconi, O.; Mancinelli, A.C.; Cotozzolo, E.; Benincasa, P. Effect of heat- and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 2019, 99, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Giuberti, G.; Rocchetti, G.; Sigolo, S.; Fortunati, P.; Lucini, L.; Gallo, A. Exploitation of alfalfa seed (Medicago sativa L.) flour into gluten-free rice cookies: Nutritional, antioxidant and quality characteristics. Food Chem. 2018, 239, 679–687. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Martino, M.; Mattioli, S.; Marconi, O.; Sileoni, V.; Ruggeri, S.; Tei, F.; Benincasa, P. The effect of dietary alfalfa and flax sprouts on rabbit meat antioxidant content, lipid oxidation and fatty acid composition. Meat Sci. 2015, 106, 31–37. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Martino, M.; Ruggeri, S.; Marconi, O.; Sileoni, V.; Falcinelli, B.; Castellini, C.; Benincasa, P. Alfalfa and flax sprouts supplementation enriches the content of bioactive compounds and lowers the cholesterol in hen egg. J. Funct. Foods 2016, 22, 454–462. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.I.; Kim, J.A.; Park, S.C.; Jeong, M.J. Sound waves increases the ascorbic acid content of alfalfa sprouts by affecting the expression of ascorbic acid biosynthesis-related genes. Plant Biotechnol. Rep. 2017, 11, 355–364. [Google Scholar] [CrossRef]
- Fiutak, G.; Michalczyk, M.; Filipczak-Fiutak, M.; Fiedor, L.; Surówka, K. The impact of LED lighting on the yield, morphological structure and some bioactive components in alfalfa (Medicago sativa L.) sprouts. Food Chem. 2019, 285, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Fiutak, G.; Tarko, T. Effect of hot water treatment of seeds on quality indicators of alfalfa sprouts. LWT-Food Sci. Technol. 2019, 113, 108270. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, A.; Gallo, A.; Lucini, L.; Giuberti, G.; Patrone, V. In vitro large intestine fermentation of gluten-free rice cookies containing alfalfa seed (Medicago sativa L.) flour: A combined metagenomic/metabolomic approach. Food Res. Int. 2019, 120, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Woch, W.; Hawrylak-Nowak, B. Selected antioxidant properties of alfalfa, radish, and white mustard sprouts biofortified with selenium. Acta Agrobot. 2019, 72, 1768. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Thomas, R.; Butler, E.; Macchi, F.; Williams, M. Phytochemicals in cancer prevention and therapy? BJMP. 2015, 8, a815. [Google Scholar]
- Nanditha, B.; Prabhasankar, P. Antioxidants in bakery products: A review. Crit. Rev. Food Sci. Nutr. 2009, 49, 1–27. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Dai, B.; An, Y.; Yu, L. Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from Alfalfa (Medicago sativa L.) stem. J. Agric. Food Chem. 2015, 63, 3219–3228. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Sun, H.; Wang, Y.; Yang, Z.; Zhang, G.; Jiang, S.; Yang, W. Polysaccharide from alfalfa activates RAW 264.7 macrophages through MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 126, 960–968. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Zhang, C.Y.; Li, M.; Lee, Y.; Zhang, G.G. Extract methods, molecular characteristics, and bioactivities of polysaccharide from Alfalfa (Medicago sativa L.). Nutrients 2019, 11, 1181. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Yang, W.; Yang, Z.; Jiang, S.; Zhang, C.; Zhang, G. Alfalfa polysaccharide prevents H2O2-induced oxidative damage in MEFs by activating MAPK/Nrf2 signaling pathways and suppressing NF-κB signaling pathways. Sci. Rep. 2019, 9, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Y.; Gan, L.P.; Du, M.Y.; Shang, Q.H.; Xie, Y.H.; Zhang, G.G. Effects of dietary supplementation of alfalfa polysaccharides on growth performance, small intestinal enzyme activities, morphology, and large intestinal selected microbiota of piglets. Livest. Sci. 2019, 223, 47–52. [Google Scholar] [CrossRef]
- Adams, S.; Xiangjie, K.; Hailong, J.; Guixin, Q.; Sossah, F.L.; Dongsheng, C. Prebiotic effects of alfalfa (Medicago sativa) fiber on cecal bacterial composition, short-chain fatty acids, and diarrhea incidence in weaning piglets. RSC Adv. 2019, 9, 13586–13599. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Kong, X.; Che, D.; Qin, G.; Jiang, H. Effects of dietary supplementation of alfalfa (Medicago Sativa) fibre on the blood biochemistry, nitrogen metabolism, and intestinal morphometry in weaning piglets. Appl. Ecol. Environ. Res. 2019, 17, 2275–2295. [Google Scholar] [CrossRef]
- Almaráz-Abarca, N.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Uribe-Soto, J.N.; González-Valdez, L.S. The phenols of the genus Agave (Agavaceae). J. Biomater. Nanobiotechnol. 2013, 4, 9–16. [Google Scholar] [CrossRef]
- Soto-Simental, S.; Caro, I.; Quinto, E.J.; Mateo, J. Effect of cooking lamb using maguey leaves (Agave salmiana) on meat volatile composition. Int. Food Res. J. 2016, 23, 1212–1216. [Google Scholar]
- Santos-Zea, L.; Leal-Diaz, A.; Cortes-Ceballos, E.; Gutierrez- Uribe, J. Agave (Agave spp.) and its traditional products as a source of bioactive compounds. Curr. Bioact. Compd. 2012, 8, 218–231. [Google Scholar] [CrossRef]
- Narvaez-Zapata, J.A.; Sánchez-Teyer, L.F. Agaves as a raw material: Recent technologies and applications. Recent Pat. Biotechnol. 2009, 3, 185–191. [Google Scholar] [CrossRef]
- Gutiérrez-Uribe, J.A.; Serna-Saldivar, S. Agave Syrup Extract Having Anticancer Activity. U.S. Patent 20090124685, B2, 14 May 2009. (Grant Date 26 June 2013). [Google Scholar]
- Ortiz-Torres, D.L.; Galván-Valencia, M.; Delgadillo-Ruíz, L.; Cabral-Arellano, F.J.; Bañuelos-Valenzuela, R.; León-Esparza-Ibarra, E. Extraction of saponins from leaves of Agaves. In Sustainable and Integral Exploitation of Agave; Gutiérrez-Mora, A., Ed.; CIATEJ: Guadalajara, Mexico, 2014; pp. 100–103. Available online: http://www.ciatej.net.mx/agave/1.7agave.pdf (accessed on 10 January 2022).
- Ortiz-Torres, D.; Galván-Valencia, M.; Delgadillo-Ruíz, L.; Huerta-García, J.; Cabral-Arellano, F.; Bañuelos-Valenzuela, R.; Esparza-Ibarra, E. Extracción y obtención de fracciones de saponinas a partir de hojas de los Agaves salmiana y tequilana weber de Zacatecas. In Proceedings of the XIII Simposium-Taller Nacional y VI Internacional “Producción y Aprovechamiento Del Nopal y Maguey”, Monterrey, Nuevo León, Mexico, 10 October 2014; pp. 169–182. [Google Scholar]
- García-Pedraza, L.G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Pinos-Rodríguez, J.M.; Martínez, J.F.; Santoyo, M.E. Effects of Agave salmiana Otto ex Salm-Dick high-fructose syrup on non-diabetic and streptozotocin-diabetic rats. J. Med. Plants Res. 2009, 3, 932–940. [Google Scholar]
- Gutiérrez-Uribe, J.A.; Santos-Zea, L.; Serna-Saldivar, S.R.O. Agave Syrup Extract Having Anticancer Activity. U.S. Patent 20130209588, B2, 2013. (Grant Date 7 March 2017). [Google Scholar]
- Santos-Zea, L.; Fajardo-Ramírez, O.R.; Romo-López, I.; Gutiérrez-Uribe, J.A. Fast centrifugal partition chromatography fractionation of concentrated Agave (Agave salmiana) sap to obtain saponins with apoptotic effect on colon cancer cells. Plant Foods Hum. Nutr. 2016, 71, 57–63. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Ríos, I.O.; González-García, G.; Mellado-Mojica, E.; Veloz García, R.A.; Dzul Cauich, J.G.; López, M.G.; García-Vieyra, M.I. Phytochemical profiles and classification of Agave syrups using 1H-NMR and chemometrics. Food Sci. Nutr. 2018, 7, 3–13. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Leal-Díaz, A.M.; Jacobo-Velázquez, D.A.; Rodríguez-Rodríguez, J.; García-Lara, S.; Gutiérrez-Uribe, J.A. Characterization of concentrated agave saps and storage effects on browning, antioxidant capacity and amino acid content. J. Food Compos. Anal. 2016, 45, 113–120. [Google Scholar] [CrossRef]
- Tovar-Robles, C.L.; Perales-Segovia, C.; Cedillo, A.N.; Valera-Montero, L.L.; Gómez-Leyva, J.F.; Guevara-Lara, F.; Hernández-Duque, J.L.M.; Silos-Espino, H. Effect of aguamiel (agave sap) on hematic biometry in rabbits and its antioxidant activity determination. Ital. J. Anim. Sci. 2011, 10, e21. [Google Scholar] [CrossRef]
- Dakah, A.; Zaid, S.; Suleiman, M.; Abbas, S.; Wink, M. In vitro propagation of the medicinal plant Ziziphora tenuior L. and evaluation of its antioxidant activity. Saudi J. Biol. Sci. 2014, 21, 317–323. [Google Scholar] [CrossRef]
- Campos, H.; Trejo, C.; Peña-Valdivia, C.B.; García-Nava, R.; Conde-Martínez, F.V.; Cruz-Ortega, M.D.R. Photosynthetic acclimation to drought stress in Agave salmiana Otto ex Salm-Dyck seedlings is largely dependent on thermal dissipation and enhanced electron flux to photosystem I. Photosynth. Res. 2014, 122, 23–39. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Gutiérrez-Mora, A.; García-Lara, S. Micropropagation of Agave salmiana: Means to production of antioxidant and bioactive principles. Front. Plant Sci. 2015, 6, 1026. [Google Scholar] [CrossRef]
- Santos-Zea, L.; Gutiérrez-Uribe, J.A.; Benedito, J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercrit. Fluids 2019, 144, 98–107. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Gutiérrez-Mora, A.; García-Lara, S. Effects on saponin, flavonol and antioxidant activity in vitro plants of Agave salmiana. In Sustainable and Integral Exploitation of Agave; CIATEJ: Guadalajara, Mexico, 2014; pp. 27–31. Available online: https://www.ciatej.mx/files/divulgacion/divulgacion_5b084050b05ed.pdf (accessed on 10 January 2022).
- Puente-Garza, C.A.; García-Lara, S.; Gutiérrez-Uribe, J.A. Enhancement of saponins and flavonols by micropropagation of Agave salmiana. Ind. Crops Prod. 2017, 105, 225–230. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Puente-Garza, C.A.; Espinosa-Leal, C.A.; García-Lara, S. Steroidal saponin and flavonol content and antioxidant activity during sporophyte development of maguey (Agave salmiana). Plant Foods Hum. Nutr. 2018, 73, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Leal-Díaz, A.M.; Santos-Zea, L.; Martínez-Escobedo, H.C.; Guajardo-Flores, D.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Effect of Agave americana and Agave salmiana ripeness on saponin content from aguamiel (agave sap). J. Agric. Food Chem. 2015, 63, 3924–3930. [Google Scholar] [CrossRef] [PubMed]
- Medina-Galván, M.I.; Bernardino-Nicanor, A.; Castro-Rosas, J.; Negrete-Rodríguez, M.L.X.; Conde-Barajas, E.; González-Cruz, L. Antimicrobial and antioxidant activity of flower scape extracts of Agave salmiana: Effect of the extraction solvent and development stage. Res. J. Biotechnol. 2018, 13, 12. [Google Scholar]
- Vargas-Rodríguez, L.; García-Vieyra, M.I.; León-Bata, B.I. Lozano-Sotomayor, P. Physical properties and microscopic structure of the Agave Salmiana cuticle (mixiote). Rev. Chapingo Ser. Zonas Áridas 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Garcia-Hernandez, M.H.; Delgado-Portales, R.E.; Corral-Fernandez, N.E.; Cortez-Espinosa, N.; Ruiz-Cabrera, M.A.; Portales-Perez, D.P. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int. J. Biol. Macromol. 2014, 63, 181–187. [Google Scholar] [CrossRef]
- Koenen, M.E.; Cruz Rubio, J.M.; Mueller, M.; Venema, K. The effect of agave fructan products on the activity and composition of the microbiota determined in a dynamic in vitro model of the human proximal large intestine. J. Funct. Foods 2016, 22, 201–210. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; Akkerman, R.; Oerlemans, M.M.P.; Logtenberg, M.J.; Schols, H.A.; Silva-Lagos, L.A.; López-Velázquez, G.; de Vos, P. β(2→6)-Type fructans attenuate proinflammatory responses in a structure dependent fashion via Toll-like receptors. Carbohydr. Polym. 2022, 277, 118893. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Urías-Silvas, J.E.; Morales-Hernández, N. The role of agave fructans in health and food applications: A review. Trends Food Sci. Technol. 2021, 114, 585–598. [Google Scholar] [CrossRef]
- Castañeda-Sánchez, A. Propiedades nutricionales y antioxidantes del maíz azul (Zea mays L.). TSIA 2011, 5, 75–83. [Google Scholar]
- Gyori, Z. Chapter 11, Corn: Grain-quality characteristics and management of quality requirements. In Cereal Grains: Assessing and Managing Quality, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Wrigley, C., Batey, I., Miskelly, D., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 257–290. ISBN 9780081007198. [Google Scholar] [CrossRef]
- Camelo-Méndez, G.A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Functional study of raw and cooked blue maize flour: Starch digestibility, total phenolic content and antioxidant activity. J. Cereal Sci. 2017, 76, 179–185. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.; Álvarez-Parrilla, E. Alimentos Vegetales Autóctonos Iberoamericanos Subutilizados. Copyright © Red ALSUB-CYTED, Fabro Editores. 2018. Available online: https://alimentos-autoctonos.fabro.com.mx/legal.html (accessed on 16 February 2022).
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of nutrient profile of quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus), and purple corn (Zea mays L.) consumed in the North of Argentina: Proximates, minerals and trace elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; Martínez-Bustos, F.; Soto-Hernández, M.; Ortega-Paczka, R.; Arellano-Vázquez, J.L. Efecto de la nixtamalización sobre las antocianinas del grano de maíces pigmentados. Agrociencia 2003, 37, 617–628. [Google Scholar]
- Mendoza-Díaz, S.; Ortiz-Valerio, M.C.; Castaño-Tostado, E.; Figueroa-Cárdenas, J.D.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Campos-Vega, R.; Loarca-Piña, G. Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented creole maize races (Zea mays L.). Plant Foods Hum. Nutr. 2012, 67, 442–449. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT-Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Lopez-Martinez, L.X.; Parkin, K.L.; Garcia, H.S. Phase II-Inducing, polyphenols content and antioxidant capacity of corn (Zea mays L.) from phenotypes of white, blue, red and purple colors processed into masa and tortillas. Plant Foods Hum. Nutr. 2011, 66, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Moreno, Y.; Pérez-Alonso, J.J.; Vázquez-Carrillo, G.; Aragón-Cuevas, F.; Velázquez-Cardelas, G.A. Anthocyanins and antioxidant act in maize grains of chalqueño elotes cónicos and bolita races. Agrociencia 2012, 46, 693–706. [Google Scholar]
- Žilić, S.; Serpen, A.; Akillioǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Flores-Silva, P.C.; Camelo-Méndez, G.A.; Paredes-López, O.; de Figueroa-Cárdenas, J.D. Effect of the nixtamalization process on the dietary fiber content, starch digestibility, and antioxidant capacity of blue maize tortilla. Cereal Chem. 2015, 92, 265–270. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Arufe, S.; Rubinos, S.N. Physicochemical characterization of white, yellow and purple maize flours and rheological characterization of their doughs. J. Food Sci. Technol. 2015, 52, 7954–7963. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Serna-Saldivar, S.O.; Muy-Rangel, M.D.; Valdez-Torres, J.B. Total phenolics, total anthocyanins and antioxidant capacity of native and elite blue maize hybrids (Zea mays L.). CYTA-J. Food 2015, 13, 336–339. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Camelo-Mendez, G.A.; Agama-Acevedo, E.; Utrilla-Coello, R.G. Aspectos nutracéuticos de los maíces pigmentados: Digestibilidad de los carbohidratos y antocianinas. Agrociencia 2016, 50, 1041–1063. [Google Scholar]
- Mora-Rochín, S.; Gaxiola-Cuevas, N.; Gutiérrez-Uribe, J.A.; Milán-Carrillo, J.; Milán-Noris, E.M.; Reyes-Moreno, C.; Serna-Saldivar, S.O.; Cuevas-Rodríguez, E.O. Effect of traditional nixtamalization on anthocyanin content and profile in Mexican blue maize (Zea mays L.) landraces. LWT-Food Sci. Technol. 2016, 68, 563–569. [Google Scholar] [CrossRef]
- Mansilla, P.S.; Nazar, M.C.; Pérez, G.T. Flour functional properties of purple maize (Zea mays L.) from Argentina. Influence of environmental growing conditions. Int. J. Biol. Macromol. 2020, 146, 311–319. [Google Scholar] [CrossRef] [PubMed]
- González-Manzano, S.; Pérez-Alonso, J.J.; Salinas-Moreno, Y.; Mateus, N.; Silva, A.M.S.; de Freitas, V.; Santos-Buelga, C. Flavanol-anthocyanin pigments in corn: NMR characterisation and presence in different purple corn varieties. J. Food Compost. Anal. 2008, 21, 521–526. [Google Scholar] [CrossRef]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and phenolic acids of hybrid and native blue maize (Zea mays L.) extracts and their antiproliferative activity in mammary (MCF7), liver (HepG2), colon (Caco2 and HT29) and prostate (PC3) cancer cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef]
- Hu, Q.P.; Xu, J.G. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef]
- Pedreschi, R.; Cisneros-Zevallos, L. Phenolic profiles of andean purple corn (Zea mays L.). Food Chem. 2007, 100, 956–963. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef]
- Ruiz Canizales, J.; Heredia, J.B.; Domínguez Avila, J.A.; Madera Santana, T.J.; Villegas Ochoa, M.A.; Robles Sánchez, R.M.; González Aguilar, G.A. Microencapsulation of blue maize (Zea mays L.) polyphenols in two matrices: Their stability during storage and in vitro digestion release. J. Food Meas. Charact. 2019, 13, 892–900. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef]
- Villacres Poveda, C.E.; Tanquina Páramo, I.M.; Yáñez Guzmán, C.F.; Quelal Tapia, M.B.; Alvarez Murillo, M.J.; Ramos Moya, M.R. Impacto del procesamiento sobre los compuestos con propiedades antioxidantes de dos variedades de maíz (Zea mays L.). ACI Adv. Cienc. Ing. 2019, 11, 104–115. [Google Scholar] [CrossRef]
- Ronceros, G.; Ramos, W.; Arroyo, J.; Galarza, C.; Gutiérrez, E.L.; Ortega-Loayza, A.G.; La Rosa, C.; Cucho, C.; Palma, L. Estudio comparativo del maíz morado (Zea mays L.) y simvastatina en la reducción de lípidos séricos de pacientes diabéticos normotensos con dislipidemia. An. Med. 2012, 73, 113. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Dia, V.P.; West, L.; West, M.; Singh, V.; Wang, Z.; Allen, C. Temperature dependency of shelf and thermal stabilities of anthocyanins from corn distillers’ dried grains with solubles in different ethanol extracts and a commercially available beverage. J. Agric. Food Chem. 2015, 63, 10032–10041. [Google Scholar] [CrossRef]
- Li, C.Y.; Kim, H.W.; Li, H.; Lee, D.C.; Rhee, H.I. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem. 2014, 152, 592–596. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Oat beta-glucan: Its role in health promotion and prevention of diseases. Compr. Rev. Food Sci. Food Saf. 2012, 11, 355–365. [Google Scholar] [CrossRef]
- Bae, I.Y.; Kim, S.M.; Lee, S.; Lee, H.G. Effect of enzymatic hydrolysis on cholesterol-lowering activity of oat β-glucan. New Biotechnol. 2010, 27, 85–88. [Google Scholar] [CrossRef]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B.R. β-Glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Hooda, S.; Matte, J.J.; Vasanthan, T.; Zijlstra, R.T. Dietary purified oat β-glucan reduces peak glucose absorption and portal insulin release in portal-vein catheterized grower pigs. Livest. Sci. 2010, 134, 15–17. [Google Scholar] [CrossRef]
- Dong, J.; Cai, F.; Shen, R.; Liu, Y. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chem. 2011, 129, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, U.; Cummins, E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition 2011, 27, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yang, X.; Niu, X.; Liu, S.; Ren, G. Chemical characterization of the avenanthramide-rich extract from oat and its effect on D-galactose-induced oxidative stress in mice. J. Agric. Food Chem. 2011, 59, 206–211. [Google Scholar] [CrossRef]
- Choromanska, A.; Kulbacka, J.; Rembialkowska, N.; Pilat, J.; Oledzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat beta-glucan—An in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef]
- Choromańska, A.; Kulbacka, J.; Harasym, J.; Dubińska-Magiera, M.; Saczko, J. Anticancer activity of oat β-glucan in combination with electroporation on human cancer cells. Acta Pol. Pharm. 2017, 74, 616–623. [Google Scholar]
- Choromanska, A.; Kulbacka, J.; Harasym, J.; Oledzki, R.; Szewczyk, A.; Saczko, J. High- and low-molecular weight oat beta-glucan reveals antitumor activity in human epithelial lung cancer. Pathol. Oncol. Res. 2018, 24, 583–592. [Google Scholar] [CrossRef]
- Sunderam, V.; Mohammed, S.S.S.; Madhavan, Y.; Dhinakaran, M.; Sampath, S.; Patteswaran, N.; Thangavelu, L.; Lawrence, A.V. Free radical scavenging activity and cytotoxicity study of fermented oats (Avena sativa). Int. J. Res. Pharm. Sci. 2020, 11, 1259–1262. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J.Β. 1,3-Glucan in cancer treatment. Am. J. Immunol. 2012, 8, 38–43. [Google Scholar] [CrossRef]
- Perrelli, A.; Goitre, L.; Salzano, A.M.; Moglia, A.; Scaloni, A.; Retta, S.F. Biological activities, health benefits, and therapeutic properties of avenanthramides: From skin protection to prevention and treatment of cerebrovascular diseases. Oxidative Med. Cell. Longev. 2018, 2018, 6015351. [Google Scholar] [CrossRef]
- Guo, W.; Nie, L.; Wu, D.; Wise, M.L.; Collins, F.W.; Meydani, S.N.; Meydani, M. Avenanthramides inhibit proliferation of human colon cancer cell lines in vitro. Nutr. Cancer 2010, 62, 1007–1016. [Google Scholar] [CrossRef]
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Battilana, P.; Ornstein, K.; Minehira, K.; Schwarz, J.M.; Acheson, K.; Schneiter, P.; Burri, J.; Jéquier, E.; Tappy, L. Mechanisms of action of β-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. 2001, 55, 327–333. [Google Scholar] [CrossRef]
- Meydani, M. Potential health benefits of avenanthramides of oats. Nutr. Rev. 2009, 67, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, Y.; Yerke, A.; Wise, M.L.; Johnson, J.; Chu, Y.; Sang, S. Oat avenanthramides induce heme oxygenase-1 expression via Nrf2-mediated signaling in HK-2 cells. Mol. Nutr. Food Res. 2015, 59, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zubik, L.; Collins, F.W.; Marko, M.; Meydani, M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis 2004, 175, 39–49. [Google Scholar] [CrossRef]
- Cerio, R.; Dohil, M.; Downie, J.; Magina, S.; Mahé, E.; Stratigos, A.J. Mechanism of action and clinical benefits of colloidal oatmeal for dermatologic practice. J. Drugs Dermatol. 2010, 9, 1116–1120. [Google Scholar]
- Wu, J. Anti-inflammatory ingredients. J. Drugs Dermatol. 2008, 7, s13-6. [Google Scholar]
- Reynertson, K.A.; Garay, M.; Nebus, J.; Chon, S.; Kaur, S.; Mahmood, K.; Kizoulis, M.; Southall, M.D. Anti-inflammatory activities of colloidal oatmeal (Avena sativa) contribute to the effectiveness of oats in treatment of itch associated with dry, irritated skin. J. Drugs Dermatol. 2015, 14, 43–48. [Google Scholar]
- Krag, A.; Munkholm, P.; Israelsen, H.; Von Ryberg, B.; Andersen, K.K.; Bendtsen, F. Profermin is efficacious in patients with active ulcerative colitis—A randomized controlled trial. Inflamm. Bowel Dis. 2013, 19, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, S.; Nasiri, A.; Waghei, Y.; Morshedi, J. Comparison of Avena Sativa, vinegar, and hydroxyzine for uremic pruritus of hemodialysis patients a crossover randomized clinical trial. Iran. J. Kidney Dis. 2015, 9, 316–322. [Google Scholar] [PubMed]
- Ilnytska, O.; Kaur, S.; Chon, S.; Reynertson, K.A.; Nebus, J.; Garay, M.; Mahmood, K.; Southall, M.D. Colloidal oatmeal (Avena Sativa) improves skin barrier through multi-therapy activity. J. Drugs Dermatol. 2016, 15, 684–690. [Google Scholar] [PubMed]
| No. | Crop | Area (Ha) | Production (Tons) | Yield (Tons Ha−1) | Harvest | ||
|---|---|---|---|---|---|---|---|
| Sown | Harvested | Loss | Obtained | Obtained | |||
| 1 | Grain corn (white) | 210,343.55 | 201,453.30 | 8890.25 | 618,156.85 | 3.07 | Agricultural year |
| 2 | Green forage oats | 24,161.78 | 24,049.78 | 112.00 | 324,378.96 | 13.49 | |
| 3 | Grain barley | 106,956.06 | 106,113.16 | 842.90 | 223,595.43 | 2.11 | |
| 4 | Beans | 17,420.18 | 17,381.18 | 39.00 | 13,338.79 | 0.77 | |
| 1 | Green alfalfa | 43,829.00 | 43,829.00 | 0.00 | 4,477,712.05 | 102.16 | Perennial |
| 2 | Pulquero maguey (honey water: thousands of liters) | 4842.20 | 1372.20 | 0.00 | 110,411.07 | 80.46 | |
| 3 | Orange (Valencia) | 5747.90 | 5478.50 | 0.00 | 65,627.47 | 11.98 | |
| 4 | Cherry coffe | 23,069.50 | 23,014.50 | 0.00 | 29,301.60 | 1.27 | |
| Treatments | Conditions | Effects | References |
|---|---|---|---|
| Alfalfa sprout supplementation in a rabbit diet. | Ninety mixed white rabbits were fed for 50 days, divided into 3 groups:
| The alfalfa sprouts presents increase in the total content of fatty acids (PUFA) (linoleic acid by 38.46% and linolenic acid by 70.05%), isoflavones (daidzein) in diets. The linolenic acid content in muscle of the alfalfa group was three times higher than control group. α-tocopherol content and α-tocotrienol, were up. | [12] |
| Dietary supplementation with alfalfa sprouts. | Dietary supplementation with alfalfa sprouts (40 g d−1) and quantification of bioactive compounds and cholesterol in chicken and chicken eggs. | Decreased cholesterol in chicken plasma from 79.2 to 65.2 mg dL−1 and in egg yolk from 11.5 to 10.4 mg g−1. | [13] |
| Sprouts alfalfa exposed to sound wave | Frequencies (250, 500, 800, 1000, and 1500 Hz) for two 1-h periods until 6 days. | Increase (24–50%) in the expression of genes that promote the production of L-ascorbic acid in sprouts (to 500 and 1000 Hz). The treatments increased the concentration of ascorbic acid and the antioxidant enzyme superoxide dismutase. | [14] |
| Substitution with alfalfa seed flour. | Adding alfalfa seed flour (0, 15, 30, 45% w/w) in rice flour biscuits (gluten free). | Increased linearly in: crude protein, total dietary fiber, total polyunsaturated, hardness, total phenolic content (22.9 to 112.9 mg GAE 100 g−1 DW for control and 45% substituted flour), and resistant starch. The antioxidant capacity increased proportionally from 14.7 to 194.6 μmol GAE 100 g−1 DW (FRAP) and from 739.3 to 3627.7 μmol TE 100 g−1 DW (ORAC), for control and 45% of alfalfa flour, respectively. | [11] |
| Different types of LED lighting in alfalfa sprout composition (FMC: fresh mass of cotyledons). | Four variants using cold white (10,032 K), warm white (3279 K), red green blue (RGB) LEDs with two chips activated: red and blue, which combined gave a violet colour. | Chlorophyll (a) up to 998.9 mg kg−1 in FMC with cold LED. β-carotene up to 44.6 mg kg−1 in FMC with red-green-blue LED (RGB). Chlorophyll a up to 843.3 mg kg−1 FMC, chlorophyll b up to 256.7 mg kg−1 FMC, β-carotene up to 21.6 mg kg−1 FMC, lutein up to 82.6 mg kg−1 FMC, neoxanthin up to 15 mg kg−1 FMC and violaxanthin up to 43.7 mg kg−1 FMC in shoots with sunlight. Total phenols up to 697 mg GAE kg−1 in FMC with blue LED (RGB). Ascorbic acid up to 155 mg kg−1 with sunlight. | [15] |
| Dry alfalfa sprouts (dry heat, freeze-dried). | Heat-dried samples (HD): stove at 60 °C for 24 h, and the freeze-dried samples (FD) for 24 h under vacuum (50 mTorr). | Greater decrease in isoflavone composition with the application of oven heat drying than with lyophilization. The lyophilisate increased the sterols concentration (41.82% for stigmasterol). The presence of carotenoids (zeaxanthin, β-carotene, retinol, lutein) was only detected after drying processes (not fresh). | [10] |
| Soak in water of seeds alfalfa. | Seeds were disinfected with hot water: soak at 85 °C, for 10 s; at 85 °C, for 40 s; at 90 °C, for 10 s; and at 100 °C for 10 s. | No effect on: lutein (23.4–26.6 mg kg−1), violaxanthin (16.0–17.2 mg kg−1), neoxanthin (3.5–4.1 mg kg−1), β-carotene (10.1–11.7 mg kg−1), total phenols (486.5–599.4 mg kg−1) and chlorophyll b (64.7–72.8 mg kg−1). The application of 100 °C caused a decrease in the content of ascorbic acid (from 84.5 a 67.5 mg kg−1) and a increased phenolic content (from 537.1 to 599.4 mg kg−1). | [16] |
| Supplementation, digestion and in vitro fermentation. | Adding alfalfa seed flour (0, 30, 45% w/w) in rice flour biscuits (gluten free). Simulated in vitro digestion and fermentation process. | Cookies with 30 and 45% of alfalfa seed flour presented the highest total phenolic content (0.42 and 0.56 mg g−1, respectively) (control 0.15 mg g−1). The in vitro fermentation of 8–48 h increased the concentration of lignans and phenolic acids, whose bioaccessibility at 24 h of in vitro fermentation were 16.2 and 12.2%, respectively. | [17] |
| Incorporation of selenium to alfalfa crop. | Inorganic selenium was used in two chemical forms: selenite (Na2SeO3) or selenate (Na2SeO4) (0, 20, or 200 μmol L−1). | Increase in anthocyanins in alfalfa (29%) after of 20 µmol L−1 selenite solution (≈8% reduction of DPPH). | [18] |
| Ultrasound in fresh alfalfa leaves. | Study factors and ranges: Solvent/raw material ratio (mL g−1): 5, 10, 15. Time (h): 1, 2, 3. Temperature (°C): 50, 65, 80. Power (W): 50, 100, 150. Ethanol concentration (%): 60, 75, 90. | Better yield (up to 1.61%) and bioaccessibility (up to 19.7%) of saponins. Conditions: solvent/raw material (9.5 mL g−1), extraction time (2.90 h), extraction temperature (79.1 °C), ultrasound power (111.0 W), ethanol concentration (88.2%). | [8] |
| Treatments | Conditions | Effects | Reference |
|---|---|---|---|
| Micropropagation | In vitro of plants from young germinated plantlets by axillary shoots. | Wild plants showed the highest phenolic content (13.06 mg EGA g−1). The antioxidant capacity was higher in vitro (369.84 µmol TE g−1 DW) than in normal ex vitro conditions (184.13 µmol TE g−1 DW) and with ex vitro irrigation (143.38 µmol TE g−1 DW) and than in wild conditions (130.39 μmol TE g−1 DW). Glycosylated flavanols were detected in plants with ex vitro irrigation (quercetin) and under normal ex vitro conditions (kaempferol). Saponins were detected: hecogenin (0.418–5.227 mg EHe g−1), tigogenin (18.821–31 mg EHe g−1), mannogenin (0.288–0.861 mg EHe g−1), and chlorogenin (0.339–2.042 mg EHe g−1). | [47] |
| Micropropagation was from axillary shoots. Leaf tissue samples were taken from the in vitro plants, ex vitro acclimated plants obtained from open environment conditions, and plants obtained from a natural population. | The total phenolic acids were 35 and 40% higher in plants propagated in vitro (11.8 mg GAE g−1 DW) and ex vitro (10.8 mg GAE g−1 DW), compared with the wild type (7 mg GAE g−1 DW). The saponin content of plants in vitro (77.1 mg PE g−1 DW) and ex vitro (63.3 mg PE g−1 DW) were higher than those of wild type plants (2.1 mg PE g−1 DW). The antioxidant capacity (ORAC) of the plants in vitro (369 μmol TE g−1 DW) was higher compared to ex vitro and wild type (184 and 146 μmol TE g−1 DW, respectively). | [45] | |
| Hydrometanolic extraction was applied to the foliar tissues and the content of flavonols and saponins was analyzed. | The plants propagated in vitro presented a higher concentration of flavonols and saponins, quantifying 7 flavanols and 5 saponins. Herbacetin (most abundant flavonol found): wild plants (14.7 mg 100 g−1 DW), in vitro (16.3 mg 100 g−1 DW), in an open environment (38.4 mg 100 g−1). Tigogenin (most abundant saponin found and only detected in plants propagated): in vitro with 6895.2 mgPE 100 g−1 DW and 4997.8 mgPE 100 g−1 DW. | [48] | |
| In vitro drought stress effect, generated by polyethylene glycol. | Stress medium: Murashige and Skoog (4.4 g L−1, pH 5.8, 30 g L−1 sucrose, and L2 vitamins) with polyethylene-glycol (0, 10, 20, 30%, 27 °C, photoperiod of 12:12 h light:dark, 60 days). | Plants grown with polyethylene glycol (30%) showed the lowest flavonol content, but the highest saponin content (tigogenin glycoside, 163 mg PE g−1 DW) and the highest antioxidant capacity (ORAC) (≈1000 mmol TE g−1 DW). | [49] |
| Ultrasonically-assisted supercritical fluid extraction (USFE). | Bagasse of Agave salmiana (part not indicated; 10 g). Process factors were pressure (150–450 bar), temperature (40–60 °C), and amount of co-solvent (5–10%). | Increased antioxidant capacity (FRAP) with the use of multiplate (US) transducer geometry of extracts at 20.91 μmol TE g−1 and saponin content at 61.59 μg g−1; comparing with the cylinder geometry (with 12.18 TE g−1 and 19.05 μg g−1, respectively). | [46] |
| Corn | Treatments | Compounds and/or Products | Bioactivity | Reference |
|---|---|---|---|---|
| Mexican corn (13 pigmented grain): Arrocillo Amarillo (red, blue), Bolita (red, blue), Chihuahua Crystal Blue (blue, red), chalqueño corn (red, blue). | Nixtamalization with alkali (0.8% of the grain weight) for 30 min, followed by resting for 14–16 h, ambient drying, grinding, and sieving (0.5 mm). | Decreases the anthocyanin concentration of corn grains in the pericarp by 73 to 100%, varying according to the type of corn and portion. | [63] | |
| Mexican corn (18 phenotypes). | White corn. Yellow corn. | Total phenolic content:
Total anthocyanin content:
| Antioxidant capacity:
| [65] |
| White, red, blue and purple corn (var Ver 42) | Ethanolic extracts (95%) from nixtamalized grains. Tortilla with nixtamalized grain. | The treatments reduced total phenols, and anthocyanins. Total phenolic content and anthocyanin (respectively):
| The processing negatively affected the capacities of the grains. Quinone reductase induction (QR): purple > White > red > blue (anticancer activity). The purple genotype (Ver 42) and its products (dough and tortilla) showed the highest antioxidant capacity (70% by ABTS, 55% by PRAC) and QR (induction twice at 125 g mL−1). | [66] |
| Creole maize races (Zea mays L.) and pigmented varieties (yellow, red and blue). | Nixtamalization (alkaline boiling) and production of dough (grinding, drying) and tortillas. | Carotenoid content (μg of β-carotene eq g−1 extract) of raw maize grains and their products (masa, tortilla) respectively:
For white grains, nixtamalization reduced carotenoids by 53 to 56%. Yellow grain suffered the highest losses from anthocyanins (174.44 to 10.30 mg of c3-GE 100 g−1 DW), not detectable in white maize and its products. The anthocyanin content of all grains was 174.44 to 963.00 mg of c3-GE 100 g−1 DW. | White corn (≈30%) and products (dough ≈20%, tortilla ≈25%) had higher antiradical (DPPH) activity than BHT (≈10% to 100 µM). Yellow corn (≈22%) and products (dough ≈18%, tortilla ≈29%) had higher antiradical activity (DPPH) than BHT (≈10% to 100 µM). Red (50%) and blue (40%) maize grain showed the highest antiradical activity. The antimutagenic activity (S. typhimurium TA98) of the grains:
| [64] |
| 18 samples of blue/purple grain of conical corn (EC), Chalqueño (CHAL), and Bolita (BOL) maize races. | 40 grains without the germ, crushed, sieved (0.5 mm), and dried in an oven (40 °C, 18 h). Analysis extract by methanol (acidified to 1% with trifluoroacetic acid) and sonicated for 15 min. | Anthocyanins totals (AT) content (CHAL): varied from 579.4 to 1046.1 mg c3-GE kg−1 DW. The total soluble phenols (TSP) (CHAL): varied from 918.9 to 1479.2 mg GAE kg−1 DW. AT content (EC): varied from 997.8 to 1332.2 mg c3-GE kg−1 DW. TSP (EC): varied from 1328.6 to 1626.7 mg GAE kg−1 DW. AT content (BOL): varied from 304.1 to 528.0 mg c3-GE kg−1 DW. TSP (BOL): varied from 875.0 to 1276.2 mg GAE kg−1 DW. | Antioxidant activity (AA) (Chalqueño): 34 to 60.3% by DPPH. AA (Elote cónico): 46.6 to 60.4% by DPPH. AA (Bolita): 21.0 to 39.5% by DPPH. | [67] |
| Whole grains of 10 different colored corn (Zea mays L.) genotypes (landrace and an inbred line, over the year 2010). | Combined extracts: acetone/methanol/water (7:7:6, v/v/v), with alkaline hydrolysis and extracted with ethyl acetate and diethyl ether (1:1, v/v). | White and yellow corn:
| White and yellow showed antioxidant capacity (ABTS) between 15 and 20 mmol Trolox kg−1 DW. The light blue genotype had the highest scavenging activity (ABTS: 35.66 mmol Trolox kg−1 DW). | [68] |
| Blue corn flour | Nixtamalization. Maize grain in cooking (1:2, grain: water), 1.0% (w/w) of calcium hydroxide to 90 °C for 23 min, was soaked for 16 h at ambient temperature, was grounded, and passed through a flash dryer (260 °C for 4 s), the obtained flour was grounded in a mill using a hammer head and a 0.5 mm mesh screen. | Not change the resistant starch content or slow digestion. | The tortilla made with blue corn nixtamalized presented a lower glycemic index (58) and presented antioxidant capacity in the different fractions. They suggest a direct relationship between polyphenol content and antioxidant activity. | [69] |
| Spanish maize kernels, white (WF, Rebordanes variety), yellow (YF, Sarreaus variety) and purple (PF, Meiro variety). | Air-drying the maize kernels using a pilot-scale tray dryer (45 °C, 2 m s−1, 30% relative humidity, 5 kg m−2 of loading density, until an average maize moisture content of 11% DW), crushed, ground, and sieved (200 y 500 µm). | Total starch (TS, % w/w, DW) content of tested maize flours, yellow, white, and purple, ranged from 60.1 (whole flour 500 µm) up to 75.2 (purple 200 µm) and no clear differences between varieties were found. | No significant differences were observed among water desorption isotherms of maize varieties. | [70] |
| Five blue hybrid maize genotypes and Chalqueño and conic kernels were used as native genotypes cultivated in the highlands of Mexico. | Homogenized with 80% ethanol for 10 min, alkaline digestion (2 M NaOH), acidification (HCl), extraction with ethyl acetate. | The total anthocyanins and anthocyanins in free phenolics of the natives, chalqueño and conic are 646 and 892 mg c3-G kg−1 and 48.7 and 60.3%, respectively. The total anthocyanins and anthocyanins in free phenolics of the hybrid genotypes are in the range of 835–1052 mg c3-G kg−1 and 62.4–80.6%, respectively. | Antioxidant capacity (free and bound phenols, respectively):
| [71] |
| White corn. Yellow corn. | White corn kernel (anthocyanin free). Yellow corn kernel (702 mg c3-GE kg−1) | Antioxidant capacity of:
| [72] | |
| Native Mexican blue corn (Zea mays L.). | Nixtamalization (maize kernels were cooked (1:3, maize grains/water) with 5.4 g of Ca(OH)2 L−1 water; 31 min, 85 °C, 8.1 h). Wet nixtamal was dried (55 °C/12 h), cooled, and milled to pass through an 80-US mesh (0.180 mm). | Increases the relative percentage of glycosylated anthocyanins and decreases acylated anthocyanins. The most abundant compounds (cyanidin-3-(6″-succinylglucoside) (Cy-Suc-Glu) and cyanidin-3-(6″-disuccinylglucoside) (Cy-diSuc-Glu)). | [73] | |
| Blue and white cornmeal | Cooked samples were prepared in water (1:10 w/v) by a heating bath with shaking for 30 min. | Extractable polyphenols:
| Antioxidant capacity and alpha-amylase inhibition (AAI):
| [60] |
| Purple corn grain flours (control: White corn) | Mixtures of various families (genotypes) of purple corn. Homogenized with ethanol (96%)/HCl (1 N) (85:15 v/v), 30 min. | White corn presented a total phenol concentration: 319 mg GAE 100 g−1. Total phenols (mixtures genotype) (range): 438 to 1933 mg GAE 100 g−1. Total phenols (original genotype): 1328 mg GAE 100 g−1. | [74] |
| Crop | Compounds and/or Products | Conditions of Bioactivity Detected | Bioactivity | Reference |
|---|---|---|---|---|
| Oat (no variety reported) | Oat bran concentrate containing 43% β-glucan. | Oat β-glucan hydrolysate was prepared by adding Celluclast (840 EGU g−1) to oat bran concentrate suspension (6.25% (w/v), 50 °C, pH 4.8). | Anti-cholesterol activity: reduced rat serum triglycerides, reduced weight gain, high-density cholesterol (HDL-C) in serum increased up to 42–62% and reduced low-density cholesterol (LDL) by 25–31%. | [89] |
| Oat (Derby variety) | β-glucan | Two β-glucan extracts were separately added to test solutions at concentrations of 0.1–0.5% (w/w). β-glucan fractions: 78.5% (E3, E4) content of extracts (w/w). | Decreased intestinal absorption of fatty acids (18:2 mainly). Inhibition of postprandial rise in glucose and insulin. | [90] |
| Oat (no variety reported) | β-glucan | Consumption in pigs of 3 and 6% in the diet. | Net glucose absorption reduction from 22 to 51%, relative to the intake percentage. | [91] |
| Oat (Avena sativa L.) | β-glucan | Dosage of 2000 mg kg−1 in reduction of hyperglycemia. Intake dose of 70 mg mL−1 for 6 weeks for enzyme inhibition. | Reduction of hyperglycemia. Inhibition of intestinal enzymes, sucrase (70.72%), maltase (83.33%) and lactase (89.43%), in diabetic mice. Similar protective effect to the diabetic mice as metformin (1% w/v metformin solution). | [92] |
| Oat (no variety reported) | β-glucan | Extract viscosity of 3 mPa, with the presence of starch of 40 g. | Glucose absorption reduction. | [93] |
| Oat (no variety reported) | β-glucan | Consumption of 3 g d−1 of oat or barley β-glucan is sufficient to decrease blood cholesterol. | There was a significant inverse relation in total cholesterol (−0.60 mmol L−1, −0.85 to −0.34), low−density lipoprotein (−0.66 mmol L−1, −0.96 to −0.36), and triglyceride/triacylglycerol (−0.04 mmol L−1, −0.15 to 0.07) after consumption of β-glucan. | [94] |
| Oat (Avena sativa L.) | Extract avenanthramides (EA) is: 6.07% N-(3′,4′-dihydroxycinamoyl)-5-hydroxyanthranilic acid, 4.37% N-(4′-hydroxycinnamoyl)-5-hydroxyanthranilic acid, 4.37% N-(4′-hydroxycinnamoyl)-5-hydroxyanthranilic acid, and 5.36% N-(4′-hydroxy-3′-methoxycinnamoyl)-5-hydroxyanthranilic acid. Phenols: vanillic acid (0.60%), caffeic acid (0.50%), syringic acid (0.54%), p-coumaric acid (0.16%), ferulic acid (0.08%), and sinapic acid (0.03%). | Mice in three experimental groups were (7th week) given EA at 250, 500, and 1000 mg (kg body weight)−1 d−1 by intragastric gavage (2 weeks). Mice were sacrificed and the liver was collected and stored until analysis. | Antioxidant effect against oxidative stress induced by D-galactose (50 mg kg−1 DW d−1) in mice, noted by increased antioxidant enzyme activity (dose-dependent mode) and the regulation of antioxidant gene expression. | [95] |
| Oat (no variety reported) | β-glucan (low molecular weight) | Concentration of 400 μg mL−1. | Deceased cancer cells viability (human pigmented malignant melanoma (Me45) and the human epidermoid carcinoma A431 cell line), while for the normal cells it was non-toxic. | [96] |
| Oat (no variety reported) | β-glucan | β-glucan (200 µg mL−1) with electroporation. | Antitumor activity due to decreased cell viability (human melanoma cell line (Me45)) of 12.5%. Not present toxic effects on normal cells. | [97] |
| Oat (no variety reported) | β-glucan (high and low molecular weight). | Decreased viability of cancer cells (human lung A549, H69AR) (about 50% decrease at 200 µg mL−1). | [98] | |
| Oat (Avena sativa L.) | Avenantramide | 100 μL of Lactobacillus acidophilus was added to finely powdered oats (solution 1 g/50 mL water) for fermented oats. And control was measured (non-fermented). | In vitro studies revealed that fermented and non-fermented oats displayed higher antioxidant activity, having a corresponding IC50 value of 201.03 μL and 236.46 μL, respectively. The colon cancer cell (HT29) death percentage, varied in the range of 41.81% and 87.48%, with the highest cytotoxic activity being for non-fermented oats (25 µg mL−1). | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones-Muñoz, T.A.; Villanueva-Rodríguez, S.J.; Torruco-Uco, J.G. Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms. Metabolites 2022, 12, 806. https://doi.org/10.3390/metabo12090806
Quiñones-Muñoz TA, Villanueva-Rodríguez SJ, Torruco-Uco JG. Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms. Metabolites. 2022; 12(9):806. https://doi.org/10.3390/metabo12090806
Chicago/Turabian StyleQuiñones-Muñoz, Tannia A., Socorro J. Villanueva-Rodríguez, and Juan G. Torruco-Uco. 2022. "Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms" Metabolites 12, no. 9: 806. https://doi.org/10.3390/metabo12090806
APA StyleQuiñones-Muñoz, T. A., Villanueva-Rodríguez, S. J., & Torruco-Uco, J. G. (2022). Nutraceutical Properties of Medicago sativa L., Agave spp., Zea mays L. and Avena sativa L.: A Review of Metabolites and Mechanisms. Metabolites, 12(9), 806. https://doi.org/10.3390/metabo12090806