Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors
Abstract
:1. Introduction
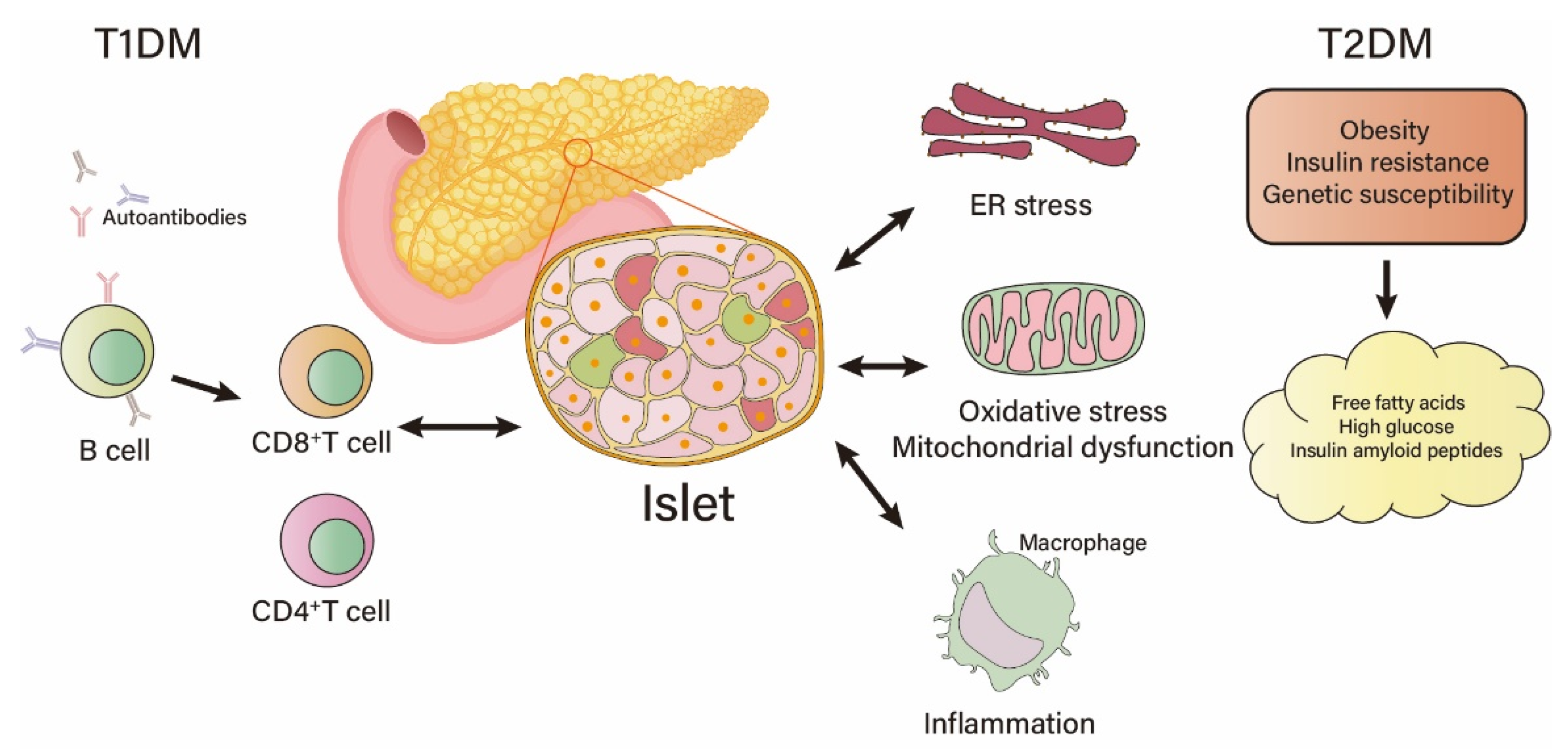
2. Factors Causing Islet β-Cell Damage
3. Role of DYRK1A in the Proliferation and Function of Islet β-Cells
3.1. DYRK1A and NFAT
3.2. DYRK1A and IRS2
3.3. DYRK1A and DREAM
3.4. DYRK1A and P27
3.5. DYRK1A and Cyclin D
4. DYRK1A Inhibitors from Natural Products
4.1. Harmine and Its Derivatives
4.2. Epigallocatechin-3-Gallate (EGCG)
4.3. Desmethylbellidifolin (DMB)
4.4. 2-Aminoimidazolone Alkaloids
4.5. Aristolactam BIII
4.6. 4-Cresol
4.7. Licocoumarone
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Katsarou, A.; Gudbjornsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, A. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Inaishi, J.; Saisho, Y. Beta-Cell Mass in Obesity and Type 2 Diabetes, and Its Relation to Pancreas Fat: A Mini-Review. Nutrients 2020, 12, 3846. [Google Scholar] [CrossRef] [PubMed]
- Marfil-Garza, B.A.; Shapiro, A.M.J.; Kin, T. Clinical islet transplantation: Current progress and new frontiers. J. Hepatobiliary Pancreat. Sci. 2021, 28, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, L.; Fang, Y.; Huang, H.; You, X.; Wu, J. Advances in Encapsulation and Delivery Strategies for Islet Transplantation. Adv. Healthc. Mater. 2021, 10, e2100965. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.W.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef]
- Buteau, J. GLP-1 receptor signaling: Effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008, 34 (Suppl. 2), S73–S77. [Google Scholar] [CrossRef]
- Kumar, K.; Suebsuwong, C.; Wang, P.; Garcia-Ocana, A.; Stewart, A.F.; DeVita, R.J. DYRK1A Inhibitors as Potential Therapeutics for beta-Cell Regeneration for Diabetes. J. Med. Chem. 2021, 64, 2901–2922. [Google Scholar] [CrossRef]
- Lee, J.H.; Mellado-Gil, J.M.; Bahn, Y.J.; Pathy, S.M.; Zhang, Y.E.; Rane, S.G. Protection from beta-cell apoptosis by inhibition of TGF-beta/Smad3 signaling. Cell Death Dis. 2020, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Anshul, F.; Malhotra, D.K.; Jaume, J.; Dworkin, L.D.; Gong, R. Microdose Lithium Protects against Pancreatic Islet Destruction and Renal Impairment in Streptozotocin-Elicited Diabetes. Antioxidants 2021, 10, 138. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Miao, X.Y.; Ma, L.C.; Gao, J.J.; Gong, Y.P.; Li, C.L. Maintenance of cellular annexin A1 level is essential for PI3K/AKT/mTOR-mediated proliferation of pancreatic beta cells. J. Biol. Regul. Homeost. Agents 2021, 35, 1011–1019. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, Y.G.; Kim, K.; Osonoi, S.; Wang, S.; Saunders, D.C.; Wang, J.; Yang, K.; Kim, H.; Lee, J.; et al. Serotonin Regulates Adult beta-Cell Mass by Stimulating Perinatal beta-Cell Proliferation. Diabetes 2020, 69, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ishiwatari, C.; Kaneko, Y.K.; Ishikawa, Y.; Kimura, Y.; Watanabe, N.; Aoshima, I.; Matsuda, Y.; Nakayama, T.; Chiba, R.; et al. Diacylglycerol kinase delta functions as a proliferation suppressor in pancreatic beta-cells. FASEB J. 2021, 35, e21420. [Google Scholar] [CrossRef] [PubMed]
- Kaise, T.; Fukui, M.; Sueda, R.; Piao, W.; Yamada, M.; Kobayashi, T.; Imayoshi, I.; Kageyama, R. Functional rejuvenation of aged neural stem cells by Plagl2 and anti-Dyrk1a activity. Genes Dev. 2022, 36, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yabut, O.; Domogauer, J.; D’Arcangelo, G. Dyrk1A overexpression inhibits proliferation and induces premature neuronal differentiation of neural progenitor cells. J. Neurosci. 2010, 30, 4004–4014. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, M.F.; Meijer, L. Dual-Specificity, Tyrosine Phosphorylation-Regulated Kinases (DYRKs) and cdc2-Like Kinases (CLKs) in Human Disease, an Overview. Int. J. Mol. Sci. 2021, 22, 6047. [Google Scholar] [CrossRef]
- Wang, P.; Alvarez-Perez, J.C.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocana, A.; et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Ling, X.; Ji, K.; Huang, H.; Liu, X.; Yin, C.; Zhu, H.; Guo, Y.; Mo, Y.; Lu, Y.; et al. (1)H NMR-based urinary metabonomic study of the antidiabetic effects of Rubus suavissimus S. Lee in STZ-induced T1DM rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1158, 122347. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ding, Y.P.; Gao, J.; Tanaka, Y.; Zhang, W. Risk factors and primary prevention trials for type 1 diabetes. Int. J. Biol. Sci. 2013, 9, 666–679. [Google Scholar] [CrossRef] [Green Version]
- Rahier, J.; Guiot, Y.; Goebbels, R.M.; Sempoux, C.; Henquin, J.C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008, 10 (Suppl. 4), 32–42. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Pasquali, L.; Cnop, M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nat. Rev. Endocrinol. 2020, 16, 349–362. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Q.; Qiao, J.T.; Liu, F.Q.; Wang, J.B.; Sha, S.; He, Q.; Cui, C.; Song, J.; Zang, N.; Wang, L.S.; et al. The STING-IRF3 pathway is involved in lipotoxic injury of pancreatic beta cells in type 2 diabetes. Mol. Cell. Endocrinol. 2020, 518, 110890. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Hua, H.; Zhu, Z.; Wu, T.; Jia, Z.; Liu, Q. Artemisinin and dihydroartemisinin promote beta-cell apoptosis induced by palmitate via enhancing ER stress. Apoptosis 2020, 25, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to beta-Cell Dysfunction Through Loss of beta-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Alvarez-Perez, J.C.; Avizonis, D.; Kin, T.; Ficarro, S.B.; Choi, D.W.; Karakose, E.; Badur, M.G.; Evans, L.; Rosselot, C.; et al. Glucose-dependent partitioning of arginine to the urea cycle protects beta-cells from inflammation. Nat. Metab. 2020, 2, 432–446. [Google Scholar] [CrossRef]
- Mukhuty, A.; Fouzder, C.; Mukherjee, S.; Malick, C.; Mukhopadhyay, S.; Bhattacharya, S.; Kundu, R. Palmitate induced Fetuin-A secretion from pancreatic beta-cells adversely affects its function and elicits inflammation. Biochem. Biophys. Res. Commun. 2017, 491, 1118–1124. [Google Scholar] [CrossRef]
- Rosselot, C.; Baumel-Alterzon, S.; Li, Y.; Brill, G.; Lambertini, L.; Katz, L.S.; Lu, G.; Garcia-Ocana, A.; Scott, D.K. The many lives of Myc in the pancreatic beta-cell. J. Biol. Chem. 2021, 296, 100122. [Google Scholar] [CrossRef]
- Kaneto, H.; Sharma, A.; Suzuma, K.; Laybutt, D.R.; Xu, G.; Bonner-Weir, S.; Weir, G.C. Induction of c-Myc expression suppresses insulin gene transcription by inhibiting NeuroD/BETA2-mediated transcriptional activation. J. Biol. Chem. 2002, 277, 12998–13006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karslioglu, E.; Kleinberger, J.W.; Salim, F.G.; Cox, A.E.; Takane, K.K.; Scott, D.K.; Stewart, A.F. cMyc is a principal upstream driver of beta-cell proliferation in rat insulinoma cell lines and is an effective mediator of human beta-cell replication. Mol. Endocrinol. 2011, 25, 1760–1772. [Google Scholar] [CrossRef] [Green Version]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Feki, A.; Hibaoui, Y. DYRK1A Protein, A Promising Therapeutic Target to Improve Cognitive Deficits in Down Syndrome. Brain Sci. 2018, 8, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowjat, K.; Adayev, T.; Wojda, U.; Brzozowska, K.; Barczak, A.; Gabryelewicz, T.; Hwang, Y.W. Abnormalities of DYRK1A-Cytoskeleton Complexes in the Blood Cells as Potential Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 1059–1075. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, X.; Tian, L.; Gao, Y.; Liu, W.; Chen, H.; Jiang, X.; Xu, Z.; Ding, H.; Zhao, Q. Design, synthesis and biological evaluation of harmine derivatives as potent GSK-3beta/DYRK1A dual inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113554. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Xiao, Y.; Wei, L.; Mo, M.; Chen, X.; Li, S.; Yang, X.; Huang, Q.; Qu, S.; Pei, Z.; et al. Association of DYRK1A polymorphisms with sporadic Parkinson’s disease in Chinese Han population. Neurosci. Lett. 2016, 632, 39–43. [Google Scholar] [CrossRef]
- Bai, Z.; Du, Y.; Cong, L.; Cheng, Y. The USP22 promotes the growth of cancer cells through the DYRK1A in pancreatic ductal adenocarcinoma. Gene 2020, 758, 144960. [Google Scholar] [CrossRef]
- Malinge, S.; Bliss-Moreau, M.; Kirsammer, G.; Diebold, L.; Chlon, T.; Gurbuxani, S.; Crispino, J.D. Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J. Clin. Investig. 2012, 122, 948–962. [Google Scholar] [CrossRef] [Green Version]
- Tarpley, M.; Oladapo, H.O.; Strepay, D.; Caligan, T.B.; Chdid, L.; Shehata, H.; Roques, J.R.; Thomas, R.; Laudeman, C.P.; Onyenwoke, R.U.; et al. Identification of harmine and beta-carboline analogs from a high-throughput screen of an approved drug collection; profiling as differential inhibitors of DYRK1A and monoamine oxidase A and for in vitro and in vivo anti-cancer studies. Eur. J. Pharm. Sci. 2021, 162, 105821. [Google Scholar] [CrossRef]
- Booiman, T.; Loukachov, V.V.; van Dort, K.A.; van‘t Wout, A.B.; Kootstra, N.A. DYRK1A Controls HIV-1 Replication at a Transcriptional Level in an NFAT Dependent Manner. PLoS ONE 2015, 10, e0144229. [Google Scholar] [CrossRef] [Green Version]
- Hutterer, C.; Milbradt, J.; Hamilton, S.; Zaja, M.; Leban, J.; Henry, C.; Vitt, D.; Steingruber, M.; Sonntag, E.; Zeittrager, I.; et al. Inhibitors of dual-specificity tyrosine phosphorylation-regulated kinases (DYRK) exert a strong anti-herpesviral activity. Antivir. Res. 2017, 143, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rozen, E.J.; Roewenstrunk, J.; Barallobre, M.J.; Di Vona, C.; Jung, C.; Figueiredo, A.F.; Luna, J.; Fillat, C.; Arbones, M.L.; Graupera, M.; et al. DYRK1A Kinase Positively Regulates Angiogenic Responses in Endothelial Cells. Cell Rep. 2018, 23, 1867–1878. [Google Scholar] [CrossRef]
- Ren, D.; Sun, J.; Mao, L.; Ye, H.; Polonsky, K.S. BH3-only molecule Bim mediates beta-cell death in IRS2 deficiency. Diabetes 2014, 63, 3378–3387. [Google Scholar] [CrossRef] [Green Version]
- Catalano-Iniesta, L.; Iglesias-Osma, M.C.; Sanchez-Robledo, V.; Carretero-Hernandez, M.; Blanco, E.J.; Carretero, J.; Garcia-Barrado, M.J. Variations in adrenal gland medulla and dopamine effects induced by the lack of Irs2. J. Physiol. Biochem. 2018, 74, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Mao, N.; Gao, D.; Hu, W.; Gadal, S.; Hieronymus, H.; Wang, S.; Lee, Y.S.; Sullivan, P.; Zhang, Z.; Choi, D.; et al. Oncogenic ERG Represses PI3K Signaling through Downregulation of IRS2. Cancer Res. 2020, 80, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Ma, L.; Shan, P.; Liu, A.; Yu, X.; Jiang, W.; Wang, X.; Zhao, X.; Ye, X.; Wang, T. DYRK1A aggravates beta cell dysfunction and apoptosis by promoting the phosphorylation and degradation of IRS2. Exp. Gerontol. 2019, 125, 110659. [Google Scholar] [CrossRef]
- Lang, L.; Pettko-Szandtner, A.; Tuncay Elbasi, H.; Takatsuka, H.; Nomoto, Y.; Zaki, A.; Dorokhov, S.; de Jaeger, G.; Eeckhout, D.; Ito, M.; et al. The DREAM complex represses growth in response to DNA damage in Arabidopsis. Life Sci. Alliance 2021, 4, e202101141. [Google Scholar] [CrossRef]
- Fajas, L.; Annicotte, J.S.; Miard, S.; Sarruf, D.; Watanabe, M.; Auwerx, J. Impaired pancreatic growth, beta cell mass, and beta cell function in E2F1 (-/-)mice. J. Clin. Investig. 2004, 113, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litovchick, L.; Florens, L.A.; Swanson, S.K.; Washburn, M.P.; DeCaprio, J.A. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev. 2011, 25, 801–813. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.G.; Kay, E.P. PI 3-kinase/Rac1 and ERK1/2 regulate FGF-2-mediated cell proliferation through phosphorylation of p27 at Ser10 by KIS and at Thr187 by Cdc25A/Cdk2. Investig. Ophthalmol. Vis. Sci. 2011, 52, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppa, U.; Schumacher, J.; Florencio Ortiz, V.; Pasqualon, T.; Tejedor, F.J.; Becker, W. The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle 2014, 13, 2084–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdolazimi, Y.; Zhao, Z.; Lee, S.; Xu, H.; Allegretti, P.; Horton, T.M.; Yeh, B.; Moeller, H.P.; Nichols, R.J.; McCutcheon, D.; et al. CC-401 Promotes beta-Cell Replication via Pleiotropic Consequences of DYRK1A/B Inhibition. Endocrinology 2018, 159, 3143–3157. [Google Scholar] [CrossRef] [PubMed]
- Fatrai, S.; Elghazi, L.; Balcazar, N.; Cras-Meneur, C.; Krits, I.; Kiyokawa, H.; Bernal-Mizrachi, E. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006, 55, 318–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polager, S.; Ginsberg, D. E2F—At the crossroads of life and death. Trends Cell Biol. 2008, 18, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhu, W.; Zhao, H.; Zeng, F.; Wang, E.; Wang, H.; Chen, J.; Li, M.; Huang, C.; Ren, L.; et al. Placenta-Derived Osteoprotegerin Is Required for Glucose Homeostasis in Gestational Diabetes Mellitus. Front. Cell Dev. Biol. 2020, 8, 563509. [Google Scholar] [CrossRef]
- Kondegowda, N.G.; Fenutria, R.; Pollack, I.R.; Orthofer, M.; Garcia-Ocana, A.; Penninger, J.M.; Vasavada, R.C. Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-kappaB Ligand Pathway. Cell Metab. 2015, 22, 77–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebrian, A.; Garcia-Ocana, A.; Takane, K.K.; Sipula, D.; Stewart, A.F.; Vasavada, R.C. Overexpression of parathyroid hormone-related protein inhibits pancreatic beta-cell death in vivo and in vitro. Diabetes 2002, 51, 3003–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guthalu Kondegowda, N.; Joshi-Gokhale, S.; Harb, G.; Williams, K.; Zhang, X.Y.; Takane, K.K.; Zhang, P.; Scott, D.K.; Stewart, A.F.; Garcia-Ocana, A.; et al. Parathyroid hormone-related protein enhances human ss-cell proliferation and function with associated induction of cyclin-dependent kinase 2 and cyclin E expression. Diabetes 2010, 59, 3131–3138. [Google Scholar] [CrossRef] [Green Version]
- El Ouaamari, A.; Dirice, E.; Gedeon, N.; Hu, J.; Zhou, J.Y.; Shirakawa, J.; Hou, L.; Goodman, J.; Karampelias, C.; Qiang, G.; et al. SerpinB1 Promotes Pancreatic beta Cell Proliferation. Cell Metab. 2016, 23, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, S.B.; Schisler, J.C.; Hohmeier, H.E.; An, J.; Sun, A.Y.; Pitt, G.S.; Newgard, C.B. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet beta-cell survival and function. Cell Metab. 2012, 16, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Untereiner, A.; Xu, J.; Bhattacharjee, A.; Cabrera, O.; Hu, C.; Dai, F.F.; Wheeler, M.B. gamma-aminobutyric acid stimulates beta-cell proliferation through the mTORC1/p70S6K pathway, an effect amplified by Ly49, a novel gamma-aminobutyric acid type A receptor positive allosteric modulator. Diabetes Obes. Metab. 2020, 22, 2021–2031. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Yu, S. Pharmacological effects of harmine and its derivatives: A review. Arch. Pharm. Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Ogawa, Y.; Nonaka, Y.; Goto, T.; Ohnishi, E.; Hiramatsu, T.; Kii, I.; Yoshida, M.; Ikura, T.; Onogi, H.; Shibuya, H.; et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010, 1, 86. [Google Scholar] [CrossRef] [Green Version]
- Shen, W.; Taylor, B.; Jin, Q.; Nguyen-Tran, V.; Meeusen, S.; Zhang, Y.Q.; Kamireddy, A.; Swafford, A.; Powers, A.F.; Walker, J.; et al. Inhibition of DYRK1A and GSK3B induces human beta-cell proliferation. Nat. Commun. 2015, 6, 8372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirice, E.; Walpita, D.; Vetere, A.; Meier, B.C.; Kahraman, S.; Hu, J.; Dancik, V.; Burns, S.M.; Gilbert, T.J.; Olson, D.E.; et al. Inhibition of DYRK1A Stimulates Human beta-Cell Proliferation. Diabetes 2016, 65, 1660–1671. [Google Scholar] [CrossRef] [Green Version]
- Allegretti, P.A.; Horton, T.M.; Abdolazimi, Y.; Moeller, H.P.; Yeh, B.; Caffet, M.; Michel, G.; Smith, M.; Annes, J.P. Generation of highly potent DYRK1A-dependent inducers of human beta-Cell replication via Multi-Dimensional compound optimization. Bioorg. Med. Chem. 2020, 28, 115193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Wang, P.; Sanchez, R.; Swartz, E.A.; Stewart, A.F.; DeVita, R.J. Development of Kinase-Selective, Harmine-Based DYRK1A Inhibitors that Induce Pancreatic Human beta-Cell Proliferation. J. Med. Chem. 2018, 61, 7687–7699. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Wang, P.; Wilson, J.; Zlatanic, V.; Berrouet, C.; Khamrui, S.; Secor, C.; Swartz, E.A.; Lazarus, M.; Sanchez, R.; et al. Synthesis and Biological Validation of a Harmine-Based, Central Nervous System (CNS)-Avoidant, Selective, Human beta-Cell Regenerative Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase A (DYRK1A) Inhibitor. J. Med. Chem. 2020, 63, 2986–3003. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Chen, M.; Wang, J.; Xie, B.; Sun, Z. (-)-Epigallocatechin-3-gallate (EGCG) inhibits starch digestion and improves glucose homeostasis through direct or indirect activation of PXR/CAR-mediated phase II metabolism in diabetic mice. Food Funct. 2018, 9, 4651–4663. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, X.; Tian, H.; Liu, H.; Li, J.; Qi, G.; Liu, X. EGCG stimulates the recruitment of brite adipocytes, suppresses adipogenesis and counteracts TNF-alpha-triggered insulin resistance in adipocytes. Food Funct. 2018, 9, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, Q.; Zhang, C.; Wu, C.; Yang, K.; Song, Z.; Wang, Q.; Li, C.; Zhou, Y.; Chen, J.; et al. A natural DYRK1A inhibitor as a potential stimulator for beta-cell proliferation in diabetes. Clin. Transl. Med. 2021, 11, e494. [Google Scholar] [CrossRef]
- Loaec, N.; Attanasio, E.; Villiers, B.; Durieu, E.; Tahtouh, T.; Cam, M.; Davis, R.A.; Alencar, A.; Roue, M.; Bourguet-Kondracki, M.L.; et al. Marine-Derived 2-Aminoimidazolone Alkaloids. Leucettamine B-Related Polyandrocarpamines Inhibit Mammalian and Protozoan DYRK & CLK Kinases. Mar. Drugs 2017, 15, 316. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, A.K.; Ham, Y.; Lee, J.Y.; Kim, D.; Yang, A.; Jo, M.J.; Yoon, E.; Heo, J.N.; Han, S.B.; et al. Aristolactam BIII, a naturally derived DYRK1A inhibitor, rescues Down syndrome-related phenotypes. Phytomedicine 2021, 92, 153695. [Google Scholar] [CrossRef]
- Brial, F.; Alzaid, F.; Sonomura, K.; Kamatani, Y.; Meneyrol, K.; Le Lay, A.; Pean, N.; Hedjazi, L.; Sato, T.A.; Venteclef, N.; et al. The Natural Metabolite 4-Cresol Improves Glucose Homeostasis and Enhances beta-Cell Function. Cell Rep. 2020, 30, 2306–2320.e5. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Wang, D.; Gao, Z.; Kan, H.; Qiu, F.; Chen, L.; Li, H. Licocoumarone induces BxPC-3 pancreatic adenocarcinoma cell death by inhibiting DYRK1A. Chem. Biol. Interact. 2020, 316, 108913. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Tan, S.; Wang, C.; Fan, S.; Huang, C. Harmine is an inflammatory inhibitor through the suppression of NF-kappaB signaling. Biochem. Biophys. Res. Commun. 2017, 489, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; He, J.; Huang, J.; Yu, T.; Shi, X.; Zhang, T.; Yan, G.; Chen, S.; Peng, C. Harmine induces anticancer activity in breast cancer cells via targeting TAZ. Int. J. Oncol. 2019, 54, 1995–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, D.; Meechoovet, B.; Wang, T.; Gately, S.; Giorgetti, M.; Shcherbakova, I.; Dunckley, T. beta-carboline compounds, including harmine, inhibit DYRK1A and tau phosphorylation at multiple Alzheimer’s disease-related sites. PLoS ONE 2011, 6, e19264. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Wu, H.; Wei, Y.; Liu, W.; Huang, F.; Shi, H.; Zhang, B.; Wu, X.; Wang, C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015, 768, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Kolecka, B.; Golabek, A.; Kida, E.; Rabe, A.; Hwang, Y.W.; Adayev, T.; Wegiel, J.; Flory, M.; Kaczmarski, W.; Marchi, E.; et al. Effect of DYRK1A activity inhibition on development of neuronal progenitors isolated from Ts65Dn mice. J. Neurosci. Res. 2012, 90, 999–1010. [Google Scholar] [CrossRef]
- Wang, P.; Karakose, E.; Liu, H.; Swartz, E.; Ackeifi, C.; Zlatanic, V.; Wilson, J.; Gonzalez, B.J.; Bender, A.; Takane, K.K.; et al. Combined Inhibition of DYRK1A, SMAD, and Trithorax Pathways Synergizes to Induce Robust Replication in Adult Human Beta Cells. Cell Metab. 2019, 29, 638–652.e5. [Google Scholar] [CrossRef] [Green Version]
- Ackeifi, C.; Wang, P.; Karakose, E.; Manning Fox, J.E.; Gonzalez, B.J.; Liu, H.; Wilson, J.; Swartz, E.; Berrouet, C.; Li, Y.; et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human beta cell regeneration. Sci. Transl. Med. 2020, 12, aaw9996. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortsater, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjoholm, A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Pournourmohammadi, S.; Grimaldi, M.; Stridh, M.H.; Lavallard, V.; Waagepetersen, H.S.; Wollheim, C.B.; Maechler, P. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ss-cells: A potential beneficial effect in the pre-diabetic state? Int. J. Biochem. Cell Biol. 2017, 88, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Bain, J.; McLauchlan, H.; Elliott, M.; Cohen, P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003, 371, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yuan, X.; Huang, H.; Zhang, B.; Cao, C.; Zhao, H.P. Chemical constituents from Swertia mussotii Franch. (Gentianaceae). Nat. Prod. Res. 2017, 31, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liu, M.; Yu, H.; Chen, Y.; Liu, Y.; Chen, S.; Ruan, J.; Da, A.; Zhang, Y.; Wang, T. Desmethylbellidifolin From Gentianella acuta Ameliorate TNBS-Induced Ulcerative Colitis through Antispasmodic Effect and Anti-Inflammation. Front. Pharmacol. 2019, 10, 1104. [Google Scholar] [CrossRef] [PubMed]
- Burgy, G.; Tahtouh, T.; Durieu, E.; Foll-Josselin, B.; Limanton, E.; Meijer, L.; Carreaux, F.; Bazureau, J.P. Chemical synthesis and biological validation of immobilized protein kinase inhibitory Leucettines. Eur. J. Med. Chem. 2013, 62, 728–737. [Google Scholar] [CrossRef]
- Hu, H.; Lee-Fong, Y.; Peng, J.; Hu, B.; Li, J.; Li, Y.; Huang, H. Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry. Molecules 2021, 26, 960. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.L.; Kim, J.K.; Choi, S.U.; Min, Y.K.; Bae, M.A.; Kim, B.T.; Heo, J.N. Synthesis of aristolactam analogues and evaluation of their antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 3036–3040. [Google Scholar] [CrossRef]
- Chia, Y.C.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Aristolactams and dioxoaporphines from Fissistigma balansae and Fissistigma oldhamii. J. Nat. Prod. 2000, 63, 1160–1163. [Google Scholar] [CrossRef]
- Meijers, B.K.; Bammens, B.; de Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Fan, Y.; Fan, C.; Yu, Y.; Sun, L.; Jin, Y.; Zhang, Y.; Ye, R.D. Licocoumarone isolated from Glycyrrhiza uralensis selectively alters LPS-induced inflammatory responses in RAW 264.7 macrophages. Eur. J. Pharmacol. 2017, 801, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xu, Y.; Zhang, L.; Chen, Y.; Wu, T.; Liu, R.; Sui, W.; Zhu, Q.; Zhang, M. Licorice extract ameliorates hyperglycemia through reshaping gut microbiota structure and inhibiting TLR4/NF-kappaB signaling pathway in type 2 diabetic mice. Food Res. Int. 2022, 153, 110945. [Google Scholar] [CrossRef]
- Rathwa, N.; Patel, R.; Palit, S.P.; Parmar, N.; Rana, S.; Ansari, M.I.; Ramachandran, A.V.; Begum, R. beta-cell replenishment: Possible curative approaches for diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, X.; Jie, Z.; Zhu, L.; Yang, J.Y.; Ko, C.J.; Gao, T.; Jain, A.; Jung, S.Y.; Baran, N.; et al. DYRK1a mediates BAFF-induced noncanonical NF-kappaB activation to promote autoimmunity and B-cell leukemogenesis. Blood 2021, 138, 2360–2371. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, R.S.; Rammohan, M.; Lee, P.; Laurent, A.P.; Wen, Q.; Suraneni, P.; Yip, B.H.; Tsai, Y.C.; Jenni, S.; Bornhauser, B.; et al. DYRK1A regulates B cell acute lymphoblastic leukemia through phosphorylation of FOXO1 and STAT3. J. Clin. Investig. 2021, 131, e135937. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. MicroRNA-215-5p inhibits the proliferation of keratinocytes and alleviates psoriasis-like inflammation by negatively regulating DYRK1A and its downstream signalling pathways. Exp. Dermatol. 2021, 30, 932–942. [Google Scholar] [CrossRef]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef]
- Masaki, S.; Kii, I.; Sumida, Y.; Kato-Sumida, T.; Ogawa, Y.; Ito, N.; Nakamura, M.; Sonamoto, R.; Kataoka, N.; Hosoya, T.; et al. Design and synthesis of a potent inhibitor of class 1 DYRK kinases as a suppressor of adipogenesis. Bioorg. Med. Chem. 2015, 23, 4434–4441. [Google Scholar] [CrossRef] [Green Version]
- Saunders, D.C.; Brissova, M.; Phillips, N.; Shrestha, S.; Walker, J.T.; Aramandla, R.; Poffenberger, G.; Flaherty, D.K.; Weller, K.P.; Pelletier, J.; et al. Ectonucleoside Triphosphate Diphosphohydrolase-3 Antibody Targets Adult Human Pancreatic beta Cells for In Vitro and In Vivo Analysis. Cell Metab. 2019, 29, 745–754.e4. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, N.; Zang, S.; Liu, H.; Wang, P.; Ji, C.; Sun, X. Tumor suppressor DYRK1A effects on proliferation and chemoresistance of AML cells by downregulating c-Myc. PLoS ONE 2014, 9, e98853. [Google Scholar] [CrossRef] [PubMed]

| Compound | Source | IC50 for DYRK1A | Model | Effects | Ref. | |
|---|---|---|---|---|---|---|
| Harmine | Peganum harmala L. | 28 nM | In vitro | RIN-m5f INS-1 Human β-cells | Increasing 8% Ki67 labeling for rat β-cells. Increasing 1% Ki67 labeling for human β-cell at 10 μM. | [16] |
| In vivo | Human islet transplanted in STZ-diabetic NOD-SCID mice | Increasing 3-fold Ki67 labeling for human β-cells. | ||||
| Harmine Analogue 2-2 | 54.8 nM | In vitro | Human islet | Increasing 2.5% Ki67 labeling for human β-cells at 30 μM. | [65] | |
| Harmine Analogue 2-2c | 25 nM | In vitro | Human islet | Increasing 1.5% Ki67 labeling for human β-cells at 3 μM. | [66] | |
| In vivo | Human islet transplanted in NOD-SCID mice | Increasing 1.75% Ki67 labeling for human β-cells. | ||||
| Epicatechin-3-gallate | Green tea | 330 nM | In vitro | HepG2 and 3T3-L1 cells | Suppressing oxidative stress and regulating mitochondrial function. | [67,68] |
| In vivo | T2DM mice induced by high-fat diet and streptozotocin induced diabetes mice | Repressing gluconeogenesis and lipogenesis in the liver. | ||||
| Desmethylbellidifolin (DMB) | Gentianella acuta | 370 nM | In vitro | INS-1 cells | Increasing 30% EDU labeling for rat β-cells. | [69] |
| In vivo | db/db mice | Increasing 6% ki67 labeling for rat β-cells. | ||||
| Polyandrocarpamines A Polyandrocarpamines B Leucettine L41 | Marine Calcareous sponges Leucetta and Clathrina | 270 nM 470 nM 32 nM | In vitro | SH-SY5Y neuroblastoma cells | Increasing Thr286-cyclin D1 phosphorylation. | [70] |
| Aristolactam BIII | Fissistigma oldhamii | 9.67 nM | In vitro | Primary fibroblast cells of DYRK1A TG mice | Decreasing cyclin D1 at 100 nM. 2–3 fold increase in BrdU labeling at 100 nM. | [71] |
| In vivo | DYRK1A TG mice | Lowering Tau phosphorylation in the hippocampus and frontal cortex. Improving the locomotive and exploratory behavior of DYRK1A-overexpressing mice in the open field test. | ||||
| 4-Cresol | Metabolite produced by intestinal bacteria | ND | In vitro | Primary islet cells of c57BL/6 mice | Increasing 2.38-fold ki67 labeling at 10 nM. | [72] |
| In vivo | Mice fed high-fat diet | Increasing glucose-stimulated secretion of insulin, Reducing liver triglycerides. | ||||
| Licocoumarone | Glycyrrhiza uralensis Fisch | 12.56 μM | In vitro | Human pancreatic adenocarcinoma cell line (BxPC-3) | Suppressing proliferation and inducing cell apoptosis. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, L.; Yao, Y.; Li, H. Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors. Metabolites 2023, 13, 51. https://doi.org/10.3390/metabo13010051
Guo Y, Li L, Yao Y, Li H. Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors. Metabolites. 2023; 13(1):51. https://doi.org/10.3390/metabo13010051
Chicago/Turabian StyleGuo, Yichuan, Lingqiao Li, Yuanfa Yao, and Hanbing Li. 2023. "Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors" Metabolites 13, no. 1: 51. https://doi.org/10.3390/metabo13010051
APA StyleGuo, Y., Li, L., Yao, Y., & Li, H. (2023). Regeneration of Pancreatic β-Cells for Diabetes Therapeutics by Natural DYRK1A Inhibitors. Metabolites, 13(1), 51. https://doi.org/10.3390/metabo13010051






