Moderate Effects of Hypoxic Training at Low and Supramaximal Intensities on Skeletal Muscle Metabolic Gene Expression in Mice
Abstract
:1. Introduction
2. Materials and Methods
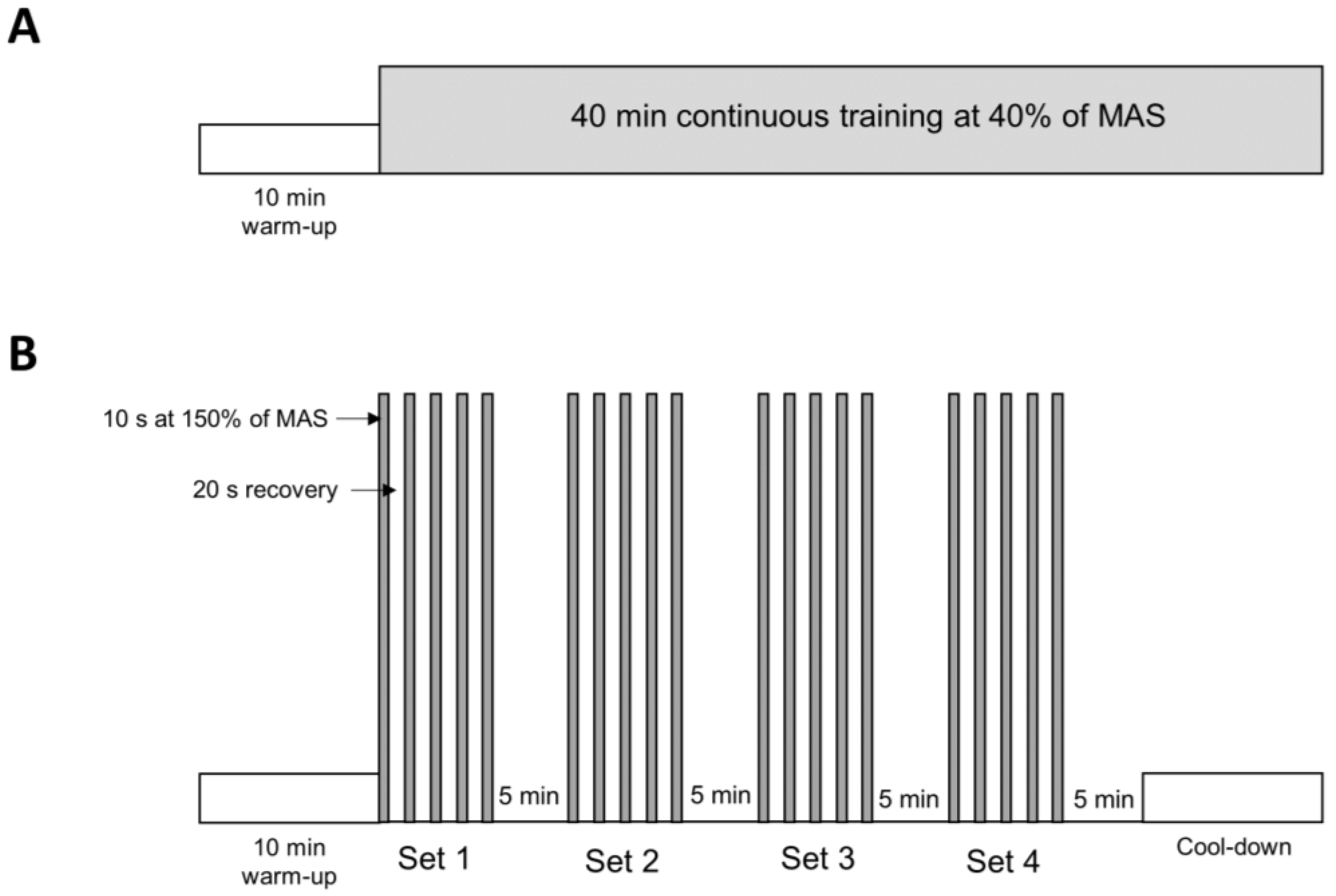
2.1. Animal and Experimental Protocol
2.2. Quantitative Real-Time Polymerase Chain Reaction
2.3. Statistical Analysis
3. Results
3.1. Effect on Performance and Body Weight
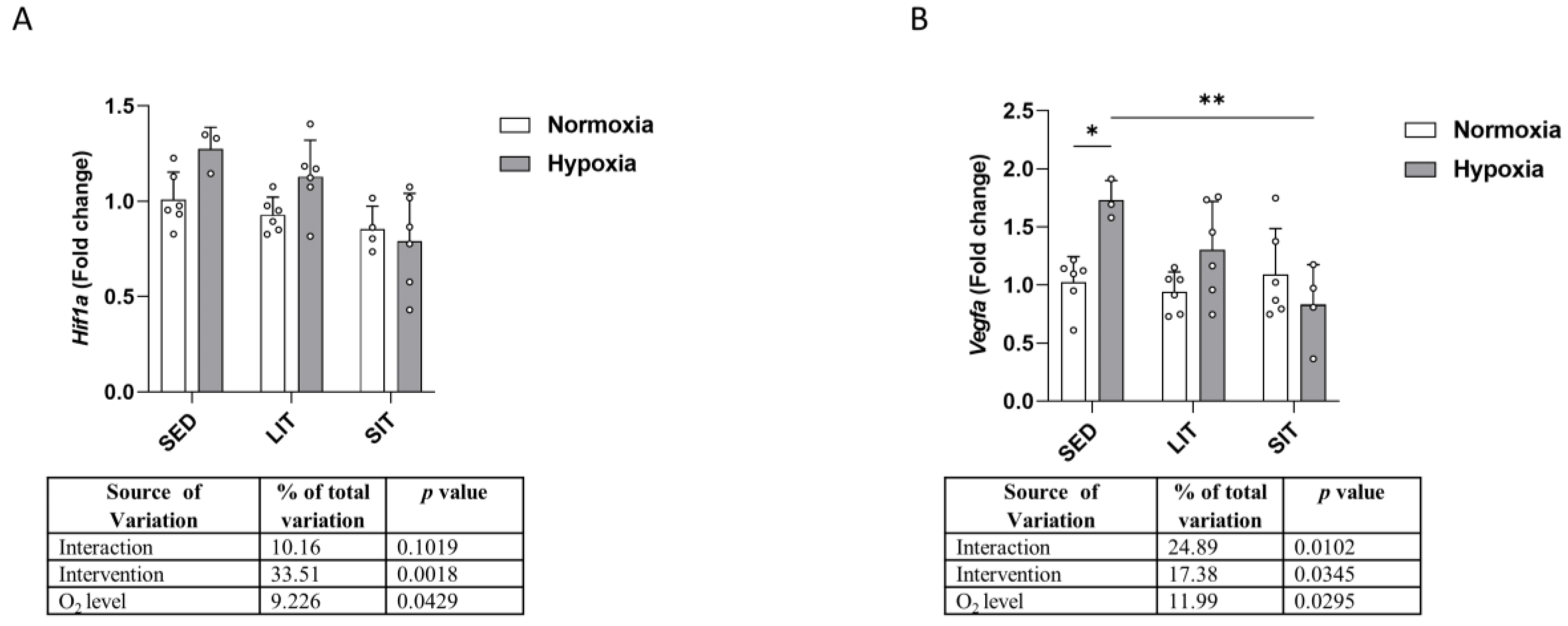
3.2. Hypoxia Signaling Pathway
3.3. Glucose Metabolism
3.4. Lactate Metabolism
3.5. Mitochondrial Biogenesis and Krebs Cycle
3.6. Mitochondrial Respiratory Chain Complex
3.7. Fatty Acid β-Oxidation
4. Discussion
- (1)
- None of the 4-week training protocols, either in normoxia or hypoxia, led to an increase in exercise performance, suggesting an insufficient training load.
- (2)
- Supramaximal exercise training in normoxia caused upregulation of some genes involved in glucose and lactate transport as well as some genes responsible for mitochondrial biogenesis and fatty acid oxidation.
- (3)
- The exposure to hypoxia induced a higher expression of genes involved in glucose metabolism and mitochondrial biogenesis, mainly in the sedentary mice.
- (4)
- Exercise training performed in hypoxia had a moderate effect on these transcriptional adaptations.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balan, E.; Schwalm, C.; Naslain, D.; Nielens, H.; Francaux, M.; Deldicque, L. Regular Endurance Exercise Promotes Fission, Mitophagy, and Oxidative Phosphorylation in Human Skeletal Muscle Independently of Age. Front. Physiol. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.E.; Huang, T.Y.; Simon, J.; Batdorf, H.M.; Essajee, N.M.; Scott, M.C.; Waskom, C.M.; Brown, J.M.; Burke, S.J.; Collier, J.J.; et al. Low-intensity exercise induces acute shifts in liver and skeletal muscle substrate metabolism but not chronic adaptations in tissue oxidative capacity. J. Appl. Physiol. (1985) 2019, 127, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; MacInnis, M.J. Physiological basis of brief, intense interval training to enhance maximal oxygen uptake: A mini-review. Am. J. Physiol. Cell Physiol. 2022, 323, C1410–C1416. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Granata, C.; Oliveira, R.S.; Little, J.P.; Renner, K.; Bishop, D.J. Sprint-interval but not continuous exercise increases PGC-1alpha protein content and p53 phosphorylation in nuclear fractions of human skeletal muscle. Sci. Rep. 2017, 7, 44227. [Google Scholar] [CrossRef] [PubMed]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia. Br. J. Sports Med. 2013, 47 (Suppl. 1), i45–i50. [Google Scholar] [CrossRef]
- Lundby, C.; Calbet, J.A.L.; Robach, P. The response of human skeletal muscle tissue to hypoxia. Cell. Mol. Life Sci. 2009, 66, 3615–3623. [Google Scholar] [CrossRef]
- Gnimassou, O.; Fernández-Verdejo, R.; Brook, M.; Naslain, D.; Balan, E.; Sayda, M.; Cegielski, J.; Nielens, H.; Decottignies, A.; Demoulin, J.B.; et al. Environmental hypoxia favors myoblast differentiation and fast phenotype but blunts activation of protein synthesis after resistance exercise in human skeletal muscle. FASEB J. 2018, 32, 5272–5284. [Google Scholar] [CrossRef]
- Vogt, M.; Billeter, R.; Hoppeler, H. Effect of hypoxia on muscular performance capacity: “living low--training high”. Ther. Umsch. 2003, 60, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Brocherie, F.; Millet, G.P.; D’Hulst, G.; Van Thienen, R.; Deldicque, L.; Girard, O. Repeated maximal-intensity hypoxic exercise superimposed to hypoxic residence boosts skeletal muscle transcriptional responses in elite team-sport athletes. Acta Physiol. 2018, 222, e12851e12989. [Google Scholar] [CrossRef] [PubMed]
- Brocherie, F.; Girard, O.; Faiss, R.; Millet, G.P. Effects of Repeated-Sprint Training in Hypoxia on Sea-Level Performance: A Meta-Analysis. Sports Med. 2017, 47, 1651–1660. [Google Scholar] [CrossRef]
- Nava, R.C.; McKenna, Z.; Fennel, Z.; Berkemeier, Q.; Ducharme, J.; de Castro Magalhaes, F.; Amorim, F.T.; Mermier, C. Repeated sprint exercise in hypoxia stimulates HIF-1-dependent gene expression in skeletal muscle. Eur. J. Appl. Physiol. 2022, 122, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Delfan, M.; Vahed, A.; Bishop, D.J.; Amadeh Juybari, R.; Laher, I.; Saeidi, A.; Granacher, U.; Zouhal, H. Effects of two workload-matched high intensity interval training protocols on regulatory factors associated with mitochondrial biogenesis in the soleus muscle of diabetic rats. Front. Physiol. 2022, 13, 927969. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J. Effects of exercise training with short-duration intermittent hypoxia on endurance performance and muscle metabolism in well-trained mice. Physiol. Rep. 2019, 7, e14182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Guo, W.; Gao, B.H. Hypoxic training upregulates mitochondrial turnover and angiogenesis of skeletal muscle in mice. Life Sci. 2022, 291, 119340. [Google Scholar] [CrossRef]
- Lavier, J.; Beaumann, M.; Menetrey, S.; Bouzourene, K.; Rosenblatt-Velin, N.; Pialoux, V.; Mazzolai, L.; Peyter, A.C.; Pellegrin, M.; Millet, G.P. High-intensity exercise in hypoxia improves endothelial function via increased nitric oxide bioavailability in C57BL/6 mice. Acta Physiol. 2021, 233, e13700. [Google Scholar] [CrossRef]
- Lavier, J.; Beaumann, M.; Menetrey, S.; Mazzolai, L.; Peyter, A.C.; Pellegrin, M.; Millet, G.P. Supramaximal Intensity Hypoxic Exercise and Vascular Function Assessment in Mice. J. Vis. Exp. 2019, 145, e58708. [Google Scholar] [CrossRef]
- Pellegrin, M.; Bouzourene, K.; Poitry-Yamate, C.; Mlynarik, V.; Feihl, F.; Aubert, J.F.; Gruetter, R.; Mazzolai, L. Experimental peripheral arterial disease: New insights into muscle glucose uptake, macrophage, and T-cell polarization during early and late stages. Physiol. Rep. 2014, 2, e00234. [Google Scholar] [CrossRef]
- Nino, M.; Kim, H.; Massett, M.P. Response to Endurance Training Using Critical Speed: Influence of Genetic Background and Exercise Intensity. FASEB J. 2022, 36. Experimental Biology Meeting, Philadelphia, USA, April 2-5 2022. [Google Scholar] [CrossRef]
- Avila, J.J.; Kim, S.K.; Massett, M.P. Differences in Exercise Capacity and Responses to Training in 24 Inbred Mouse Strains. Front. Physiol. 2017, 8, 974. [Google Scholar] [CrossRef] [PubMed]
- Massett, M.P.; Matejka, C.; Kim, H. Systematic Review and Meta-Analysis of Endurance Exercise Training Protocols for Mice. Front. Physiol. 2021, 12, 782695. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Oliveira, A.R.; Aquino-Junior, J.; Abbasi, A.; Santos-Dias, A.; Oliveira-Junior, M.C.; Alberca-Custodio, R.W.; Rigonato-Oliveira, N.C.; Salles-Dias, L.P.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; et al. Effects of aerobic exercise on molecular aspects of asthma: Involvement of SOCS-JAK-STAT. Exerc. Immunol. Rev. 2019, 25, 50–62. [Google Scholar] [PubMed]
- Flores-Opazo, M.; McGee, S.L.; Hargreaves, M. Exercise and GLUT4. Exerc. Sport. Sci. Rev. 2020, 48, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Asad, M.R.; Rahimi, M.; Souri, R.; Ahvar, A. The Effect of Exercise with Different Intensity on Glucose Transporter 4 (GLUT4) Gene Expression in Skeletal Muscle of Obese Male Rats. J. Arak Univ. Med. Sci. 2018, 21, 98–108. [Google Scholar]
- Dill, R.P.; Chadan, S.G.; Li, C.; Parkhouse, W.S. Aging and glucose transporter plasticity in response to hypobaric hypoxia. Mech. Ageing Dev. 2001, 122, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Pescador, N.; Villar, D.; Cifuentes, D.; Garcia-Rocha, M.; Ortiz-Barahona, A.; Vazquez, S.; Ordoñez, A.; Cuevas, Y.; Saez-Morales, D.; Garcia-Bermejo, M.L.; et al. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS ONE 2010, 5, e9644. [Google Scholar] [CrossRef]
- Ferrara, C.M.; Goldberg, A.P.; Ortmeyer, H.K.; Ryan, A.S. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 480–487. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Steenberg, D.E.; Hostrup, M.; Birk, J.B.; Larsen, J.K.; Santos, A.; Kjøbsted, R.; Hingst, J.R.; Schéele, C.C.; Murgia, M.; et al. Author Correction: Deep muscle-proteomic analysis of freeze-dried human muscle biopsies reveals fiber type-specific adaptations to exercise training. Nat. Commun. 2021, 12, 1600. [Google Scholar] [CrossRef]
- Ikeda, S.; Abe, F.; Matsuda, Y.; Kitadate, A.; Takahashi, N.; Tagawa, H. Hypoxia-inducible hexokinase-2 enhances anti-apoptotic function via activating autophagy in multiple myeloma. Cancer Sci. 2020, 111, 4088–4101. [Google Scholar] [CrossRef]
- Riddle, S.R.; Ahmad, A.; Ahmad, S.; Deeb, S.S.; Malkki, M.; Schneider, B.K.; Allen, C.B.; White, C.W. Hypoxia induces hexokinase II gene expression in human lung cell line A549. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L407–L416. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Green, H.J.; Sutton, J.R.; Coates, G.; Cymerman, A.; Young, P.; Houston, C.S. Operation Everest II: Structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol. Scand. 1991, 142, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bigard, A.X.; Brunet, A.; Guezennec, C.Y.; Monod, H. Skeletal muscle changes after endurance training at high altitude. J. Appl. Physiol. (1985) 1991, 71, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Dehne, N.; Kerkweg, U.; Otto, T.; Fandrey, J. The HIF-1 response to simulated ischemia in mouse skeletal muscle cells neither enhances glycolysis nor prevents myotube cell death. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1693–R1701. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Klarskov, C.; Nielsen, J.J.; Krustrup, P.; Mohr, M.; Bangsbo, J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E245–E251. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.J.; Roels, B.; Thomas, C.; Ives, R.; Mercier, J.; Millet, G.; Cameron-Smith, D. The relationship between monocarboxylate transporters 1 and 4 expression in skeletal muscle and endurance performance in athletes. Eur. J. Appl. Physiol. 2009, 106, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, R.; Wang, J.; Yu, S.; Wang, J.; Zhang, Y. Nrf2 Activation Enhances Muscular MCT1 Expression and Hypoxic Exercise Capacity. Med. Sci. Sports Exerc. 2020, 52, 1719–1728. [Google Scholar] [CrossRef]
- Millet, G.; Bentley, D.J.; Roels, B.; Mc Naughton, L.R.; Mercier, J.; Cameron-Smith, D. Effects of intermittent training on anaerobic performance and MCT transporters in athletes. PLoS ONE 2014, 9, e95092. [Google Scholar] [CrossRef]
- Butcher, L.R.; Thomas, A.; Backx, K.; Roberts, A.; Webb, R.; Morris, K. Low-intensity exercise exerts beneficial effects on plasma lipids via PPARgamma. Med. Sci. Sports Exerc. 2008, 40, 1263–1270. [Google Scholar] [CrossRef]
- Thomas, A.W.; Davies, N.A.; Moir, H.; Watkeys, L.; Ruffino, J.S.; Isa, S.A.; Butcher, L.R.; Hughes, M.G.; Morris, K.; Webb, R. Exercise-associated generation of PPARγ ligands activates PPARγ signaling events and upregulates genes related to lipid metabolism. J. Appl. Physiol. (1985) 2012, 112, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Gillen, J.B.; Percival, M.E.; Safdar, A.; Tarnopolsky, M.A.; Punthakee, Z.; Jung, M.E.; Gibala, M.J. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J. Appl. Physiol. (1985) 2011, 111, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Percival, M.E.; Tricarico, S.; Little, J.P.; Cermak, N.; Gillen, J.B.; Tarnopolsky, M.A.; Gibala, M.J. Intermittent and continuous high-intensity exercise training induce similar acute but different chronic muscle adaptations. Exp. Physiol. 2014, 99, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Little, J.P.; Tarnopolsky, M.A.; Gibala, M.J. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. J. Appl. Physiol. (1985) 2010, 108, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High intensity interval training and molecular adaptive response of skeletal muscle. Sports Med. Health Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Tsuji, K.; Horii, N.; Hasegawa, N.; Fujie, S.; Homma, T.; Uchida, M.; Hamaoka, T.; Kanehisa, H.; Tabata, I.; et al. Gene expression profile of muscle adaptation to high-intensity intermittent exercise training in young men. Sci. Rep. 2018, 8, 16811. [Google Scholar] [CrossRef]
- Tunstall, R.J.; Mehan, K.A.; Wadley, G.D.; Collier, G.R.; Bonen, A.; Hargreaves, M.; Cameron-Smith, D. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E66–E72. [Google Scholar] [CrossRef]
- Picca, A.; Lezza, A.M.S.; Leeuwenburgh, C.; Pesce, V.; Calvani, R.; Landi, F.; Bernabei, R.; Marzetti, E. Fueling Inflamm-Aging through Mitochondrial Dysfunction: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2017, 18, 933. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Arany, Z. The many roles of PGC-1α in muscle—recent developments. Metabolism 2014, 63, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hock, M.B.; Kralli, A. Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.C.; Han, D.H.; Garcia-Roves, P.M.; Geiger, P.C.; Jones, T.E.; Holloszy, J.O. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. 2007, 282, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Lundby, C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. (1985) 2013, 114, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Roca, D.; Ríos-Kristjánsson, J.G.; Núñez-Espinosa, C.; Santos-Alves, E.; Magalhães, J.; Ascensão, A.; Pagès, T.; Viscor, G.; Torrella, J.R. Modulation of mitochondrial biomarkers by intermittent hypobaric hypoxia and aerobic exercise after eccentric exercise in trained rats. Appl. Physiol. Nutr. Metab. 2017, 42, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.H.; Johnson, M.L.; Dasari, S.; LeBrasseur, N.K.; Vuckovic, I.; Henderson, G.C.; Cooper, S.A.; Manjunatha, S.; Ruegsegger, G.N.; Shulman, G.I.; et al. TFAM Enhances Fat Oxidation and Attenuates High-Fat Diet-Induced Insulin Resistance in Skeletal Muscle. Diabetes 2019, 68, 1552–1564. [Google Scholar] [CrossRef]
- Pellegrin, M.; Bouzourene, K.; Aubert, J.F.; Bielmann, C.; Gruetter, R.; Rosenblatt-Velin, N.; Poitry-Yamate, C.; Mazzolai, L. Impact of aerobic exercise type on blood flow, muscle energy metabolism, and mitochondrial biogenesis in experimental lower extremity artery disease. Sci. Rep. 2020, 10, 14048. [Google Scholar] [CrossRef]
- Holm, C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003, 31, 1120–1124. [Google Scholar] [CrossRef]
- Kang, S.; Park, K.M.; Sung, K.Y.; Yuan, Y.; Lim, S.T. Effect of Resistance Exercise on the Lipolysis Pathway in Obese Pre- and Postmenopausal Women. J. Pers. Med. 2021, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Alsted, T.J.; Nybo, L.; Schweiger, M.; Fledelius, C.; Jacobsen, P.; Zimmermann, R.; Zechner, R.; Kiens, B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E445–E453. [Google Scholar] [CrossRef] [PubMed]
- Louche, K.; Badin, P.M.; Montastier, E.; Laurens, C.; Bourlier, V.; de Glisezinski, I.; Thalamas, C.; Viguerie, N.; Langin, D.; Moro, C. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J. Clin. Endocrinol. Metab. 2013, 98, 4863–4871. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, G.; Zhao, X.; Huang, Z.; Li, P.; Zhang, H. Post-exercise Effects and Long-Term Training Adaptations of Hormone Sensitive Lipase Lipolysis Induced by High-Intensity Interval Training in Adipose Tissue of Mice. Front. Physiol. 2020, 11, 535722. [Google Scholar] [CrossRef] [PubMed]
- Enevoldsen, L.H.; Stallknecht, B.; Langfort, J.; Petersen, L.N.; Holm, C.; Ploug, T.; Galbo, H. The effect of exercise training on hormone-sensitive lipase in rat intra-abdominal adipose tissue and muscle. J. Physiol. 2001, 536, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Padmanabha Das, K.M.; Wechselberger, L.; Liziczai, M.; De la Rosa Rodriguez, M.; Grabner, G.F.; Heier, C.; Viertlmayr, R.; Radler, C.; Lichtenegger, J.; Zimmermann, R.; et al. Hypoxia-inducible lipid droplet-associated protein inhibits adipose triglyceride lipase. J. Lipid Res. 2018, 59, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Feng, W.; An, X.; Lin, W.; Tang, C. Hypoxia-Induced Adipose Lipolysis Requires Fibroblast Growth Factor 21. Front. Pharmacol. 2020, 11, 1279. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.; Goulet, N.; Mauger, J.-F.; Imbeault, P. Physiological Responses to Hypoxia on Triglyceride Levels. Front. Physiol. 2021, 12, 730935. [Google Scholar] [CrossRef]
- Bensaad, K.; Favaro, E.; Lewis, C.A.; Peck, B.; Lord, S.; Collins, J.M.; Pinnick, K.E.; Wigfield, S.; Buffa, F.M.; Li, J.L.; et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014, 9, 349–365. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdovska, S.; Zanou, N.; Lavier, J.; Mazzolai, L.; Millet, G.P.; Pellegrin, M. Moderate Effects of Hypoxic Training at Low and Supramaximal Intensities on Skeletal Muscle Metabolic Gene Expression in Mice. Metabolites 2023, 13, 1103. https://doi.org/10.3390/metabo13101103
Drozdovska S, Zanou N, Lavier J, Mazzolai L, Millet GP, Pellegrin M. Moderate Effects of Hypoxic Training at Low and Supramaximal Intensities on Skeletal Muscle Metabolic Gene Expression in Mice. Metabolites. 2023; 13(10):1103. https://doi.org/10.3390/metabo13101103
Chicago/Turabian StyleDrozdovska, Svitlana, Nadège Zanou, Jessica Lavier, Lucia Mazzolai, Grégoire P. Millet, and Maxime Pellegrin. 2023. "Moderate Effects of Hypoxic Training at Low and Supramaximal Intensities on Skeletal Muscle Metabolic Gene Expression in Mice" Metabolites 13, no. 10: 1103. https://doi.org/10.3390/metabo13101103





