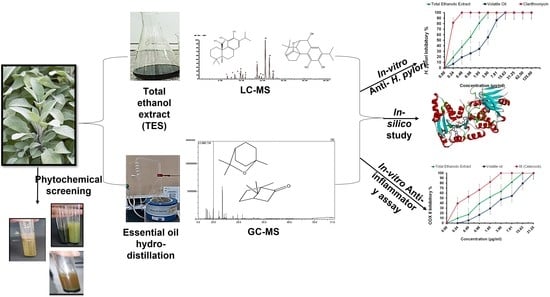
Anti-Heliobacter pylori and Anti-Inflammatory Potential of Salvia officinalis Metabolites: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Experimental Design
2.1. Plant Material
2.2. Phytochemical Screening
2.3. Total Ethanolic Extract (TES)
2.4. Essential Oil Extraction
2.5. GC-MS Analysis for Essential Oil
2.6. LC/MS Analysis
2.7. In Vitro Evaluation of Anti-H. pylori Activity
2.8. In Silico Evaluation of Anti-H. pylori Activity
2.9. Anti-Inflammatory Assay
3. Results
3.1. Phytochemical Screening
3.2. LC/MS of the Ethanolic Extract
3.3. Extraction and GC/MS of the S. officinal Essential Oil
3.4. In Vitro Anti-H. pylori Activity of the TES and the Essential oil of S. officinalis
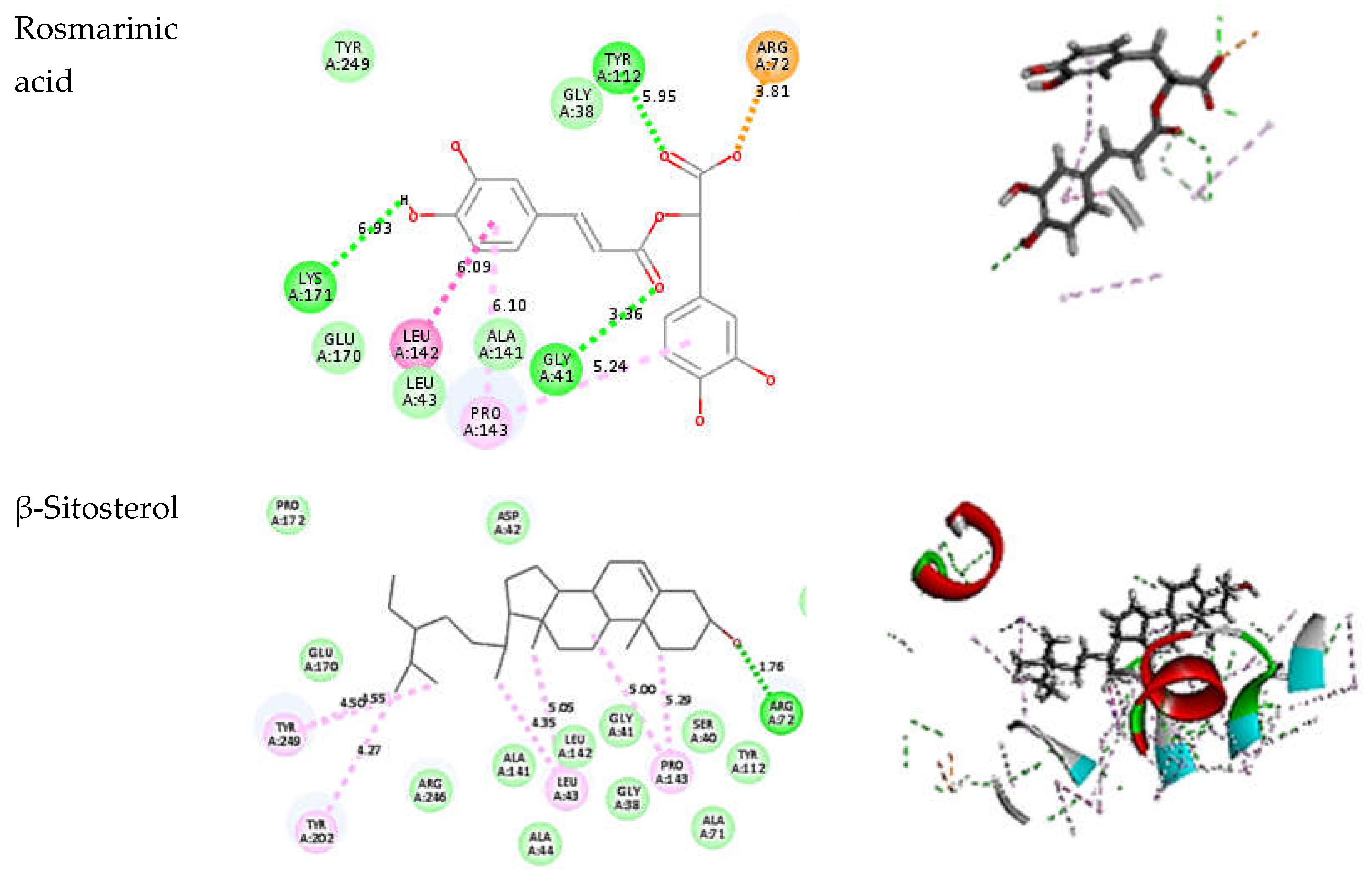
3.5. In Silico Evaluation of Anti-H. pylori Activity
3.6. COX-2 Inhibition Assay (Anti-Inflammatory Assay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abidullah, S.; Rauf, A.; Zaman, W.; Ullah, F.; Ayaz, A.; Batool, F.; Saqib, S. Consumption of wild food plants among tribal communities of Pak-Afghan border, near Bajaur, Pakistan. Acta Ecol. Sin. 2021, 1–17. [Google Scholar] [CrossRef]
- Jakovljević, M.; Jokić, S.; Molnar, M.; Jašić, M.; Babić, J.; Jukić, H.; Banjari, I. Bioactive profile of various Salvia officinalis L. preparations. Plants 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alomar, H.A.; Fathallah, N.; Abdel-Aziz, M.M.; Ibrahim, T.A.; Elkady, W.M. GC-MS Profiling, Anti-Helicobacter pylori, and Anti-Inflammatory Activities of Three Apiaceous Fruits’ Essential Oils. Plants 2022, 11, 2617. [Google Scholar] [CrossRef]
- Peter, S.; Beglinger, C. Helicobacter pylori and gastric cancer: The causal relationship. Digestion 2007, 75, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Marras, G.; Rocchi, C.; Soro, S.; Loria, M.F.; Bassotti, G.; Graham, D.Y.; Malaty, H.M.; Pes, G.M. Changing prevalence of Helicobacter pylori infection and peptic ulcer among dyspeptic Sardinian patients. Intern. Emerg. Med. 2015, 10, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Tonkic, A.; Tonkic, M.; Lehours, P.; Mégraud, F. Epidemiology and Diagnosis of Helicobacter pylori Infection. Helicobacter 2012, 17, 1–8. [Google Scholar] [CrossRef]
- Wang, A.Y.; Peura, D.A. The prevalence and incidence of Helicobacter pylori–associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest. Endosc. Clin. 2011, 21, 613–635. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M. Treatment of Helicobacter pylori infection by herbal drugs; a review on current data. J. Prev. Epidemiol. 2016, 1, e06. [Google Scholar]
- Graham, D.Y.; Shiotani, A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 321–331. [Google Scholar] [CrossRef]
- Bytzer, P.; O’Morain, C. Treatment of Helicobacter pylori. Helicobacter 2005, 10, 40–46. [Google Scholar] [CrossRef]
- Botanica, A. Micromorphological studies of Lallemantia L. (Lamiaceae) species growing in Turkey. Acta Biol. Crac. Ser. Bot. 2009, 51, 45–54. [Google Scholar]
- Camele, I.; Gruľová, D.; Elshafie, H.S. Chemical composition and antimicrobial properties of Mentha piperita cv.‘Kristinka’essential oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef] [PubMed]
- Jafari, B.; Fatemi, S.; Pashazadeh, M.; Al-Snafi, A.E.; Shariat, A. Antibacterial effects of Thymus vulgaris, Mentha pulegium, Crocus sativus and Salvia officinalis on pathogenic bacteria: A brief review study based on gram-positive and gram-negative bacteria. Jorjani Biomed. J. 2020, 8, 58–74. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Miraj, S.; Kiani, S. A review study of therapeutic effects of Salvia officinalis L. Der Pharm. Lett. 2016, 8, 299–303. [Google Scholar]
- Ben Farhat, M.; Jordan, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in essential oil, phenolic compounds, and antioxidant activity of tunisian cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Mitić-Ćulafić, D.; Vuković-Gačić, B.S.; Knežević-Vukčević, J.B.; Stanković, S.; Simić, D.M. Comparative study on the antibacterial activity of volatiles from sage (Salvia officinalis L.). Arch. Biol. Sci. 2005, 57, 173–178. [Google Scholar] [CrossRef]
- Mendes, F.S.F.; Garcia, L.M.; da Silva Moraes, T.; Casemiro, L.A.; de Alcantara, C.B.; Ambrósio, S.R.; Veneziani, R.C.S.; Miranda, M.L.D.; Martins, C.H.G. Antibacterial activity of Salvia officinalis L. against periodontopathogens: An in vitro study. Anaerobe 2020, 63, 102194. [Google Scholar] [CrossRef]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Stanojevic, D.; Čomić, L.; Stefanović, O.; Solujić-Sukdolak, S. In vitro synergistic antibacterial activity of Salvia officinalis L. and some preservatives. Arch. Biol. Sci. 2010, 62, 175–183. [Google Scholar] [CrossRef]
- Rus, C.; Pop, G.; Alexa, E.; Șumălan, R.M.; Copolovici, D.M. Antifungal activity and chemical composition of Salvia officinalis L. essential oil. Res. J. Agric. Sci. 2015, 47, 186–193. [Google Scholar]
- Veličković, D.T.; Ranđelović, N.V.; Ristić, M.S.; Veličković, A.S.; Šmelcerović, A.A. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from the flower, leaf and stem of Salvia officinalis L. J. Serb. Chem. Soc. 2003, 68, 17–24. [Google Scholar] [CrossRef]
- Selim, S.; Almuhayawi, M.S.; Alqhtani, H.; Al Jaouni, S.K.; Saleh, F.M.; Warrad, M.; Hagagy, N. Anti-Salmonella and Antibiofilm Potency of Salvia officinalis L. Essential Oil against Antibiotic-Resistant Salmonella enterica. Antibiotics 2022, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, M.; ur Rehman, F.; Hassanzadeh, M.A.; Beigomi, M.; Fazeli-Nasab, B. Investigating the Antimicrobial Effects of Glycyrrhiza glabra and Salvia officinalis Ethanolic Extract Against Helicobacter pylori. Int. J. Infect. 2021, 8, 1–6. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, R.Z.; Khalil, N.; Zengin, G.; Najar, B.; Khojimatov, O.K.; Al Musayeib, N.M.; Ashour, M.L.; Mamadalieva, N.Z. GC-MS Chemical Profiling, Biological Investigation of Three Salvia Species Growing in Uzbekistan. Molecules 2022, 27, 5365. [Google Scholar] [CrossRef] [PubMed]
- Lespagnol, A. Chimie des médicaments (Tome II). In Edition Technique et Documentation France; 1975; Available online: https://new.societechimiquedefrance.fr/wp-content/uploads/2019/12/1976-31-avril-livres.pdf (accessed on 20 December 2022).
- Audu, S.A.; Mohammed, I.; Kaita, H.A. Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae). Life Sci. J. 2007, 4, 75–79. [Google Scholar]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.C. Trease and Evans’ Pharmacognosy; Elsevier Health Sciences: Toronto, ON, Canada, 2009. [Google Scholar]
- Sintim, H.Y.; Burkhardt, A.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Obour, A.E.; Zheljazkov, V.D.; Schlegel, V. Hydrodistillation time affects dill seed essential oil yield, composition, and bioactivity. Ind. Crops Prod. 2015, 63, 190–196. [Google Scholar] [CrossRef]
- Pitarević, I.; Kuftinec, J.; Blažević, N.; Kuštrak, D. Seasonal variation of essential oil yield and composition of dalmatian sage, Salvia officinalis. J. Nat. Prod. 1984, 47, 409–412. [Google Scholar] [CrossRef]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef]
- Salahvarzi, S.; Shakib, P.; Pirhadi, M.; Alikord, M.; Jahed Khaniki, G. Helicobacter Phytotherapy: Medicinal Plants Affecting Helicobacter pylori Infection in Iran. Plant Biotechnol. Persa 2020, 2, 23–38. [Google Scholar] [CrossRef]
- Fathallah, N.; Raafat, M.M.; Issa, M.Y.; Abdel-Aziz, M.M.; Bishr, M.; Abdelkawy, M.A.; Salama, O. Bio-guided fractionation of prenylated benzaldehyde derivatives as potent antimicrobial and antibiofilm from Ammi majus L. fruits-associated Aspergillus amstelodami. Molecules 2019, 24, 4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, I.M.; Abdel-Aziz, M.M.; Elhady, S.S.; Bagalagel, A.A.; Malatani, R.T.; Elkady, W.M. Valorization of Pimenta racemosa Essential Oils and Extracts: GC-MS and LC-MS Phytochemical Profiling and Evaluation of Helicobacter pylori Inhibitory Activity. Molecules 2022, 27, 7965. [Google Scholar] [CrossRef] [PubMed]
- Saqib, S.; Faryad, S.; Afridi, M.I.; Arshad, B.; Younas, M.; Naeem, M.; Zaman, W.; Ullah, F.; Nisar, M.; Ali, S. Bimetallic assembled silver nanoparticles impregnated in Aspergillus fumigatus extract damage the bacterial membrane surface and release cellular contents. Coatings 2022, 12, 1505. [Google Scholar] [CrossRef]
- Petrovic, N.; Murray, M. Using N, N, N’, N’-tetramethyl-p-phenylenediamine (TMPD) to assay cyclooxygenase activity in vitro. In Advanced Protocols in Oxidative Stress II; Springer: Berlin/Heidelberg, Germany, 2010; pp. 129–140. [Google Scholar]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant capacity and phenolic contents of some Mediterranean medicinal plants and their potential role in the inhibition of cyclooxygenase-1 and acetylcholinesterase activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement. Med. 2017, 7, 360–366. [Google Scholar] [CrossRef]
- Djarmati, Z.; Jankov, R.M.; Csanádi, J.; Djordjevic, A. The isolation of carnosic acid 12-methyl ether from Salvia officinalis L. and NMR study of its methyl ester. Collect. Czechoslov. Chem. Commun. 1993, 58, 1919–1924. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Apianane terpenoids from Salvia officinalis. Phytochemistry 2001, 58, 1171–1175. [Google Scholar] [CrossRef]
- Okamura, N.; Fujimoto, Y.; Kuwabara, S.; Yagi, A. High-performance liquid chromatographic determination of carnosic acid and carnosol in Rosmarinus officinalis and Salvia officinalis. J. Chromatogr. A 1994, 679, 381–386. [Google Scholar] [CrossRef]
- Cuvelier, M.E.; Berset, C.; Richard, H. Antioxidant constituents in sage (Salvia officinalis). J. Agric. Food Chem. 1994, 42, 665–669. [Google Scholar] [CrossRef]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloukopoulou, C.; Karioti, A. A Validated Method for the Determination of Carnosic Acid and Carnosol in the Fresh Foliage of Salvia rosmarinus and Salvia officinalis from Greece. Plants 2022, 11, 3106. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2001, 18, 310–333. [Google Scholar] [CrossRef]
- Taarit, M.B.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant growth, essential oil yield and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Ind. Crops Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential oils from Dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar] [CrossRef]
- Dudai, N.; Lewinsohn, E.; Larkov, O.; Katzir, I.; Ravid, U.; Chaimovitsh, D.; Sa’ad, D.; Putievsky, E. Dynamics of yield components and essential oil production in a commercial hybrid sage (Salvia officinalis × Salvia fruticosa cv. Newe Ya’ar No. 4). J. Agric. Food Chem. 1999, 47, 4341–4345. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Oyman, B.U. Essential Oil Essential Oil Analysis of Some Plants. J. Med. Res. Health Sci. 2022, 5, 2197–2202. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Pezo, L.; Čabarkapa, I.; Trudić, A.; Stanković-Jeremić, J.; Varga, A.; Lončar, B.; Šovljanski, O.; Tešević, V. Variation of Salvia officinalis L. Essential Oil and Hydrolate Composition and Their Antimicrobial Activity. Processes 2022, 10, 1608. [Google Scholar] [CrossRef]
- Moradi, A. The Mortality and Repellency Effect of Nanoformulation of Mentha Longifollia (Laminaceae) and Salvia Mirzayanii (Lamiaceae) Essential Oils on Date Lesser Moth Batrachedra Amydraula Meyr (Lepidoptera: Batrachedridae); University of Zabol: Zabol, Iran, 2022. [Google Scholar]
- Alipour, H.-R.; Yaghmaei, P.; Ahmadian, S.; Ghobeh, M.; Ebrahim-Habibi, A. A study on alpha-terpineol in Alzheimer’s disease with the use of rodent in vivo model, restraint stress effect and in vitro Amyloid beta fibrils. Braz. J. Pharm. Sci. 2022, 58, 1–13. [Google Scholar] [CrossRef]
- Dashti, M.; Kafi, M.; Mirza, M. Chemical Variation in the Essential Oil of Salvia leriifolia Benth. in Response to Organic and Biological Fertilizers. J. Med. Plants By-Prod. 2022, 11, 93–101. [Google Scholar]
- Acimovic, M.G.; Loncar, B.L.; Jeliazkov, V.D.; Pezo, L.L.; Ljujic, J.P.; Miljkovic, A.R.; Vujisic, L.V. Comparison of volatile compounds from clary sage (Salvia sclarea L.) verticillasters essential oil and hydrolate. J. Essent. Oil Bear. Plants 2022, 25, 555–570. [Google Scholar] [CrossRef]
- Senkal, B.; Uskutoglu, T.; Fidan, H.; Stankov, S.; Dogan, H.; Stoyanova, A. Essential oil composition and mineral element content of Salvia aethiopis L. from Turkey. Bulg. Chem. Commun. 2022, 54, 57–61. [Google Scholar] [CrossRef]
- Akiel, M.A.; Alshehri, O.Y.; Aljihani, S.A.; Almuaysib, A.; Bader, A.; Al-Asmari, A.I.; Alamri, H.S.; Alrfaei, B.M.; Halwani, M.A. Viridiflorol induces anti-neoplastic effects on breast, lung, and brain cancer cells through apoptosis. Saudi J. Biol. Sci. 2022, 29, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Schlichting, B.; Schönheit, P. Glucose-6-phosphate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima: Expression of the g6pd gene and characterization of an extremely thermophilic enzyme. FEMS Microbiol. Lett. 2002, 216, 249–253. [Google Scholar] [CrossRef]
- Rowland, P.; Basak, A.K.; Gover, S.; Levy, H.R.; Adams, M.J. The three–dimensional structure of glucose 6–phosphate dehydrogenase from Leuconostoc mesenteroides refined at 2.0 Å resolution. Structure 1994, 2, 1073–1087. [Google Scholar] [CrossRef] [Green Version]
- Mendz, G.L.; Hazell, S.L. Evidence for a pentose phosphate pathway in Helicobacter pylori. FEMS Microbiol. Lett. 1991, 84, 331–336. [Google Scholar] [CrossRef]
- Ruggiero, P. Helicobacter pylori and inflammation. Curr. Pharm. Des. 2010, 16, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- El Guindi, M.A.; El Sayed, M.M. Helicobacter pylori should be treated in children with failure to thrive. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S13. [Google Scholar] [CrossRef]
- Gelband, H.; Sloan, F.A. Cancer Control Opportunities in Low-and Middle-Income Countries; National Academies Press: Washington, DC, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Frenck, R.W., Jr.; Clemens, J. Helicobacter in the developing world. Microbes Infect. 2003, 5, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Rothenbacher, D.; Brenner, H. Burden of Helicobacter pylori and H. pylori-related diseases in developed countries: Recent developments and future implications. Microbes Infect. 2003, 5, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Chuah, S.-K.; Tsay, F.-W.; Hsu, P.-I.; Wu, D.-C. A new look at anti-Helicobacter pylori therapy. World J.Gastroenterol. WJG 2011, 17, 3971. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. Response to Georgopoulos et al. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 1169–1170. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era—Searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Huang, T.-L. Screening of anti-Helicobacter pylori herbs deriving from Taiwanese folk medicinal plants. FEMS Immunol. Med. Microbiol. 2005, 43, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saari, N. Anti-Helicobacter pylori and urease inhibition activities of some traditional medicinal plants. Molecules 2013, 18, 2135–2149. [Google Scholar] [CrossRef]
- Safavi, M.; Shams-Ardakani, M.; Foroumadi, A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharm. Biol. 2015, 53, 939–960. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef]
- Jalsenjak, V.; Peljnjak, S.; Kustrak, D. Microcapsules of sage oil: Essential oils content and antimicrobial activity. Pharmazie 1987, 42, 419–420. [Google Scholar] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkütük, A.S. Antimicrobial effects of carnosic acid, kaempferol and luteolin on biogenic amine production by spoilage and food-borne pathogenic bacteria. Food Biosci. 2022, 46, 101588. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; Repetto, V.; Moreno, S. Carnosic acid is an efflux pumps modulator by dissipation of the membrane potential in Enterococcus faecalis and Staphylococcus aureus. World J. Microbiol. Biotechnol. 2013, 29, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hörnig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, N.M.; Fiorilli, G.; Guido, P.A.C.; Moreno, S. Carnosic acid acts synergistically with gentamicin in killing methicillin-resistant Staphylococcus aureus clinical isolates. Phytomedicine 2016, 23, 1337–1343. [Google Scholar] [CrossRef]
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Upregulation of Akt/NF-κB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carnosic acid nanoparticle. Int. J. Nanomed. 2016, 11, 6401. [Google Scholar] [CrossRef] [Green Version]
- Fong, P.; Hao, C.-H.; Io, C.-C.; Sin, P.-I.; Meng, L.-R. In silico and in vitro anti-Helicobacter pylori effects of combinations of phytochemicals and antibiotics. Molecules 2019, 24, 3608. [Google Scholar] [CrossRef] [Green Version]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiprono, P.C.; Kaberia, F.; Keriko, J.M.; Karanja, J.N. The in vitro anti-fungal and anti-bacterial activities of β-sitosterol from Senecio lyratus (Asteraceae). Z. Für Nat. C 2000, 55, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-C.; Lai, M.-H.; Hsu, K.-P.; Kuo, Y.-H.; Chen, J.; Tsai, M.-C.; Li, C.-X.; Yin, X.-J.; Jeyashoke, N.; Chao, L.K.-P. Identification of β-sitosterol as in vitro anti-inflammatory constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Sökmen, A.; Sökmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Ünlü, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother.Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2004, 18, 451–456. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.-P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and α-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The in vitro activity of essential oils against Helicobacter pylori growth and urease activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef]









| Active Ingredients | Test | Test Procedure | Observation | Abundance |
|---|---|---|---|---|
| Flavonoids | NaOH | Ethanol extract + 10% NaOH + dilute HCl | The yellow solution turned colorless with the addition of dilute HCl | +++ |
| NH3 | 1 drop of ethanol extract is exposed to ammonia vapor | Yellow color. | ||
| Carbohydrates | Molisch | Aqueous extract + 2 mL alcoholic α-naphthol + a few drops of concentrated H2SO4. | A Violet ring is formed | +++ |
| Tannins | Ferric chloride. | Ethanol extract + FeCl3 | A green solution | +++ |
| Saponins | Frothing test. | Aqueous Extract + distilled water was vigorously shaken | Persistent Froth for 3 min. | + |
| Sterols | Liebermann. | Ethanol extract + 2 mL CHCl3 + conc. H2SO4 to form a lower layer | A reddish-brown ring at interphase | ++ |
| Volatile oil | Filter paper stain | Press the aerial parts between filter paper | A transient stain is formed that evaporates upon standing. | ++ |
| No | Name | Rt (min) | Molecular Formula | (M-H) | M.wt | Abundance | References |
|---|---|---|---|---|---|---|---|
| 1. | Rosmarinic acid | 6.68 | C18H16O8 | 359 | 360 | 2.1% | [39] |
| 2. | Hispidulin | 8.45 | C16H12O6 | 299 | 300 | 2.8% | [40] |
| 3. | Cirsimaritin | 9.76 | C17H14O6 | 313 | 314 | 2.8% | [40] |
| 4. | 12-O-methyl carnosol | 9.86 | C20H26O5 | 345 | 346 | 6.15% | [41] |
| 5. | Rosmanol | 10.31 | C20H26O5 | 345 | 346 | 2.92% | [42] |
| 6. | β-sitosterol | 10.92 | C29H50O | 413 | 414 | 1.72% | [43] |
| 7. | Carnosol | 13.73 | C17H14O7 | 329 | 330 | 13.3% | [44] |
| 8. | Carnosol isomer | 14.45 | C17H14O7 | 329 | 330 | 1.45% | [44] |
| 9. | Rosmadial | 14.27 | C20H24O5 | 343 | 344 | 2.00% | [45] |
| 10. | Carnosic acid | 15.66 | C20H28O4 | 331 | 332 | 37.66% | [46,47] |
| 11. | Epirosmanol isomer | 16.63 | C20H26O5 | 345 | 346 | 20.65% | [45] |
| 12. | 6,8-Dihydroxykaempferol | 17.53 | C15H10O8 | 316 | 317 | 1.1% | [48] |
| Peak No | Rt | Compound | KIe | KIl | Area % | Reference |
|---|---|---|---|---|---|---|
| 1 | 7.120 | α-Pinene | 934 | 932 | 3.99 | [53] |
| 2 | 7.540 | Camphene | 949 | 946 | 3.80 | [53] |
| 3 | 8.374 | Limonene | 1051 | 1049 | 2.66 | [53] |
| 4 | 8.844 | β-Myrcene | 990 | 988 | 1.29 | [53] |
| 5 | 9.861 | Cymene | 1024 | 1020 | 0.52 | [53] |
| 6 | 10.086 | Eucalyptol (1,8-Cineole) | 1029 | 1025 | 50.04 | [53] |
| 7 | 12.207 | α-terpineol | 1188 | 1187 | 3.62 | [53] |
| 8 | 12.358 | 3-Thujanone | 1124 | 1121 | 0.97 | [54] |
| 9 | 12.700 | α-Thujone | 1102 | 1100 | 0.34 | [53] |
| 10 | 13.581 | Camphor | 1146 | 1143 | 17.75 | [55] |
| 11 | 14.056 | (E)-Pinocamphone | 1163 | 1158 | 0.50 | [25] |
| 12 | 14.252 | Endo-Borneol | 1169 | 1165 | 3.26 | [25] |
| 13 | 14.587 | 4-Terpinol | 1177 | 1176 | 0.56 | [56] |
| 14 | 15.007 | α-Terpineol | 1188 | 1184 | 2.78 | [57] |
| 15 | 15.187 | Myrtenol | 1195 | 1193 | 0.44 | [58] |
| 16 | 17.801 | Bornyl acetate | 1287 | 1286 | 1.30 | [25] |
| 18 | 21.558 | Caryophyllene | 1419 | 1422 | 2.16 | [59] |
| 19 | 22.464 | Humulene | 1457 | 1452 | 0.46 | [60] |
| 20 | 25.790 | Caryophyllene oxide | 1583 | 1582 | 0.41 | [25] |
| 21 | 26.010 | Viridiflorol | 1592 | 1590 | 0.84 | [61] |
| Total identified components | 95.89% | |||||
| Monoterpene Hydrocarbon | 12.21% | |||||
| Oxygenated monoterpene | 81.56% | |||||
| Oxygenated sesquiterpene | 1.66% | |||||
| Sesquiterpene Hydrocarbon | 0.46% | |||||
| Sample Concentration (µg/ mL) | Total Ethanolic Extract | Volatile Oil | Clarithromycin | |||
|---|---|---|---|---|---|---|
| Mean of H. pylori Inhibitory % | S.D. | Mean of H. pylori Inhibitory % | S.D. | Mean of H. pylori Inhibitory % | S.D. | |
| 125 | 100 | - | 100 | - | 100 | - |
| 62.5 | 100 | - | 100 | - | 100 | - |
| 31.25 | 100 | - | 100 | - | 100 | - |
| 15.63 | 100 | - | 100 | - | 100 | - |
| 7.81 | 100 | - | 86.32 | 1.5 | 100 | - |
| 3.9 | 100 | - | 55.34 | 2.4 | 100 | - |
| 1.95 | 81.42 | 0.85 | 34.38 | 1.3 | 100 | - |
| 0.98 | 56.37 | 1.1 | 26.34 | 0.69 | 100 | - |
| 0.48 | 38.23 | 0.63 | 19.3 | 0.95 | 100 | - |
| 0.24 | 19.38 | 1.4 | 7.2 | 0.83 | 81.64 | 0.58 |
| 0 | 0 | - | 0 | - | 0 | - |
| MIC | 3.9 | 15.63 | 0.48 | |||
| TES Compounds | Oil Compounds | ||
|---|---|---|---|
| Compound | Binding Energy ∆G (Kcal/mol) Rule-Base | Compound | Binding Energy ∆G (Kcal/mol) Rule-Base |
| Rosmarinic acid | −46.6769 | co-crystallized ligand (NADP) | −29.6914 |
| β-Sitosterol | −44.8608 | Borneyl acetate | −29.2608 |
| Carnosic acid | −40.7992 | α-Terpineol | −25.2219 |
| Epirosmanol | −40.4131 | Caryophyllene | −24.4108 |
| 12-O-methyl carnosic acid | −39.6734 | (E)-Pinocamphone | −23.0788 |
| Carnosol | −38.5699 | 4-Terpineol | −22.6625 |
| Rosmanol | −34.6064 | Myrtenol | −22.6432 |
| Dihydroxy kamepferol | −34.5906 | Thujanone | −22.5693 |
| Rosmadial | −34.5739 | Endoborneol | −22.497 |
| Cirsimaritin | −33.5894 | Camphor | −21.7282 |
| Hispidulin | −33.1293 | Cymene | −20.7157 |
| co-crystallized ligand (NADP) | −29.6914 | Myrcene | −20.2976 |
| Eucalyptol | −19.1464 | ||
| Limonene | −19.1059 | ||
| α-Pinene | −18.6418 | ||
| Sample Conc. (µg/mL) | Total Ethanolic Extract | Volatile Oil | St (Celecoxib) | |||
|---|---|---|---|---|---|---|
| Mean of COX-2 Inhibitory % | S.D. | Mean of COX-2 Inhibitory % | S.D. | Mean of COX-2 Inhibitory % | S.D. | |
| 31.25 | 100.00 | - | 100.00 | - | 100 | - |
| 15.63 | 100.00 | - | 79.35 | 0.85 | 100 | - |
| 7.81 | 82.17 | 0.71 | 54.31 | 0.74 | 100 | - |
| 3.9 | 63.74 | 0.89 | 47.32 | 0.34 | 100 | - |
| 1.95 | 52.19 | 1.3 | 29.31 | 0.82 | 82.15 | 0.63 |
| 0.98 | 39.21 | 0.96 | 16.35 | 0.14 | 66.34 | 1.2 |
| 0.49 | 17.35 | 0.74 | 5.31 | 0.52 | 52.72 | 0.58 |
| 0.24 | 9.85 | 0.82 | 0.00 | - | 39.35 | 1.2 |
| 0.00 | 0.00 | - | 0.00 | - | 0.00 | - |
| IC50 | 1.79 ± 0.27 | 5.3 ± 0.62 | 0.43 ± 0.12 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomar, H.A.; Elkady, W.M.; Abdel-Aziz, M.M.; Ibrahim, T.A.; Fathallah, N. Anti-Heliobacter pylori and Anti-Inflammatory Potential of Salvia officinalis Metabolites: In Vitro and In Silico Studies. Metabolites 2023, 13, 136. https://doi.org/10.3390/metabo13010136
Alomar HA, Elkady WM, Abdel-Aziz MM, Ibrahim TA, Fathallah N. Anti-Heliobacter pylori and Anti-Inflammatory Potential of Salvia officinalis Metabolites: In Vitro and In Silico Studies. Metabolites. 2023; 13(1):136. https://doi.org/10.3390/metabo13010136
Chicago/Turabian StyleAlomar, Hatun A., Wafaa M. Elkady, Marwa M. Abdel-Aziz, Taghreed A. Ibrahim, and Noha Fathallah. 2023. "Anti-Heliobacter pylori and Anti-Inflammatory Potential of Salvia officinalis Metabolites: In Vitro and In Silico Studies" Metabolites 13, no. 1: 136. https://doi.org/10.3390/metabo13010136
APA StyleAlomar, H. A., Elkady, W. M., Abdel-Aziz, M. M., Ibrahim, T. A., & Fathallah, N. (2023). Anti-Heliobacter pylori and Anti-Inflammatory Potential of Salvia officinalis Metabolites: In Vitro and In Silico Studies. Metabolites, 13(1), 136. https://doi.org/10.3390/metabo13010136