Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches
Abstract
1. Introduction
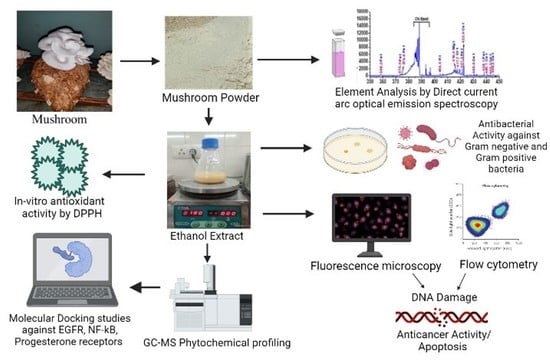
2. Material and Methods
2.1. Spawn Preparation, Cultivation and Harvesting of Mushroom
2.2. Optical Emission Spectroscopy
2.3. Ethanol Extraction of Herbs
2.4. GC-MS Analysis
2.5. Antioxidant Assay
2.6. Tested Bacterial Organisms
2.7. Antibacterial Activity
2.8. Cell Culture
2.9. In Vitro Cellular Toxicity/Cell Proliferation
2.10. Anticancer Activity Using SRB Assay
2.11. Nuclear Morphological Examination of Cancer Cells
2.12. Cell Apoptosis Assay
2.13. Molecular Docking
2.14. Statistical Analysis
3. Result
3.1. Optical Emission Spectroscopy
3.2. Chemical Profiling of Pleurotus ostreatus
3.3. Antioxidant Activity of Pleurotus ostreatus
3.4. Antimicrobial Activity of Pleurotus ostreatus
3.5. Pleurotus ostreatus Inhibits the Proliferation of Breast Cancer Cells
3.6. Effect of Pleurotus ostreatus on Cellular Morphology
3.7. Apoptotic Potential of Pleurotus ostreatus
3.8. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valverde, M.E.; Hernández-pérez, T.; Paredeslópez, O. Review article edible mushrooms: Improving human health and promoting edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M. Promising anticancer activity of polysaccharides and other macromolecules derived from oyster mushroom (Pleurotus sp.): An updated review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kumar, S.; Sharma, R.; Mehta, C.M. Bauhinia variegata: A comprehensive review on bio-active compounds, health benefits and utilization. Adv. Tradit. Med. 2021, 21, 645–653. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Chowdhury, M.M.H.; Kubra, K.; Ahmed, S.R. Screening of antimicrobial, antioxidant properties and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Hamad, D.; El-Sayed, H.; Ahmed, W.; Sonbol, H.; Ramadan, M.A.H. GC-MS Analysis of Potentially Volatile Compounds of Pleurotus ostreatus Polar Extract: In vitro Antimicrobial, Cytotoxic, Immunomodulatory, and Antioxidant Activities. Front. Microbiol. 2022, 13, 834525. [Google Scholar] [CrossRef]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-Functional Activity Relationship of β-Glucans From the Perspective of Immunomodulation: A Mini-Review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- OOyetayo, V.; Dong, C.H.; Yao, Y.J. Antioxidant and antimicrobial properties of aqueous extract from Dictyophora indusiata. Open Mycol. J. 2009, 3, 20–26. [Google Scholar] [CrossRef]
- Lall, N.; Henley-Smith, C.J.; De Canha, M.N.; Oosthuizen, C.B.; Berrington, D. Viability Reagent, PrestoBlue, in Comparison with Other Available Reagents, Utilized in Cytotoxicity and Antimicrobial Assays. Int. J. Microbiol. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Phillips, S.; Rao MR, K.; Prabhu, K.; Priya, M.; Kalaivani, S.; Ravi, A.; Dinakar, S. Preliminary GC-MS analysis of an Ayurvedic medicine “Kulathadi Kashayam”. J. Chem. Pharm. Res. 2015, 7, 393–400. [Google Scholar]
- Ren, J.; Wang, J.; Karthikeyan, S.; Liu, H.; Cai, J. Natural Anti-Phytopathogenic Fungi Compound Phenol, 2, 4-Bis (1, 1-Dimethylethyl) from Pseudomonas Fluorescens TL-1. NISCAIR-CSIR, India. 2019. Available online: http://nopr.niscpr.res.in/handle/123456789/46912 (accessed on 17 August 2022).
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Yin, W.-F.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiol. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Geng, C.-A.; Yang, T.-H.; Yang, Y.-P.; Chen, J.-J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2018, 84, 19–30. [Google Scholar] [CrossRef]
- Swantara, M.D.; Rita, W.S.; Suartha, N.; Agustina, K.K. Anticancer activities of toxic isolate of Xestospongia tes-tudinaria sponge. Vet. World 2019, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, K.J.; Sunil, C.N. GC-MS analysis and nutrient evaluation of rare, endemic and threatened species Apono-geton appendiculatus V. Bruggen of South India. Indian J. Sci. Res. 2018, 20, 7–11. [Google Scholar]
- Kumar, A.; Kaur, S.; Dhiman, S.; Singh, P.P.; Thakur, S.; Sharma, U.; Kumar, S.; Kaur, S. 1, 2-Benzenedicarboxylic Acid, Bis (2-methyl Propyl) Ester Isolated from Onosma bracteata Wall. Inhibits MG-63 Cells Proliferation via Akt-p53-Cyclin Pathway. India. 2021. Available online: https://assets.researchsquare.com/files/rs-182390/v1_covered.pdf?c=1631869103 (accessed on 4 June 2021).
- Arora, S.; Meena, S. Pharmacological Studies on Flowers of Ceropegia bulbosa Roxb. var. bulbosa and lushii (Grah.) Hook. F from Thar Desert of Rajasthan, India. Res. J. Pharmacogn. Phytochem. 2018, 10, 226. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Vats, S.; Gupta, T. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol. Mol. Biol. Plants 2017, 23, 239–248. [Google Scholar] [CrossRef]
- Rajivgandhi, G.N.; Ramachandran, G.; Li, J.-L.; Yin, L.; Manoharan, N.; Kannan, M.R.; Velanganni, A.A.J.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; et al. Molecular identification and structural detection of anti-cancer compound from marine Streptomyces akiyoshiensis GRG 6 (KY457710) against MCF-7 breast cancer cells. J. King Saud Univ. Sci. 2020, 32, 3463–3469. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Oni, J.O.; Akomaye, F.A.; Markson, A.-A.A.; Egwu, A.C. GC-MS Analysis of Bioactive Compounds in Some Wild-Edible Mushrooms from Calabar, Southern Nigeria. Eur. J. Biol. Biotechnol. 2020, 1, 2684–5199. [Google Scholar] [CrossRef]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chem-ical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 107–125. [Google Scholar]
- Eleazu, C.O. Characterization of the natural products in cocoyam (Colocasia esculenta) using GC–MS. Pharm. Biol. 2016, 54, 2880–2885. [Google Scholar] [CrossRef] [PubMed]
- Sudha, T.; Chidambarampillai, S.; Mohan, V.R. GC-MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd.(Euphorbiaceae). J. Appl. Pharm. Sci. 2013, 3, 126–130. [Google Scholar]
- Photolo, M.M.; Mavumengwana, V.; Sitole, L.; Tlou, M.G. Antimicrobial and Antioxidant Properties of a Bacterial Endophyte, Methylobacterium radiotolerans MAMP 4754, Isolated from Combretum erythrophyllum Seeds. Int. J. Microbiol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Barber, B.E.; Fernandez, M.; Patel, H.B.; Barcelo, C.; Woolley, S.D.; Patel, H.; Llewellyn, S.; Abd-Rahman, A.N.; Sharma, S.; Jain, M.; et al. Safety, pharmacokinetics, and antimalarial activity of the novel triaminopyrimidine ZY-19489: A first-in-human, randomised, placebo-controlled, double-blind, single ascending dose study, pilot food-effect study, and volunteer infection study. Lancet Infect. Dis. 2022, 22, 879–890. [Google Scholar] [CrossRef]
- Telahigue, K.; Ghali, R.; Nouiri, E.; Labidi, A.; Hajji, T. Antibacterial activities and bioactive compounds of the ethyl acetate extract of the sea cucumber Holothuria forskali from Tunisian coasts. J. Mar. Biol. Assoc. U. K. 2020, 100, 229–237. [Google Scholar] [CrossRef]
- Pansuksan, K.; Sangthong, R.; Nakamura, I.; Mii, M.; Supaibulwatana, K. Tetraploid induction of Mitracarpus hirtus L. by colchicine and its characterization including antibacterial activity. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 117, 381–391. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, N.U.; KMostafa Kamal, A.T.M.; Azad, M.O.K.; Paul, A.; Uddin, S.B.; Barlow, J.W.; Faruque, M.O.; Park, C.H.; Cho, D.H. Investigation of the Biological Activities and Characterization of Bioactive Constituents of Ophiorrhiza rugosa var. prostrata (D.Don) & Mondal Leaves through In Vivo, In Vitro, and In Silico Approaches. Molecules 2019, 24, 1367. [Google Scholar] [CrossRef]
- Sande, D.; de Oliveira, G.P.; e Moura, M.A.F.; Martins, B.D.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants-A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Sun, H.; Brocato, J.; Costa, M. Oral chromium exposure and toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Kim, H.M.; Jin, C.H.; Kang, S.-Y.; Kim, J.-B.; Jeon, Y.G.; Park, K.Y.; Lee, I.-S.; Han, A.-R. Composition and Antioxidant Activities of Volatile Organic Compounds in Radiation-Bred Coreopsis Cultivars. Plants 2020, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, G.; Murugan, M.; Mohan, V.R. GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 301–308. [Google Scholar]
- Reza, A.A.; Haque, M.A.; Sarker, J.; Nasrin, M.S.; Rahman, M.M.; Tareq, A.M.; Khan, Z.; Rashid, M.; Sadik, G.; Tsukahara, T.; et al. Antiproliferative and antioxidant potentials of bioactive edible vegetable fraction of Achyranthes ferruginea Roxb. in cancer cell line. Food Sci. Nutr. 2021, 9, 3777–3805. [Google Scholar] [CrossRef]
- Vamanu, E.; Pelinescu, D.; Avram, I.; Nita, S. An In Vitro Evaluation of Antioxidant and Colonic Microbial Profile Levels following Mushroom Consumption. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef][Green Version]
- Daniel, A.R.; Qiu, M.; Faivre, E.J.; Ostrander, J.H.; Skildum, A.; Lange, C.A. Linkage of progestin and epidermal growth factor signaling: Phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids 2007, 72, 188–201. [Google Scholar] [CrossRef]









 |  |
| Phenol, 2,4-bis (1,1-dimethylethyl)- | 1-Hexadecene |
 |  |
| Ethyl. alpha. -d-glucopyranoside | 11-Tetradecen-1-ol, acetate, (Z)- |
 |  |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | Pentadecanoic acid, ethyl ester |
 |  |
| 2-(Diethylamino)-N-(2,6-dimethylphenyl)ACE | Ethyl 9-hexadecenoate |
 |  |
| 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione | Ethyl 9-hexadecanoate |
 |  |
| Hexadecanoic acid, ethyl ester | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester |
 |  |
| 9,12-Octadecadienoyl chloride, (Z, Z)- | Linoleic acid ethyl ester |
 |  |
| Octadecanoic acid, ethyl ester | Octanoic acid, 2-dimethylaminoethyl ester |
 |  |
| 9-Octadecenal, (Z)- | 3-Cyclopentylpropionic acid, 2-dimethylaminoethyl ester |
 |  |
| Benzyl diethyl-(2,6-xylylcarbamoylmethyl)-ammonium ben | Ergosta-5,7,9(11),22-tetraen-3-ol, (3. beta.,22E)- |
 |  |
| Stigmasta-5,22-dien-3-ol, acetate, (3. beta.,22Z)- | Stigmast-5-en-3-ol, oleat |
 |  |
| Ergosta-5,7,22-trien-3-ol, (3. beta.,22E)- | Stigmasta-5,23-dien-3-ol, (3. beta.)- |
 |  |
| Gamma—Sitosterol | Progesterone, 1A28 |
 |  |
| EGFR, 5GTY | NF-kB2, 4OT9 |
| Peak | R.Time | Area | Area% | Name | Ligand Name for Molecular Docking | Biological Activity | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 9.115 | 2,518,815 | 1.66 | Phenol,2,4-Bis(1,1-Dimethylethyl) | Ligand 1 | Anticancer, antimicrobial and antioxidant | [11,12,13] |
| 2 | 10.060 | 238,480 | 0.16 | 1-Hexadecene | Ligand 2 | - | |
| 3 | 10.904 | 2,087,528 | 1.38 | Ethyl. alpha. -d-glucopyranoside | Ligand 3 | Antioxidant and anticancer | [14] |
| 4 | 12.322 | 391,627 | 0.26 | 9-Octadecenoic acid(Z)-, ethyl ester | Ligand 4 | Anticancer | [15] |
| 5 | 12.770 | 136,985 | 0.09 | 11-Tetradecen-1-ol, acetate, (Z) | Ligand 5 | Used in cosmetics | [16] |
| 6 | 13.089 | 451,454 | 0.30 | 1,2-Benzenedicarboxylicacid, bis(2-methylpropyl) ester | Ligand 6 | Anticancer, apoptotic, antimicrobial and antifouling activity | [17,18] |
| 7 | 13.361 | 3,516,405 | 2.32 | Pentadecanoic acid, ethyl ester | Ligand 7 | Antibacterial and antifungal | [19] |
| 8 | 13.471 | 4,288,819 | 2.83 | 2-(Diethylamino)-N-(2,6-Dimethylphenyl) Ace | Ligand 8 | - | |
| 9 | 14.153 | 104,349 | 0.07 | Ethyl9-hexadecenoate | Ligand 9 | Antioxidant and anti-androgenic | [20] |
| 10 | 14.211 | 170,369 | 0.11 | 7,9-Di-tert-butyl-1-oxaspiro (4,5) deca-6,9-diene-2,8-dione | Ligand 10 | Antineoplastic, antimicrobial and antiviral | [21,22] |
| 11 | 14.251 | 79,393 | 0.05 | Ethyl9-hexadecanoate | Ligand 11 | - | |
| 12 | 14.357 | 32,499,008 | 21.42 | Hexadecanoic acid, ethyl ester | Ligand 12 | Anti-inflammatory, antioxidant, hypocholesterolemic, nematicide, pesticide, lubricant and anti-androgenic | [23,24] |
| 13 | 15.327 | 1,174,559 | 0.77 | 9,12-Octadecadienoicacid (Z, Z)-, methyl ester | Ligand 13 | Antibacterial, antifungal, antioxidant and anticancer | [18,20,23] |
| 14 | 15.671 | 312,974 | 0.21 | 9,12-Octadecadienoylchloride, (Z, Z) | Ligand 14 | Antisecretory, contraceptive, antitubercular and anti-spermatogenic | [25] |
| 15 | 15.947 | 49,748,008 | 32.78 | Linoleic acid ethyl ester | Ligand 15 | Hypocholesterolemic, nematicide, antiarthritic, hepatoprotective, antiandrogenic, hypocholesterolemic, 5-alpha reductase inhibitor, antihistaminic, anti-coronary, insectifuge, anti-eczemic and anti-acne | [26] |
| 16 | 15.993 | 20,864,605 | 13.75 | (E)-9-Octadecenoicacidethylester | Ligand 16 | Antimicrobial | [26] |
| 17 | 16.223 | 7,107,650 | 4.68 | Octadecanoic acid, ethyl ester | Ligand 17 | 5-alpha reductase inhibitor, hypocholesterolemic and lubricant | [26] |
| 18 | 17.030 | 415,993 | 0.27 | Octanoicacid,2-dimethylaminoethylester | Ligand 18 | - | |
| 19 | 17.503 | 309,994 | 0.20 | Urea, N-[4-(diethylamino)-2-methoxyphenyl]-N’-(1,1-dimet | Ligand 19 | - | |
| 20 | 17.564 | 629,878 | 0.42 | 9-Octadecenal, (Z) | Ligand 20 | Antimicrobial | [27] |
| 21 | 18.450 | 6,818,740 | 4.49 | 3-Cyclopentylpropionicacid,2-dimethylaminoethylester | Ligand 21 | Antimicrobial and antifouling | [16] |
| 22 | 19.033 | 5,791,604 | 3.82 | Benzyl diethyl-(2,6-xylylcarbamoylmethyl)-ammonium | Ligand 22 | - | |
| 23 | 19.528 | 600,854 | 0.40 | Docosanoic acid, ethyl ester | Ligand 23 | Cosmetics | [20] |
| 24 | 20.279 | 328,001 | 0.22 | Octadecanoic acid, ethyl ester | Ligand 24 | 5-alpha reductase inhibitor, hypocholesterolemic, lubricant and flavor | [26] |
| 25 | 20.837 | 1,373,935 | 0.91 | cis-15-Tetracosenoicacid, propyl ester | Ligand 25 | Antimalarial activity | [28] |
| 26 | 20.999 | 1,833,453 | 1.21 | Ethyl tetracosanoate | Ligand 26 | - | |
| 27 | 22.521 | 420,128 | 0.28 | Ergosta-5,7,9(11),22-tetraen-3-ol, (3. beta.,22E) | Ligand 27 | - | |
| 28 | 23.176 | 354,005 | 0.23 | Stigmasta-5,22-dien-3-ol, acetate, (3. beta.,22Z) | Ligand 28 | Antibacterial and antifungal | [29] |
| 29 | 23.807 | 1,000,313 | 0.66 | Stigmast-5-en-3-ol, Oleat | Ligand 29 | Antihyperlipidemic and anti-tumor | [30] |
| 30 | 25.223 | 4,663,969 | 3.07 | Ergosta-5,7,22-Trien-3-ol, (3. Beta.,22E) | Ligand 30 | Not reported | |
| 31 | 25.899 | 724,079 | 0.48 | Stigmasta-5,23-Dien-3-ol, (3. Beta.) | Ligand 31 | Not reported | |
| 32 | 26.867 | 785,623 | 0.52 | Gamma. Sitosterol | Ligand 32 | Anti-hypercholesterolemic, antiviral (influenza), anti-inflammatory, anti-acne, antiprotozoal (leishmania), antibacterial and antioxidant | [31] |
| 151,741,597 | 100.00 |
| mg/mL | Staphylococcus aureus | Proteus vulgaris | Pseudomonas aeruginosa | Proteus mirabilis |
|---|---|---|---|---|
| 50 | 16.6 ± 0.1 | 20.3 ± 0.2 | 16.2 ± 0.05 | 12.5 ± 0.1 |
| 100 | 22.9 ± 0.1 | 24.3 ± 0.2 | 22.2 ± 0.1 | 16.5 ± 0.3 |
| 150 | 27.9 ± 0.3 | 25.5 ± 0.4 | 23.3 ± 0.2 | 16.7 ± 0.2 |
| 200 | 29.6 ± 0.2 | 27.4 ± 0.2 | 24.4 ± 0.3 | 16.4 ± 0.2 |
| Target Macromolecule and Binding Energy (kcal/mol) | |||
|---|---|---|---|
| Compounds | EGFR 5GTY | NF-kB 4OT9 | PROGESTERONE 1A28 |
| lig_1 | −6.8 | −4.8 | −6.7 |
| lig_2 | −5.6 | −3.8 | −4.3 |
| lig_3 | −5.6 | −4.9 | −5.9 |
| lig_5 | −5.4 | −3.9 | −3.3 |
| lig_6 | −6.8 | −4.9 | −6.6 |
| lig_7 | −4.4 | −3.1 | −3.4 |
| lig_8 | −6 | −4.5 | −5.8 |
| lig_9 | −5.1 | −3.1 | −3.3 |
| lig_10 | −7.6 | −5.9 | −6.5 |
| lig_11 | −5.1 | −3.3 | −3.4 |
| lig_12 | −5.5 | −3.5 | −4.1 |
| lig_13 | −4.9 | −3.1 | −3 |
| lig_14 | −5.4 | −3.4 | −4.4 |
| lig_15 | −13.2 | −10.4 | −11 |
| lig_17 | −6.9 | −5.2 | −6.1 |
| lig_18 | −5.5 | −3.8 | −4.1 |
| lig_20 | −4.9 | −2.2 | −3.4 |
| lig_21 | −6.2 | −4.6 | −2.9 |
| lig_22 | −7.4 | −6.3 | −5.4 |
| lig_27 | −9.7 | −6.1 | −6.7 |
| lig_28 | −9.4 | −5.8 | −8.7 |
| lig_29 | −7.9 | −6.6 | −6.3 |
| lig_30 | −8.6 | −6.4 | −6.3 |
| lig_31 | −8.2 | −5.2 | −6.2 |
| lig_32 | −8.5 | −4.9 | −5.1 |
| S.No. | Protein | Top Three Compounds with the Lowest Binding Energy and Names of Residues of Proteins that Showed Close Contact with Ligands | ||
|---|---|---|---|---|
| 1 | 5GTY | Lig15 (THR766, LEU764, LYS721, MET742, ASP831) | Lig27 (LEU694, VAL702, PHE699) | Lig28 (VAL702, MET769, LEU768, ALA719, LEU820, PHE699) |
| 2 | 4OT9 | Lig15 (ARG471, VAL510, TYR509, HIS513) | Lig29 (LEU734, THR732, HIS695, PRO756, GLU755, ALA750, ALA749, LEU688, LYS689, THR753, MET754, ALA692, ILE694) | Lig30 (GLU699, ALA667, HIS731, ASN698, ASN669, HIS673, LEU736, LEU674, GLY677) |
| 3 | 1A28 | Lig15 (GLU807, LEU883, LEU880, LEU876, ASN879) | Lig27 LYS734, VAL730, ARG740, GLN747, ILE748, ILE751, ILE744, GLN752, VAL912, GLU911 | Lig28 VAL698, VAL729, SER728, GLU695 MET692, HIS770, MET759, GLN725, TRP765, GLY762, ARG766, PRO696 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, V.; Tomar, S.; Yadav, P.; Vishwakarma, S.; Singh, M.P. Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches. Metabolites 2022, 12, 821. https://doi.org/10.3390/metabo12090821
Mishra V, Tomar S, Yadav P, Vishwakarma S, Singh MP. Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches. Metabolites. 2022; 12(9):821. https://doi.org/10.3390/metabo12090821
Chicago/Turabian StyleMishra, Vartika, Sarika Tomar, Priyanka Yadav, Shraddha Vishwakarma, and Mohan Prasad Singh. 2022. "Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches" Metabolites 12, no. 9: 821. https://doi.org/10.3390/metabo12090821
APA StyleMishra, V., Tomar, S., Yadav, P., Vishwakarma, S., & Singh, M. P. (2022). Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus ostreatus through In Vitro and In Silico Approaches. Metabolites, 12(9), 821. https://doi.org/10.3390/metabo12090821