Association of Plasma Irisin Levels with Circulating Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs) in Children Born Prematurely
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Size Calculation
2.3. Clinical Assessment
2.4. Blood Biochemistry and Plasma Irisin Levels
2.5. Flow Cytometric Analysis of Circulating Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs)
2.6. Statistical Analyses
3. Results
3.1. Correlation of Irisin with Clinical, Perinatal and Biochemical Variables
3.2. Correlation of Irisin with Circulating EMPs and EPCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Edstedt Bonamy, A.K. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef]
- Bavineni, M.; Wassenaar, T.M.; Agnihotri, K.; Ussery, D.W.; Lüscher, T.F.; Mehta, J.L. Mechanisms linking preterm birth to onset of cardiovascular disease later in adulthood. Eur. Heart J. 2019, 40, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Crump, C. Preterm birth and mortality in adulthood: A systematic review. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2020, 40, 833–843. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Namana, V.; Fanos, V.; Mercuro, G. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 70, 1943–1944. [Google Scholar] [CrossRef]
- Melville, J.M.; Moss, T.J. The immune consequences of preterm birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markopoulou, P.; Papanikolaou, E.; Loukopoulou, S.; Galina, P.; Papassotiriou, I.; Siahanidou, T. Elevated circulating endothelial microparticles (EMPs) in prepubertal children born preterm. Pediatr. Res. 2022, 91, 1754–1761. [Google Scholar] [CrossRef]
- Markopoulou, P.; Papanikolaou, E.; Loukopoulou, S.; Galina, P.; Mantzou, A.; Siahanidou, T. Increased circulating endothelial progenitor cells (EPCs) in prepubertal children born prematurely: A possible link between prematurity and cardiovascular risk. Pediatr. Res. 2021, 90, 156–165. [Google Scholar] [CrossRef]
- Bertagnolli, M.; Xie, L.F.; Paquette, K.; He, Y.; Cloutier, A.; Fernandes, R.O.; Béland, C.; Sutherland, M.R.; Delfrate, J.; Curnier, D.; et al. Endothelial Colony-Forming Cells in Young Adults Born Preterm: A Novel Link Between Neonatal Complications and Adult Risks for Cardiovascular Disease. J. Am. Heart Assoc. 2018, 7, e009720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Agouni, A.; Lagrue-Lak-Hal, A.H.; Ducluzeau, P.H.; Mostefai, H.A.; Draunet-Busson, C.; Leftheriotis, G.; Heymes, C.; Martinez, M.C.; Andriantsitohaina, R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 2008, 173, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Tramontano, A.F.; Lyubarova, R.; Tsiakos, J.; Palaia, T.; Deleon, J.R.; Ragolia, L. Circulating endothelial microparticles in diabetes mellitus. Mediat. Inflamm. 2010, 2010, 250476. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Quelhas-Santos, J. Circulating Endothelial Progenitor Cells: A Review of Definition, Characterization and Identification. RRJOB 2016, 4, 42–49. [Google Scholar]
- Burger, D.; Touyz, R.M. Cellular biomarkers of endothelial health: Microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 2012, 6, 85–99. [Google Scholar] [CrossRef]
- Bertagnolli, M.; Nuyt, A.M.; Thébaud, B.; Luu, T.M. Endothelial Progenitor Cells as Prognostic Markers of Preterm Birth-Associated Complications. Stem Cells Transl. Med. 2017, 6, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.A.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2005, 38, 1538–1544. [Google Scholar] [CrossRef]
- De Meneck, F.; Victorino de Souza, L.; Oliveira, V.; do Franco, M.C. High irisin levels in overweight/obese children and its positive correlation with metabolic profile, blood pressure, and endothelial progenitor cells. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 756–764. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, F.; Tang, Y.; Cai, L.; Zeng, C.; Yang, Y.; Yang, J. The Emerging Role of Irisin in Cardiovascular Diseases. J. Am. Heart Assoc. 2021, 10, e022453. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Hu, W.; Wang, M.; Lv, W.; Jia, T.; Xiao, Y. Irisin as a mediator between obesity and vascular inflammation in Chinese children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 320–329. [Google Scholar] [CrossRef]
- Tang, L.; Tong, Y.; Zhang, F.; Chen, G.; Zhang, Y.C.; Jobin, J.; Tong, N. The association of circulating irisin with metabolic risk factors in Chinese adults: A cross-sectional community-based study. BMC Endocr. Disord. 2019, 19, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.C.; Jeon, W.S.; Park, C.Y.; Youn, B.S. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc. Diabetol. 2016, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Gonzalez-Gil, A.M.; Villarreal-Calderón, J.R.; Tamez-Rivera, O.; Rodriguez-Gutierrez, N.A.; Castillo, E.C.; Silva-Platas, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Serum Irisin Levels, Endothelial Dysfunction, and Inflammation in Pediatric Patients with Type 2 Diabetes Mellitus and Metabolic Syndrome. J. Diabetes Res. 2020, 2020, 1949415. [Google Scholar] [CrossRef]
- Campolo, J.; Corradi, E.; Rizzardi, A.; Parolini, M.; Dellanoce, C.; Di Guglielmo, M.L.; Tarlarini, P.; Cattaneo, M.; Trivella, M.G.; De Maria, R. Irisin and markers of metabolic derangement in non-diabetic Caucasian subjects with stage I-II obesity during early aging. PLoS ONE 2020, 15, e0229152. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.B.; Kim, H.J.; Kang, J.H.; Park, S.I.; Park, K.H.; Lee, H.J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism 2017, 73, 100–108. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Song, M.; Zhou, F.; Fu, D.; Ruan, G.; Zhu, X.; Bai, Y.; Huang, L.; Pang, R.; et al. Irisin Increased the Number and Improved the Function of Endothelial Progenitor Cells in Diabetes Mellitus Mice. J. Cardiovasc. Pharmacol. 2016, 68, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, S.; Xu, F.; Wang, D.; Yin, H.; Lai, Q.; Liao, J.; Hou, X.; Hu, M. Exercise training with dietary restriction enhances circulating irisin level associated with increasing endothelial progenitor cell number in obese adults: An intervention study. PeerJ 2017, 5, e3669. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Zhang, Y.; Liu, C.; Wang, Y.; Zhang, L.; Shi, Z.; Wu, Z.; et al. Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage–related diseases. JCI Insight 2020, 5, e136277. [Google Scholar] [CrossRef]
- Koutroumpa, A.; Kanaka Gantenbein, C.; Mantzou, A.; Doulgeraki, A.; Bacopoulou, F.; Bouza, H.; Chrousos, G.; Siahanidou, T. Circulating Irisin Levels in Preadolescents and Adolescents Born Preterm. Horm. Res. Paediatr. 2021, 94, 416–425. [Google Scholar] [CrossRef]
- Mól, N.; Zasada, M.; Tomasik, P.; Klimasz, K.; Kwinta, P. Evaluation of irisin and visfatin levels in very low birth weight preterm newborns compared to full term newborns-A prospective cohort study. PLoS ONE 2018, 13, e0204835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, K.E.; Park, K.H.; Filippaios, A.; Dincer, F.; Christou, H.; Mantzoros, C.S. Cord blood irisin levels are positively correlated with birth weight in newborn infants. Metabolism 2015, 64, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64.
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef]
- Seo, D.Y.; Bae, J.H.; Kim, T.N.; Kwak, H.B.; Kha, P.T.; Han, J. Exercise-Induced Circulating Irisin Level Is Correlated with Improved Cardiac Function in Rats. Int. J. Environ. Res. Public Health 2020, 17, 3863. [Google Scholar] [CrossRef]
- Jackson, A.S.; Blair, S.N.; Mahar, M.T.; Wier, L.T.; Ross, R.M.; Stuteville, J.E. Prediction of functional aerobic capacity without exercise testing. Med. Sci. Sports Exerc. 1990, 22, 863–870. [Google Scholar] [CrossRef]
- Sipola-Leppanen, M.; Vaarasmaki, M.; Tikanmaki, M.; Matinolli, H.M.; Miettola, S.; Hovi, P.; Wehkalampi, K.; Ruokonen, A.; Sundvall, J.; Pouta, A.; et al. Cardiometabolic risk factors in young adults who were born preterm. Am. J. Epidemiol. 2015, 181, 861–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondolot, M.; Horoz, D.; Poyrazoğlu, S.; Borlu, A.; Öztürk, A.; Kurtoğlu, S.; Mazıcıoğlu, M.M. Neck Circumference to Assess Obesity in Preschool Children. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 17–23. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazdam, M.; de la Horra, A.; Pitcher, A.; Mannie, Z.; Diesch, J.; Trevitt, C.; Kylintireas, I.; Contractor, H.; Singhal, A.; Lucas, A.; et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism? Hypertension 2010, 56, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutfield, W.S.; Jefferies, C.A.; Jackson, W.E.; Robinson, E.M.; Hofman, P.L. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr. Diabetes 2003, 4, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Cheng, S.; Renard, J.M.; Larson, M.G.; Ghorbani, A.; McCabe, E.; Griffin, G.; Guerin, C.; Ho, J.E.; Shaw, S.Y.; et al. Association of circulating endothelial microparticles with cardiometabolic risk factors in the Framingham Heart Study. Eur. Heart J. 2014, 35, 2972–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visentin, S.; Grumolato, F.; Nardelli, G.B.; Di Camillo, B.; Grisan, E.; Cosmi, E. Early origins of adult disease: Low birth weight and vascular remodeling. Atherosclerosis 2014, 237, 391–399. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N.; Kim, D.W. Circulating Endothelial Microparticles: A Key Hallmark of Atherosclerosis Progression. Scientifica 2016, 2016, 8514056. [Google Scholar] [CrossRef] [Green Version]
- Deng, F.; Wang, S.; Zhang, L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: A literature review. J. Cell. Mol. Med. 2017, 21, 1698–1710. [Google Scholar] [CrossRef]
- Keleş, E.; Turan, F.F. Evaluation of cord blood irisin levels in term newborns with small gestational age and appropriate gestational age. SpringerPlus 2016, 5, 1757. [Google Scholar] [CrossRef] [Green Version]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paviotti, G.; Fadini, G.P.; Boscaro, E.; Agostini, C.; Avogaro, A.; Chiandetti, L.; Baraldi, E.; Filippone, M. Endothelial progenitor cells, bronchopulmonary dysplasia and other short-term outcomes of extremely preterm birth. Early Hum. Dev. 2011, 87, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 2000, 95, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Avogaro, A. Cell-based methods for ex vivo evaluation of human endothelial biology. Cardiovasc. Res. 2010, 87, 12–21. [Google Scholar] [CrossRef] [PubMed]
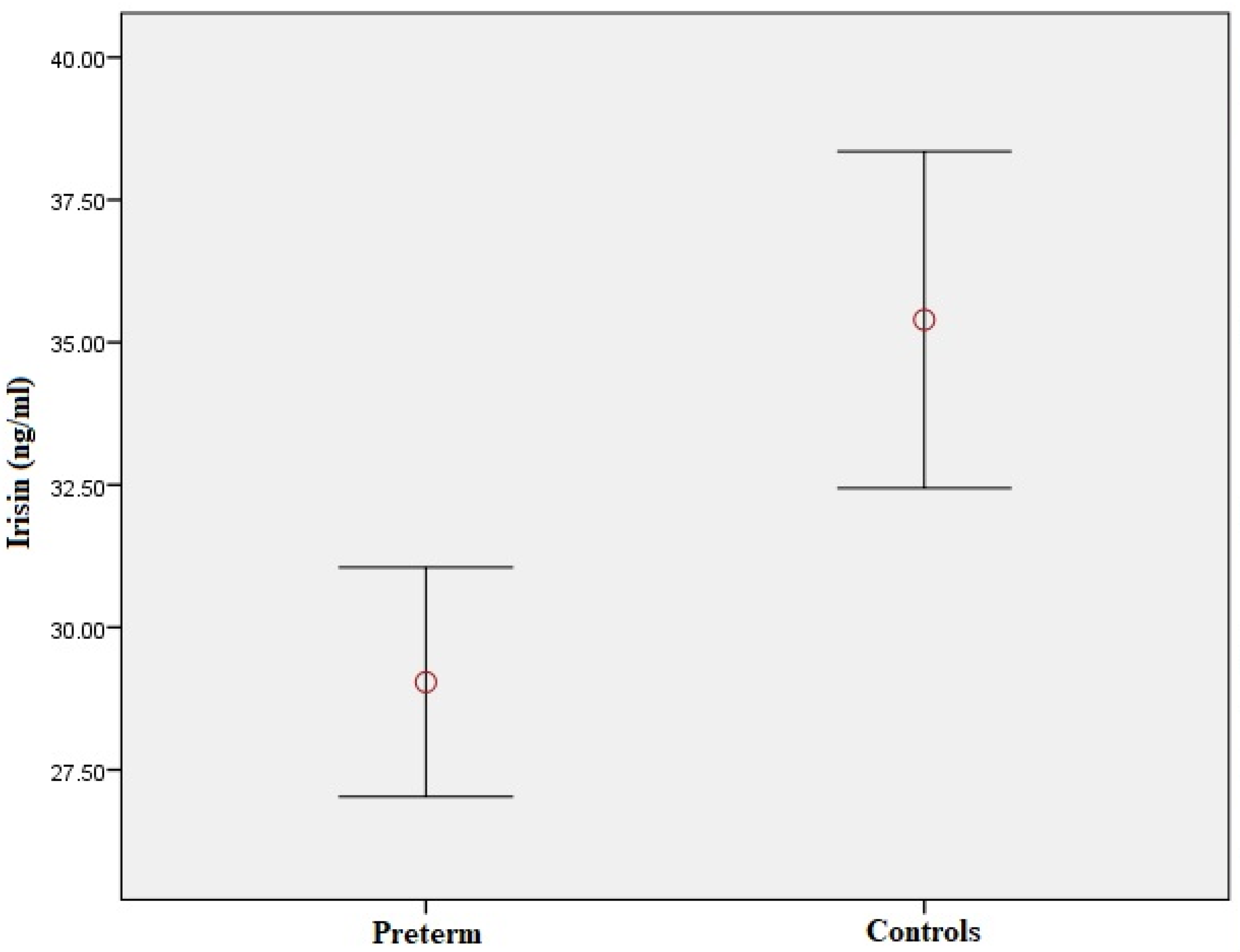


| Variable | Preterm-Born Children (n = 61) | Controls (n = 70) | p Value |
|---|---|---|---|
| Age (years) | 10.9 ± 1.8 | 10.5 ± 1.9 | 0.43 |
| Males (n) | 24 | 36 | 0.17 |
| Small for gestational age (SGA) | 10 | 8 | 0.37 |
| Gestational hypertension [n (%)] | 2 (3.3) | 0 (0) | N/A |
| Preeclampsia [n (%)] | 8 (13.1) | 1 (1.4) | 0.01 |
| Gestational diabetes [n (%)] | 10 (16.4) | 9 (12.9) | 0.57 |
| Cesarean delivery [n (%)] | 57 (93.4) | 34 (48.6) | <0.001 |
| Gestational age (weeks) | 31.7 ± 3.2 | 38.9 ± 1.0 | <0.001 |
| Birth weight (g) | 1619 ± 540 | 3230 ± 457 | <0.001 |
| Bronchopulmonary dysplasia (BPD) [n (%)] | 12 (19.7) | 0 (0) | N/A |
| Intraventricular hemorrhage (IVH) [n (%)] | 16 (26.2) | 0 (0) | N/A |
| Retinopathy of prematurity (ROP) [n (%)] | 22 (36.1) | 0 (0) | N/A |
| Patent ductus arteriosus (PDA) [n (%)] | 8 (13.1) | 0 (0) | N/A |
| Physical activity rating (PA-R) | 4 (3–7) | 5 (3–7) | 0.45 |
| Body weight (kg) | 41.6 ± 11.5 | 38.0 ± 9.6 | 0.11 |
| Height (cm) | 145.8 ± 11.9 | 142.9 ± 12.4 | 0.22 |
| Body mass index (BMI) (kg/m2) | 19.2 ± 3.3 | 18.3 ± 2.7 | 0.14 |
| Waist circumference (cm) | 71.9 ± 9.7 | 68.0 ± 8.3 | 0.02 |
| Hip circumference (cm) | 80.2 ± 10.0 | 77.2 ± 8.5 | 0.08 |
| Waist-to-hip ratio (WHR) | 0.90 ± 0.05 | 0.88 ± 0.04 | 0.05 |
| Neck circumference (cm) | 29.9 ± 2.5 | 29.0 ± 1.8 | 0.05 |
| Systolic blood pressure (SBP) (mmHg) | 107.5 ± 11.4 | 102.4 ± 9.1 | 0.01 |
| Diastolic blood pressure (DBP) (mmHg) | 66.6 ± 7.6 | 63.8 ± 7.0 | 0.04 |
| Glucose (mg/dL) | 78.6 ± 8.7 | 79.8 ± 7.0 | 0.43 |
| Insulin (μUI/mL) | 6.8 (5.1–9.8) | 6.7 (5.7–8.8) | 0.70 |
| HOMA-IR | 1.26 (0.94–1.95) | 1.30 (1.07–1.67) | 0.66 |
| Total Cholesterol (mg/dL) | 150.0 (134.5–170.0) | 156.5 (141.0–166.5) | 0.25 |
| HDL-C (mg/dL) | 63.5 ± 13.6 | 63.0 ± 14.2 | 0.79 |
| LDL-C (mg/dL) | 73.0 (60.0–93.5) | 80.0 (66.3–93.8) | 0.28 |
| Triglycerides (mg/dL) | 51.0 (40.0–68.0) | 56.5 (41.0–72.3) | 0.70 |
| Multiple Regression Analyses * | |||
|---|---|---|---|
| Dependent Variables | Independent Variables with Significant Association | Standardized Coefficient Beta | p-Value |
| Total study population | |||
| Irisin (ng/mL) | −0.23 | 0.01 |
| Irisin (ng/mL) | −0.27 | 0.002 |
| Waist-to-hip ratio | −0.21 | 0.02 | |
| Irisin (ng/mL) | −0.25 | 0.003 |
| Age (years) | 0.19 | 0.02 | |
| Triglycerides (mg/dL) | −0.25 | 0.003 | |
| Irisin (ng/mL) | −0.34 | <0.001 |
| Age (years) | 0.19 | 0.02 | |
| Triglycerides (mg/dL) | −0.30 | <0.001 | |
| Preterm-born group | |||
| Irisin (ng/mL) | −0.32 | 0.01 |
| SGA | 0.25 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markopoulou, P.; Koutroumpa, A.; Mantzou, A.; Margeli, A.; Papanikolaou, E.; Siahanidou, T. Association of Plasma Irisin Levels with Circulating Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs) in Children Born Prematurely. Metabolites 2023, 13, 120. https://doi.org/10.3390/metabo13010120
Markopoulou P, Koutroumpa A, Mantzou A, Margeli A, Papanikolaou E, Siahanidou T. Association of Plasma Irisin Levels with Circulating Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs) in Children Born Prematurely. Metabolites. 2023; 13(1):120. https://doi.org/10.3390/metabo13010120
Chicago/Turabian StyleMarkopoulou, Panagiota, Arsinoi Koutroumpa, Aimilia Mantzou, Alexandra Margeli, Eleni Papanikolaou, and Tania Siahanidou. 2023. "Association of Plasma Irisin Levels with Circulating Endothelial Microparticles (EMPs) and Endothelial Progenitor Cells (EPCs) in Children Born Prematurely" Metabolites 13, no. 1: 120. https://doi.org/10.3390/metabo13010120






