An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plant Materials
2.2. Metabolite Extraction
2.3. LC-MS/MS Analysis
2.4. Molecular Networking in the GNPS Analysis Environment
2.5. Network Pharmacology
2.5.1. Target Prediction, Data Acquisition, and Preprocessing
2.5.2. Metabolite Similarity Analysis
2.5.3. Output Preparation and Analysis
2.5.4. Compound–Target Network
2.5.5. Protein–Protein Interaction Network and Gene Ontology Enrichment Analysis
2.6. Molecular Docking
2.6.1. Protein and Ligand Preparation
2.6.2. Docking Method
3. Results and Discussion
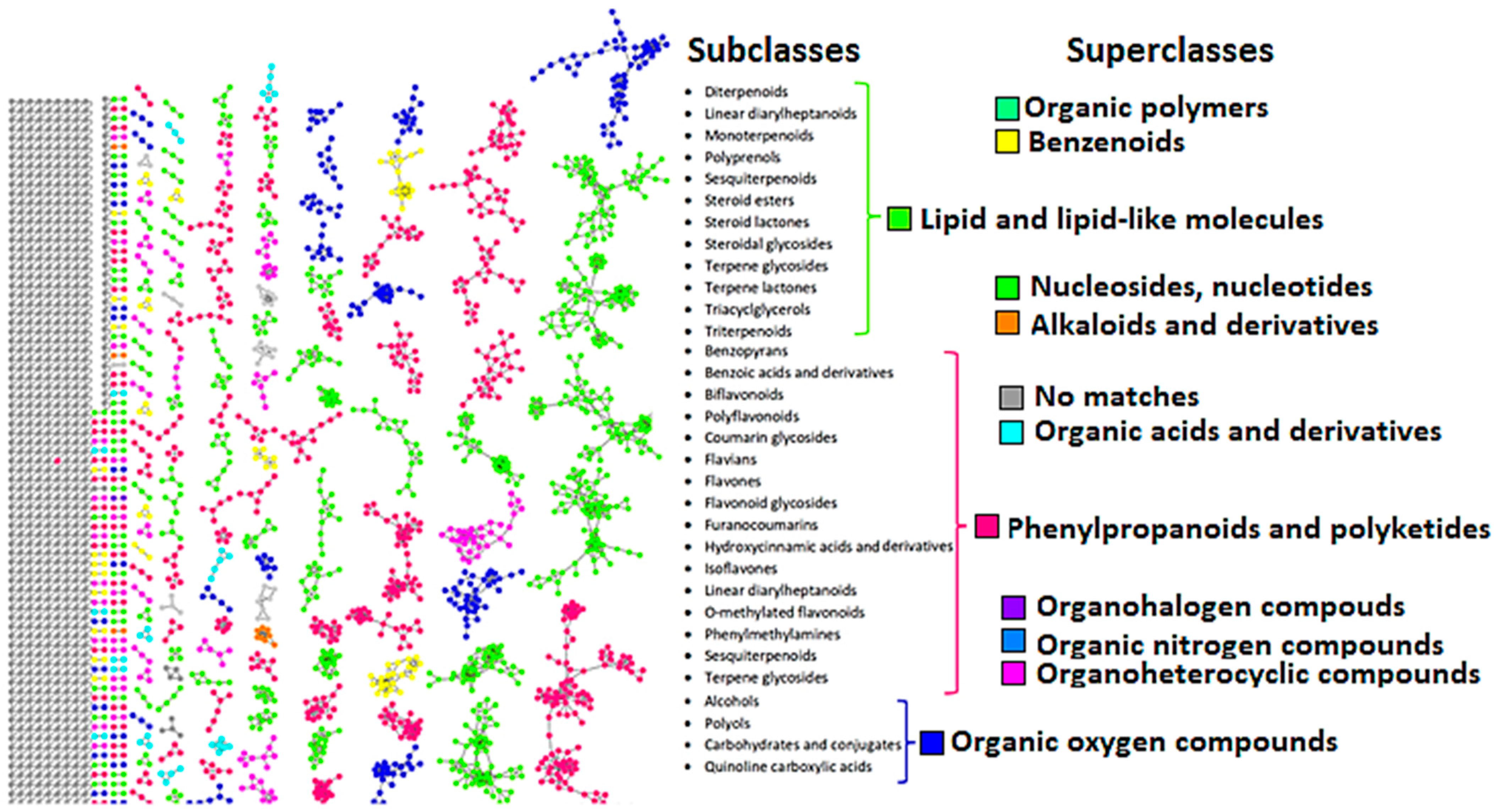
3.1. The Metabolomic Chart of H. splendidum Methanol Extracts
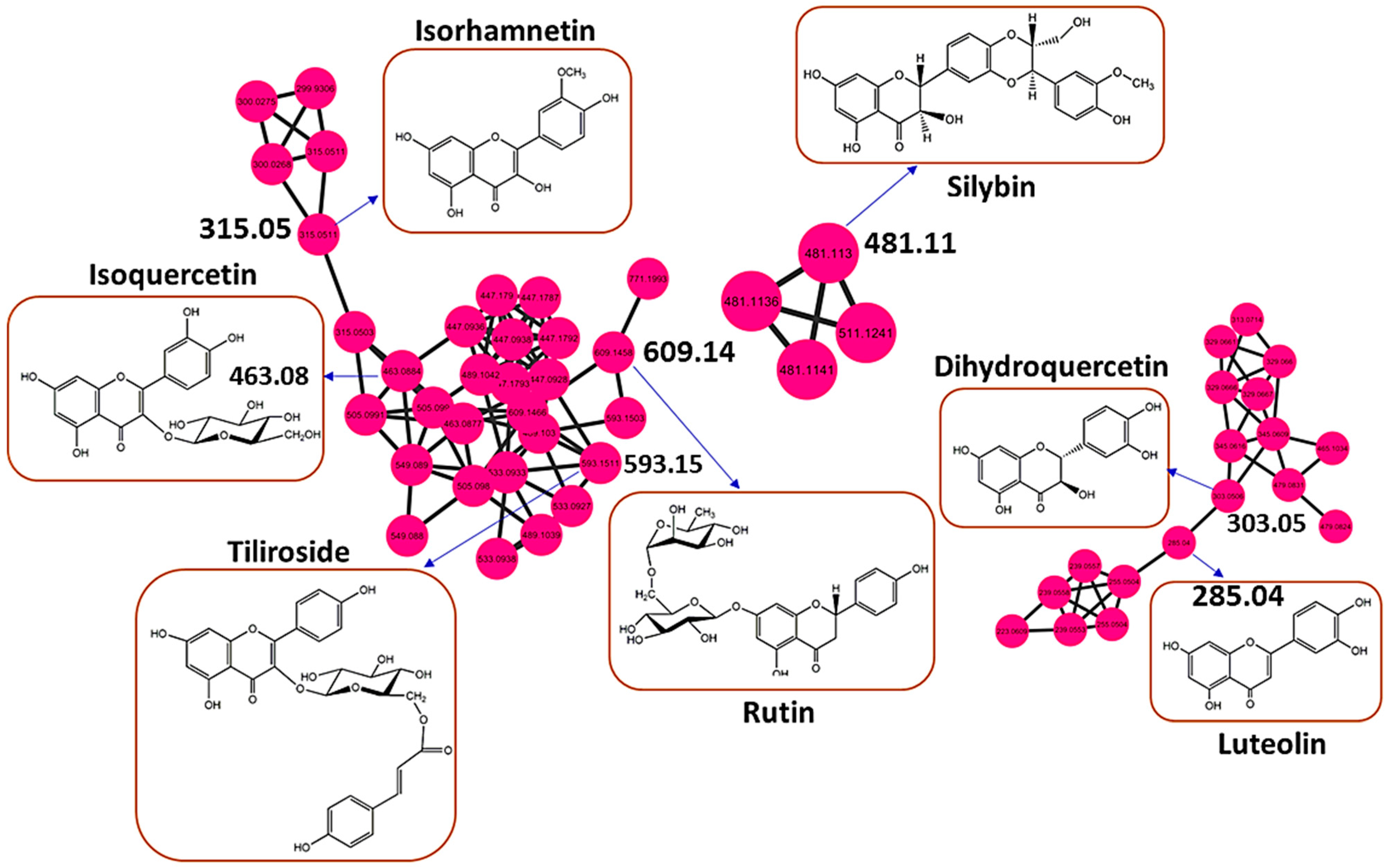
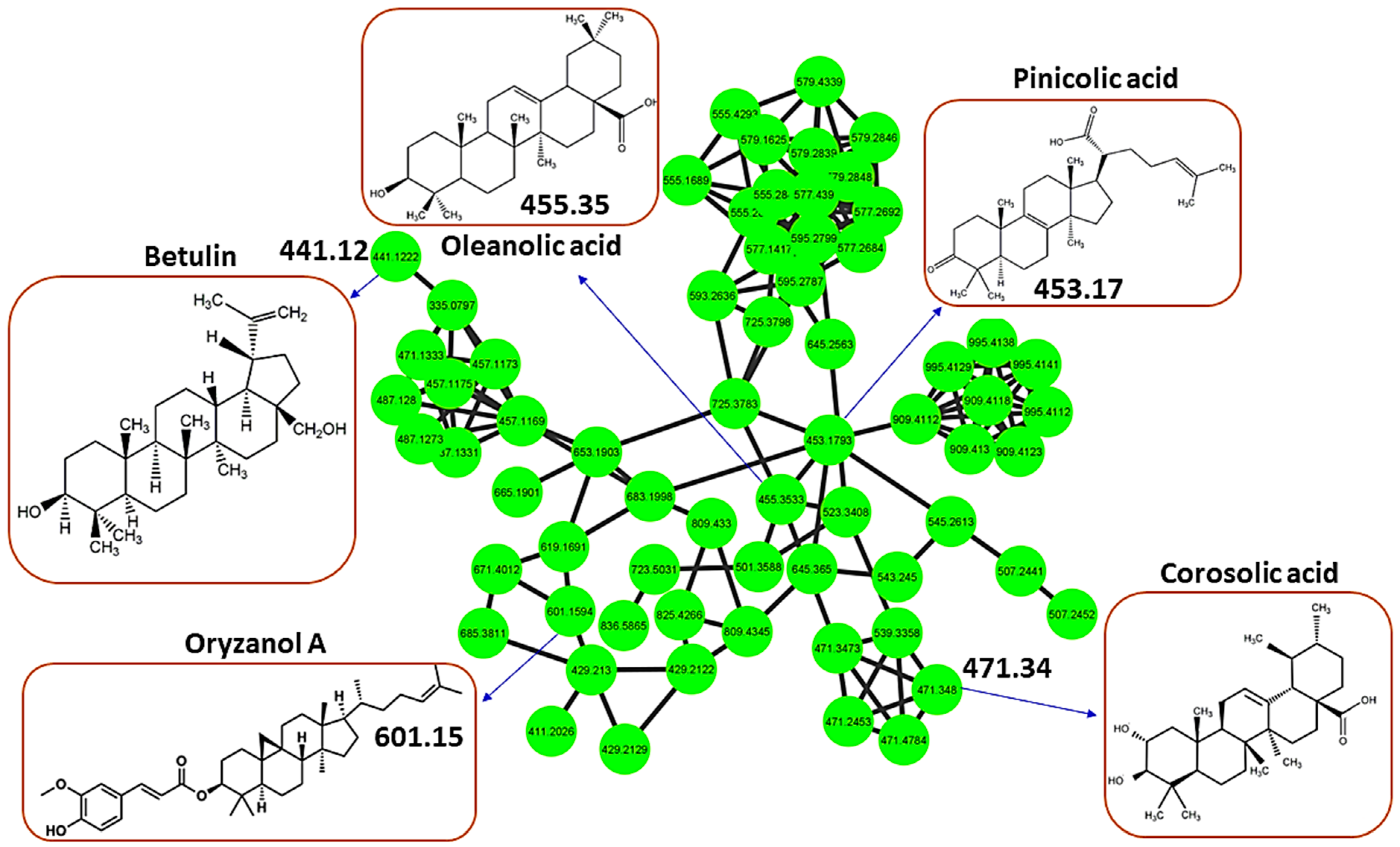
3.2. Health Benefits from the H. splendidum Chemistry: The Case of Flavonoids and Terpenoids
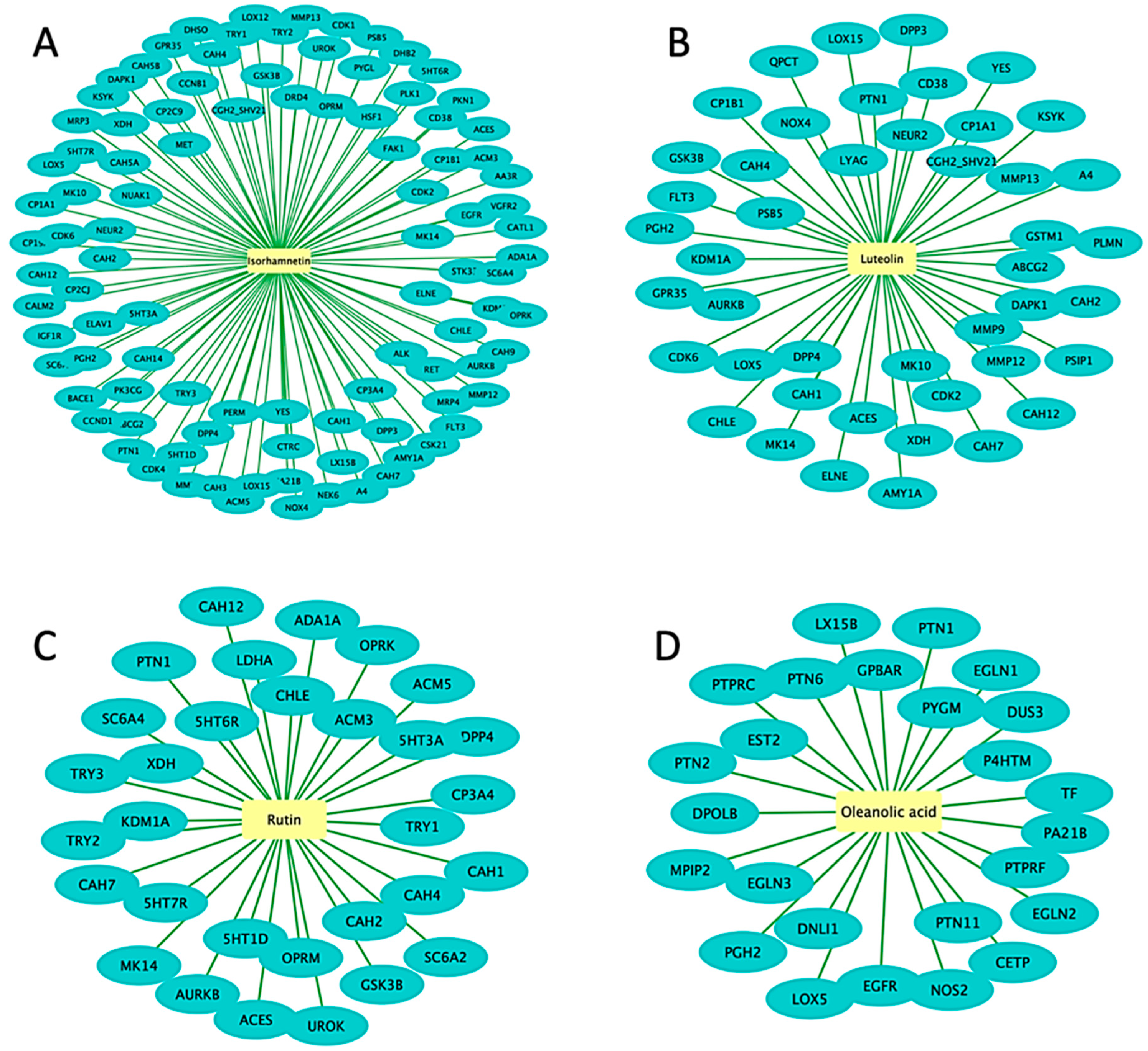
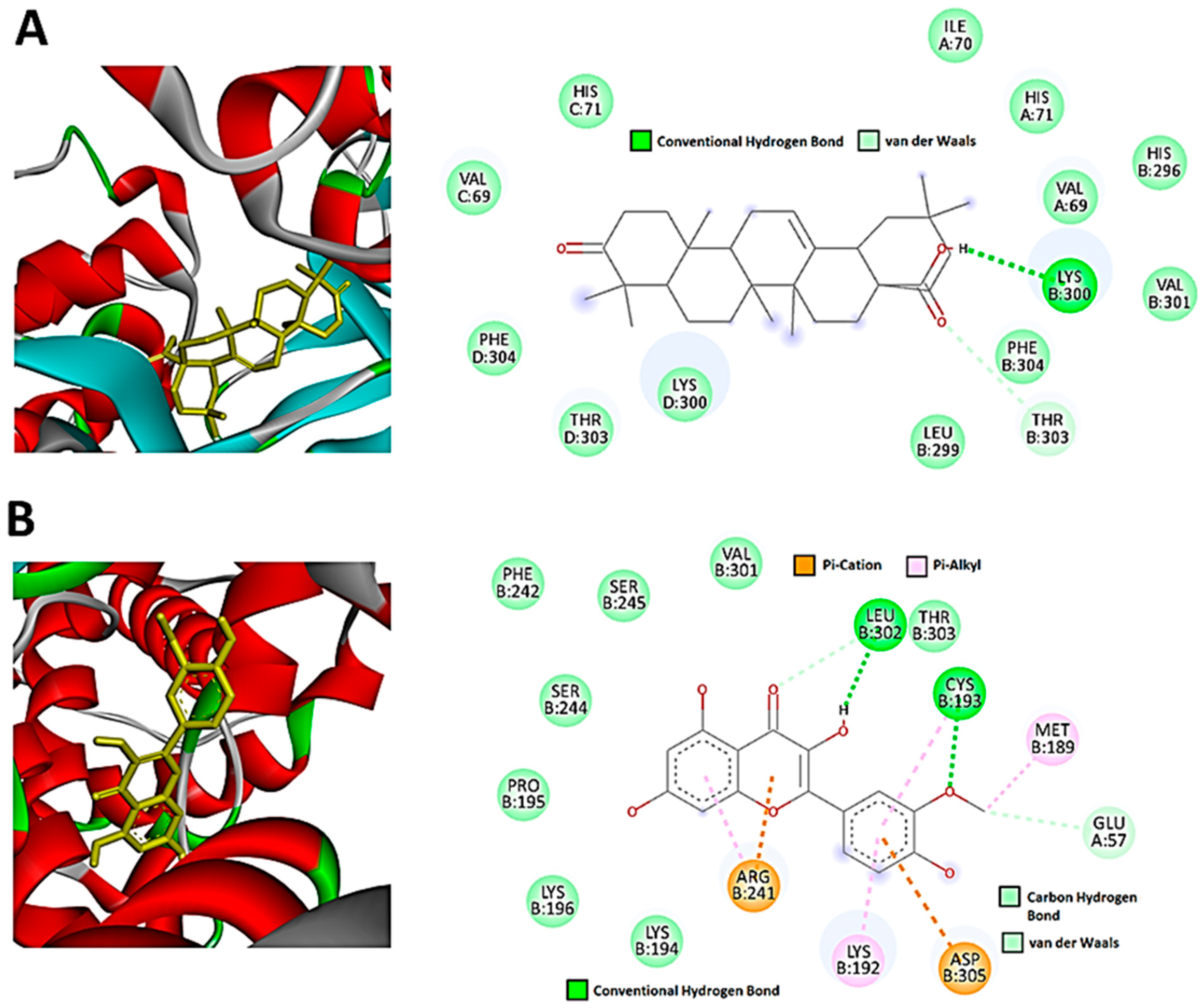
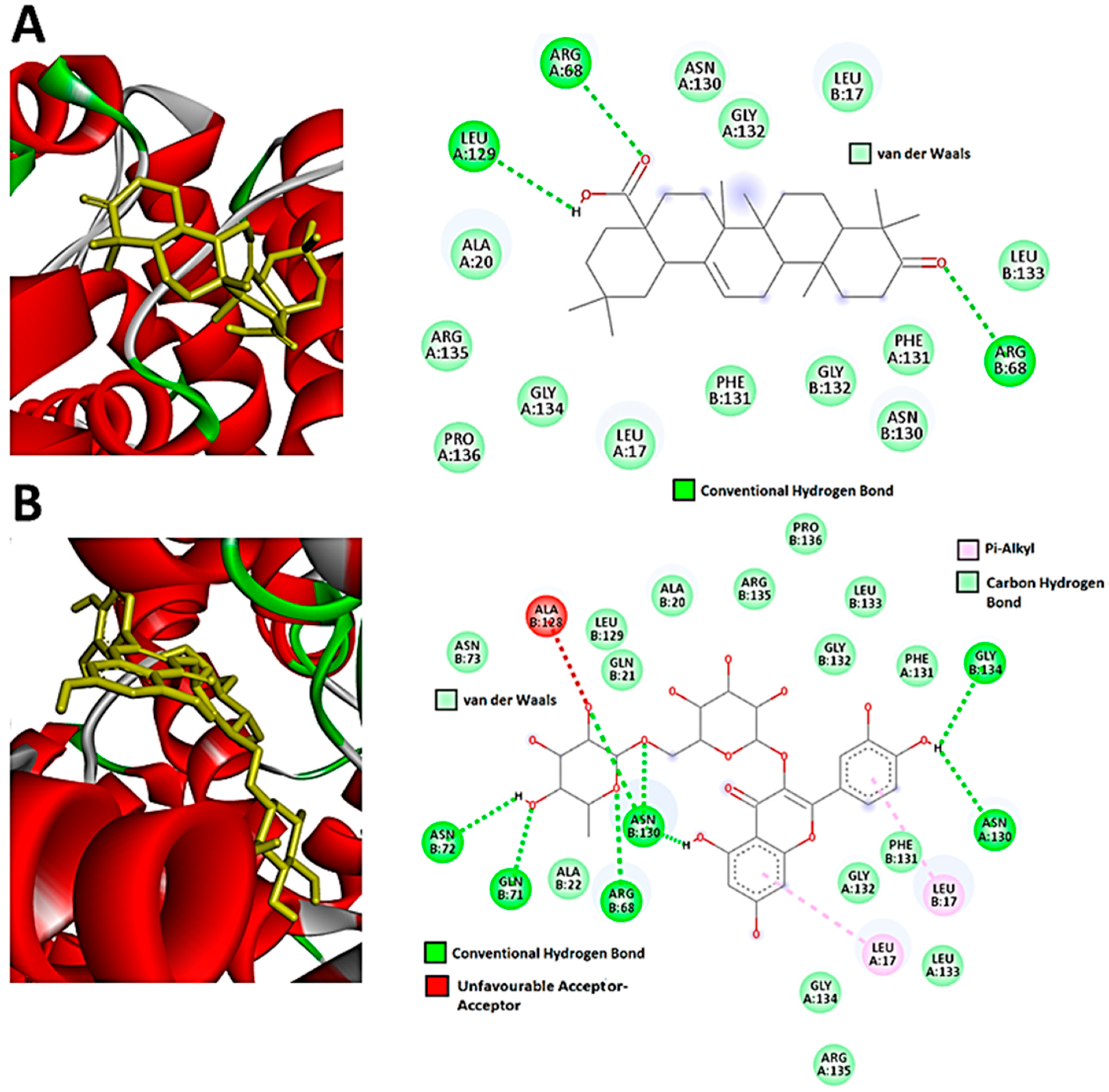
3.3. Network Pharmacology and Molecular Docking of Flavonoids and Terpenoids from H. splendidum in the Binding Pocket of CDK-2 and CCNB1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afolayan, A.J.; Meyer, J.J.M. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 57, 177–181. [Google Scholar] [CrossRef]
- Çubukçu, B.; Yüksel, V. Constituents of Anatolian Medicinal Plants; Flavonoids of Helichrysum armenium. J. Nat. Prod. 1982, 45, 137–139. [Google Scholar] [CrossRef]
- Facino, R.; Carini, M.; Franzoi, L.; Pirola, O.; Bosisio, E. Phytochemical characterization and radical scavenger activity of flavonoids from G. Don (compositae). Pharmacol. Res. 1990, 22, 709–721. [Google Scholar] [CrossRef]
- Bougatsos, C.; Ngassapa, O.; Runyoro, D.K.B.; Chinou, I.B. Chemical composition and in vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Zeitschrift Naturforsch.—Sect. C J. Biosci. 2004, 59, 368–372. [Google Scholar] [CrossRef]
- Ibara, J.R.; Elion Itou, R.D.G.; Ouamba, J.M.; Diatéwa, M.; Gbéassor, M.; Abena, A.A. Preliminary evaluation of antiulcerogenic activity of Ceiba pentandra Gaertn and Helicrysum mechowianum klatt in rats. J. Med. Sci. 2007, 7, 485–488. [Google Scholar] [CrossRef][Green Version]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crisan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crişan, G.; Păltinean, R. Phytochemical analysis, antioxidant and antimicrobial activities of helichrysum arenarium (L.) moench. and antennaria dioica (L.) gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef]
- Mao, Z.; Gan, C.; Zhu, J.; Ma, N.; Wu, L.; Wang, L.; Wang, X. Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. Moench through the pathway of anti-inflammation. Bioorganic Med. Chem. Lett. 2017, 27, 2812–2817. [Google Scholar] [CrossRef]
- Heyman, H.M.; Senejoux, F.; Seibert, I.; Klimkait, T.; Maharaj, V.J.; Meyer, J.J.M. Identification of anti-HIV active dicaffeoylquinic- and tricaffeoylquinic acids in Helichrysum populifolium by NMR-based metabolomic guided fractionation. Fitoterapia 2015, 103, 155–164. [Google Scholar] [CrossRef]
- Gouveia, S.; Castilho, P.C. Characterisation of phenolic acid derivatives and flavonoids from different morphological parts of Helichrysum obconicum by a RP-HPLC-DAD-(-)-ESI-MS n method. Food Chem. 2011, 129, 333–344. [Google Scholar] [CrossRef]
- Mari, A.; Napolitano, A.; Masullo, M.; Pizza, C.; Piacente, S. Identification and quantitative determination of the polar constituents in Helichrysum italicum flowers and derived food supplements. J. Pharm. Biomed. Anal. 2014, 96, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N.H. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar] [CrossRef] [PubMed]
- AL-Zuaidy, M.H.; Mumtaz, M.W.; Hamid, A.A.; Ismail, A.; Mohamed, S.; Razis, A.F.A. Biochemical characterization and 1H NMR based metabolomics revealed Melicope lunu-ankenda leaf extract a potent anti-diabetic agent in rats. BMC Complement. Altern. Med. 2017, 17, 359. [Google Scholar] [CrossRef]
- Jan, B.; Zahiruddin, S.; Basist, P.; Irfan, M.; Abass, S.; Ahmad, S. Metabolomic Profiling and Identification of Antioxidant and Antidiabetic Compounds from Leaves of Different Varieties of Morus alba Linn Grown in Kashmir. ACS Omega 2022, 7, 24317–24328. [Google Scholar] [CrossRef]
- García-Pérez, P.; Zhang, L.; Miras-Moreno, B.; Lozano-Milo, E.; Landin, M.; Lucini, L.; Gallego, P.P. The combination of untargeted metabolomics and machine learning predicts the biosynthesis of phenolic compounds in bryophyllum medicinal plants (Genus kalanchoe). Plants 2021, 10, 2430. [Google Scholar] [CrossRef]
- Sandra Liliana, P.D.; Manasés, G.C.; Enrique, J.F.; Rubén, R.R.; Cinthya, B.P.; Belen, M.H.G.; Alejandro, Z.; Maribel, H.R. Isolation, chemical characterization, and anti-inflammatory activity of coumarins, flavonoids, and terpenes from Tagetes lucida. Nat. Prod. Res. 2022, 36, 4751–4756. [Google Scholar] [CrossRef]
- Nagashima, F.; Tabuchi, Y.; Ito, T.; Harinantenaina, L.; Asakawa, Y. Terpenoids, flavonoids and acetogenins from some Malagasy plants. Nat. Prod. Commun. 2016, 11, 153–157. [Google Scholar] [CrossRef]
- Ofir, R. Cannabis-derived terpenes and flavonoids as potential pharmaceuticals. Isr. J. Plant Sci. 2021, 68, 29–37. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Chen, L.; Xu, S.; Yuan, G.; Xu, X. Drug-target network and polypharmacology studies of a Traditional Chinese Medicine for type II diabetes mellitus. Comput. Biol. Chem. 2011, 35, 293–297. [Google Scholar] [CrossRef]
- Chen, L.; Du, J.; Dai, Q.; Zhang, H.; Pang, W.; Hu, J. Prediction of anti-tumor chemical probes of a traditional Chinese medicine formula by HPLC fingerprinting combined with molecular docking. Eur. J. Med. Chem. 2014, 83, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2008; Volume 443, pp. 365–382. ISBN 9781588298645. [Google Scholar]
- Obakachi, V.A.; Kehinde, I.; Kushwaha, N.D.; Akinpelu, O.I.; Kushwaha, B.; Merugu, S.R.; Kayamba, F.; Kumalo, H.M.; Karpoormath, R. Structural based investigation of novel pyrazole-thiazole Hybrids as dual CDK-1 and CDK-2 inhibitors for cancer chemotherapy. Mol. Simul. 2022, 48, 687–701. [Google Scholar] [CrossRef]
- Cheung, A.; Chenoweth, A.M.; Quist, J.; Sow, H.S.; Malaktou, C.; Ferro, R.; Hoffmann, R.M.; Osborn, G.; Sachouli, E.; French, E.; et al. CDK Inhibition Primes for Anti-PD-L1 Treatment in Triple-Negative Breast Cancer Models. Cancers 2022, 14, 3361. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.-F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochamad Afendi, F.; Kawsar Parvin, A.; Ono, N.; Tanaka, K.; Hirai Morita, A.; Sato, T.; Sugiura, T.; Altaf-Ul-Amin, M.; Kanaya, S. KNApSAcK Metabolite Activity Database for Retrieving the Relationships Between Metabolites and Biological Activities. Plant Cell Physiol. 2014, 55, e7. [Google Scholar] [CrossRef]
- Pence, H.E.; Williams, A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010, 87, 1123–1124. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Whittle, M.; Willett, P.; Klaffke, W.; van Noort, P. Evaluation of Similarity Measures for Searching the Dictionary of Natural Products Database. J. Chem. Inf. Comput. Sci. 2003, 43, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.P.; Hersey, A.; Félix, E.; Landrum, G.; Gaulton, A.; Atkinson, F.; Bellis, L.J.; De Veij, M.; Leach, A.R. An open source chemical structure curation pipeline using RDKit. J. Cheminform. 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database J. Biol. Databases Curation 2010, 2010, baq020. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, 623–633. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2015; Volume 1263, pp. 243–250. ISBN 9781493922680. [Google Scholar]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef]
- Suh, M.C.; Uk Kim, H.; Nakamura, Y. Plant lipids: Trends and beyond. J. Exp. Bot. 2022, 73, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U. Lipid Metabolism in Plants. Plants 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Öztürk, B. Utilization of nanotechnology to improve the handling, storage and biocompatibility of bioactive lipids in food applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef]
- Duhan, N.; Barak, S.; Mudgil, D. Bioactive lipids: Chemistry & health benefits. Biointerface Res. Appl. Chem. 2020, 10, 6676–6687. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Fiorito, S.; Epifano, F.; Preziuso, F.; Taddeo, V.A.; Genovese, S. Biomolecular Targets of Oxyprenylated Phenylpropanoids and Polyketides. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2019; Volume 108, pp. 143–205. ISBN 9783030010980. [Google Scholar]
- Yu, O.; Jez, J.M. Nature’s assembly line: Biosynthesis of simple phenylpropanoids and polyketides. Plant J. 2008, 54, 750–762. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Cao, Y.; DePinho, R.A.; Ernst, M.; Vousden, K. Cancer research: Past, present and future. Nat. Rev. Cancer 2011, 11, 749–754. [Google Scholar] [CrossRef]
- Luo, X.; Yu, X.; Liu, S.; Deng, Q.; Liu, X.; Peng, S.; Li, H.; Liu, J.; Cao, Y. The role of targeting kinase activity by natural products in cancer chemoprevention and chemotherapy (Review). Oncol. Rep. 2015, 34, 547–554. [Google Scholar] [CrossRef]
- Süzgeç, S.; Meriçli, A.H.; Houghton, P.J.; Çubukçu, B. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia 2005, 76, 269–272. [Google Scholar] [CrossRef]
- Nile, A.; Gansukh, E.; Park, G.S.; Kim, D.H.; Hariram Nile, S. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste—In vitro and in silico approach. Food Chem. 2021, 335, 127650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Y.; Wang, Y.M.; Gong, H.; Zhao, H.; Lv, X.Y.; Yuan, G.H.; Han, S.R. Isorhamnetin flavonoid synergistically enhances the anticancer activity and apoptosis induction by cis-platin and carboplatin in non-small cell lung carcinoma (NSCLC). Int. J. Clin. Exp. Pathol. 2015, 8, 25–37. [Google Scholar] [PubMed]
- Dai, M.; Wikantyasning, E.; Wahyuni, A.; Kusumawati, I.; Saifudin, A.; Suhendi, A. Antiproliferative properties of tiliroside from Guazuma ulmifolia lamk on T47D and MCF7 cancer cell lines. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 627. [Google Scholar] [CrossRef]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-Cancer Efficacy of Silybin Derivatives—A Structure-Activity Relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef] [PubMed]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; de Almeida, T.S. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Das, A.; Baidya, R.; Chakraborty, T.; Samanta, A.K.; Roy, S. Pharmacological basis and new insights of taxifolin: A comprehensive review. Biomed. Pharmacother. 2021, 142, 112004. [Google Scholar] [CrossRef]
- Abdulla, M.; Gruber, P. Role of diet modification in cancer prevention. BioFactors 2000, 12, 45–51. [Google Scholar] [CrossRef]
- Yagura, T.; Motomiya, T.; Ito, M.; Honda, G.; Iida, A.; Kiuchi, F.; Tokuda, H.; Nishino, H. Anticarcinogenic compounds in the Uzbek medicinal plant, Helichrysum maracandicum. J. Nat. Med. 2008, 62, 174–178. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A. Introductory chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; IntechOpen: London, UK, 2018; Volume 1, pp. 1–12. [Google Scholar]
- Drag-Zalesińska, M.; Drag, M.; Poreba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer properties of ester derivatives of betulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010, 49, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, C.; Cai, Z.; Zhao, F.; He, L.; Lou, X.; Qi, X. Betulin induces cytochrome c release and apoptosis in colon cancer cells via NOXA. Oncol. Lett. 2018, 15, 7319–7327. [Google Scholar] [CrossRef] [PubMed]
- Pięt, M.; Paduch, R. Ursolic and Oleanolic Acids as Potential Anticancer Agents Acting in the Gastrointestinal Tract. Mini. Rev. Org. Chem. 2019, 16, 78–91. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Li, Y.; Tang, Y.T.; Ma, X.D.; Tang, Z.Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef] [PubMed]
- Steindl, T.M.; Schuster, D.; Laggner, C.; Langer, T. Parallel Screening: A Novel Concept in Pharmacophore Modeling and Virtual Screening. J. Chem. Inf. Model. 2006, 46, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Waszkowycz, B.; Clark, D.E.; Gancia, E. Outstanding challenges in protein–ligand docking and structure-based virtual screening. WIREs Comput. Mol. Sci. 2011, 1, 229–259. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef] [PubMed]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor-ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Wang, Y.; Bryant, S.H. Investigating the correlations among the chemical structures, bioactivity profiles and molecular targets of small molecules. Bioinformatics 2010, 26, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Safari-Alighiarloo, N.; Taghizadeh, M.; Rezaei-Tavirani, M.; Goliaei, B.; Peyvandi, A.A. Protein-protein interaction networks (PPI) and complex diseases. Gastroenterol. Hepatol. Bed Bench 2014, 7, 17–31. [Google Scholar] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, D.; Huskey, N.E.; Kusdra, L.; Wohlbold, L.; Merrick, K.A.; Zhang, C.; Creasman, K.J.; Shokat, K.M.; Fisher, R.P.; Goga, A. Chemical-genetic analysis of cyclin dependent kinase 2 function reveals an important role in cellular transformation by multiple oncogenic pathways. Proc. Natl. Acad. Sci. USA 2012, 109, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935. [Google Scholar]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Kontek, R. Cyclin-Dependent Kinase Synthetic Lethality Partners in DNA Damage Response. Int. J. Mol. Sci. 2022, 23, 3555. [Google Scholar] [CrossRef]
- Javed, A.; Malagraba, G.; Yarmohammadi, M.; Perelló-Reus, C.M.; Barceló, C.; Rubio-Tomás, T. Therapeutic Potential of Mitotic Kinases’ Inhibitors in Cancers of the Gastrointestinal System. Future Pharmacol. 2022, 2, 214–237. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Castillo, J.C.; Jiménez, E.; Portilla, J.; Insuasty, B.; Quiroga, J.; Moreno-Fuquen, R.; Kennedy, A.R.; Abonia, R. Application of a catalyst-free Domino Mannich/Friedel-Crafts alkylation reaction for the synthesis of novel tetrahydroquinolines of potential antitumor activity. Tetrahedron 2018, 74, 932–947. [Google Scholar] [CrossRef]
- Abonia, R.; Insuasty, D.; Castillo, J.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. Synthesis of novel quinoline-2-one based chalcones of potential anti-tumor activity. Eur. J. Med. Chem. 2012, 57, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sramkoski, R.M.; Jacobberger, J.W. The kinetics of G2 and M transitions regulated by B cyclins. PLoS ONE 2013, 8, 30–35. [Google Scholar] [CrossRef]
- Egloff, A.M.; Vella, L.A.; Finn, O.J. Cyclin B1 and other cyclins as tumor antigens in immunosurveillance and immunotherapy of cancer. Cancer Res. 2006, 66, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Urano, T.; Miki, Y.; Moriya, T.; Akahira, J.I.; Ishida, T.; Horie, K.; Inoue, S.; Sasano, H. Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2007, 98, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Braun, M.; Strmiska, V.; Sicinski, P. Targeting cell-cycle machinery in cancer. Cancer Cell 2021, 39, 759–778. [Google Scholar] [CrossRef]
- Aljohani, A.I.; Toss, M.S.; Green, A.R.; Rakha, E.A. The clinical significance of cyclin B1 (CCNB1) in invasive breast cancer with emphasis on its contribution to lymphovascular invasion development. Breast Cancer Res. Treat. 2023, 198, 423–435. [Google Scholar] [CrossRef]

| Compound Name | Adduct | Precursor m/z | Library m/z | Mass Diff (Da) | Chemical Formula | RT (min) | Fragment Ions |
|---|---|---|---|---|---|---|---|
| Caffeic acid hexoside | [M-H]− | 341.087 | 341.087 | 9.16 × 10−5 | C15H18O9 | 5.83 | 179, 135 |
| 4-O-Caffeoylquinic acid | [M-H]− | 353.09 | 353.09 | 0.002289 | C16H18O9 | 5.99 | 191, 179, 173, 135 |
| Quinic acid | [M-H]− | 191.056 | 191.055 | 0.000305176 | C7H12O6 | 6.03 | 191, 173, 127, 111 |
| 1,4-Dicaffeoylquinic acid | [M-H]− | 515.119 | 515.12 | 0.000610352 | C25H24O12 | 6.21 | 353, 191, 179, 135 |
| Oryzanol A | [M-H]− | 602.9 | 601.1594 | 1.7406 | C40H58O4 | 6.28 | 601, 439, 409 |
| 3-O-Feruloylquinic acid | [M-H]− | 367.103 | 367.103 | 0.000305176 | C17H20O9 | 6.54 | 191, 173, 135 |
| Pinicolic acid | [M-H]− | 453.137 | 453.1793 | 0.0424194 | C30H44O34 | 6.69 | 453 |
| 1,3-Dicaffeoylquinic acid | [M-H]− | 515.119 | 515.12 | 0.000915527 | C25H24O12 | 6.92 | 353, 191, 179, 135 |
| 5-O-Caffeoylquinic acid | [M-H]− | 353.09 | 353.0869 | 0.00198364 | C16H18O9 | 7.01 | 191, 179, 173, 135 |
| Vitamin P | [M-H]− | 609.146 | 609.146 | 0.000183105 | C27H30O16 | 7.28 | 609, 301, 300 |
| Isoquercetin | [M-H]− | 463.088 | 463.088 | 0.000396729 | C21H20O12 | 7.31 | 463, 300, 271, 255 |
| Quercetin-3-O-glucosyl-6′’-acetate | [M-H]− | 505.08 | 505.098 | 0.00189209 | C25H22O13 | 7.51 | 505, 301, 300, 271 |
| Ranolazine | [M-H]− | 429.26 | 429.21 | 0.05 | C24H33N3O4 | 7.6 | 429, 249 |
| Gibberellic acid 8 | [M-H]− | 363.145 | 363.145 | 0.000976562 | C19H22O6 | 7.71 | 363,301, 275 |
| Kaempferol-3-O-rutinoside | [M-H]− | 593.15 | 593.15 | 0.000915527 | C27H30O15 | 7.72 | 593, 285, 284, 257 |
| Kaempferol 3-glucuronide | [M-H]− | 461.072 | 461.073 | 0.000183105 | C21H18O12 | 7.76 | 285, 257 |
| Isorhamnetin-3-O-glucoside | [M-H]− | 477.104 | 477.104 | 9.16 × 10−5 | C22H22O12 | 7.82 | 314, 299, 285, 271, 243 |
| Viscidulin III | [M-H]− | 345.06 | 345.0609 | 0.00109863 | C17H14O7 | 8.68 | 330, 315, 287 |
| Isorhamnetin | [M-H]− | 315.05 | 315.05 | 0.000305176 | C16H12O7 | 8.71 | 300, 271, 255 |
| Eleutheroside E | [M-H]− | 741.239 | 741.261 | 0.0216675 | C34H46O18 | 8.71 | 741, 579, 417 |
| Dihydroquercetin | [M-H]− | 303.051 | 303.051 | 0.000396729 | C15H12O7 | 8.84 | 175,125 |
| Quercetin 3,7-dimethyl ether | [M-H]− | 329.062 | 329.066 | 0.00479126 | C17H14O7 | 9.43 | 314, 299, 271, 243 |
| Jaceidin | [M-H]− | 359.077 | 359.077 | 0.000305176 | C18H16O8 | 9.49 | 344, 329, 314, 26 |
| Cynarine | [M-H]− | 515.12 | 515.12 | 0.00012207 | C25H24O12 | 9.72 | 353, 191, 179 |
| Pinocembrine | [M-H]− | 255.066 | 255.066 | 0.000198364 | C15H12O4 | 9.74 | 255, 213, 171, 151 |
| Quercetin 3-O-glucuronide | [M-H]− | 477.067 | 477.067 | 0.000823975 | C21H18O13 | 9.86 | 301, 179, 151 |
| Betulin | [M-H]− | 442.7 | 441.1222 | 1.5778 | C30H50O2 | 9.87 | 441, 260, 245, 231 |
| Silybin | [M-H]− | 482.4 | 481.113 | 1.287 | C25H22O10 | 10.01 | 327, 315, 312 |
| 5-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-3,6,7-trimethoxy-4H-chromen-4-one | [M-H]− | 373.093 | 373.093 | 0.00088501 | C19H18O7 | 10.15 | 358, 343, 328 |
| Quercetin 3-O-malonylglucoside | [M-H]− | 549.089 | 549.089 | 0.000427246 | C24H22O15 | 10.16 | 300, 271,255 |
| Kaempferol-3-O-glucoside | [M-H]− | 447.094 | 447.093 | 0.000427246 | C21H19O11 | 10.56 | 285, 284, 255, 227 |
| 3,7-Dihydroxy-3′,4′-dimethoxyflavone | [M-H]− | 313.072 | 313.071 | 0.000183105 | C17H14O6 | 10.59 | 298, 283, 255 |
| Spiraeoside | [M-H]− | 463.089 | 463.088 | 0.000488281 | C21H20O12 | 10.6 | 301, 179, 151 |
| Velutin | [M-H]− | 313.072 | 313.071 | 0.000976562 | C17H14O6 | 10.7 | 298, 283, 255 |
| Kaempferol-3-O-glucuronoside | [M-H]− | 461.073 | 461.073 | 0.00088501 | C21H18O12 | 10.7 | 285, 257, 229 |
| Tiliroside | [M-H]− | 593.151 | 593.116 | 0.015625 | C30H26O13 | 10.79 | 593, 285, 255 |
| Rhein | [M-H]− | 283.061 | 283.025 | 0.0357056 | C15H8O6 | 11.31 | 268, 239, 211 |
| 9-hydroxy-10,12-octadecadienoic acid | [M-H]− | 295.228 | 295.227 | 0.00241089 | C16H32O3 | 11.91 | 295, 277, 195 |
| 3,4-di-O-caffeoylquinic acid | [M-H]− | 515.12 | 515.12 | 0.000183105 | C25H24O12 | 11.95 | 191, 179, 173 |
| Luteolin | [M-H]− | 285.04 | 285.04 | 0 | C15H10O6 | 12.51 | 175, 151, 133 |
| Limocitrin | [M-H]− | 345.062 | 345.062 | 0.000396729 | C17H14O8 | 12.64 | 315, 287 |
| Dodecylbenzenesulfonic acid | [M-H]− | 325.184 | 325.184 | 0.000183105 | C18H30O3S | 12.86 | 325, 183 |
| Isokaempferide | [M-H]− | 299.056 | 299.056 | 0.000213623 | C16H12O6 | 13.98 | 255, 227 |
| 4′,5,7-Trihydroxy-3,6-dimethoxyflavone | [M-H]− | 329.067 | 329.067 | 0.00088501 | C17H14O7 | 13.99 | 299, 271, 215 |
| Irigenin | [M-H]− | 359.077 | 359.077 | 0.000305176 | C18H16O8 | 14.62 | 329, 314,286, 258 |
| 3-O-Acetylpinobanksin | [M-H]− | 313.066 | 313.071 | 0.000183105 | C17H14O6 | 15.13 | 253 |
| Tricin | [M-H]− | 329.067 | 329.067 | 0.000396729 | C17H14O7 | 15.3 | 314, 299, 271 |
| Myricetin 3,7,3′,4′-tetramethyl ether | [M-H]− | 373.093 | 373.093 | 0.000183105 | C19H18O8 | 15.67 | 343, 328, 300, 285, 257 |
| Eupatilin | [M-H]− | 343.08 | 343.082 | 0.000305176 | C18H16O7 | 16.89 | 298, 285, 270, 242 |
| Decylbenzenesulfonic acid | [M-H]− | 297.142 | 297.153 | 0.0107117 | C16H26O3S | 19.05 | 297, 183 |
| Thymol-beta-d-glucoside | [M-H]− | 311.15 | 311.168 | 0.0540771 | C24H32O10 | 20.03 | 311, 183 |
| Corosolic acid | [M-H]− | 471.348 | 471.348 | 0 | C30H48O4 | 20.33 | 471, 407 |
| Hydroquinidine | [M-H]− | 325.192 | 325.184 | 0.0105286 | C20H26N2O2 | 21 | 325, 183 |
| Canrenone | [M-H]− | 339.197 | 339.2 | 0.00271606 | C22H28O3 | 22.19 | 339, 184, 183 |
| Oleanolic acid | [M-H]− | 455.353 | 455.3533 | 0.000305176 | C30H48O3 | 22.96 | 455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lephatsi, M.M.; Choene, M.S.; Kappo, A.P.; Madala, N.E.; Tugizimana, F. An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials. Metabolites 2023, 13, 1104. https://doi.org/10.3390/metabo13101104
Lephatsi MM, Choene MS, Kappo AP, Madala NE, Tugizimana F. An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials. Metabolites. 2023; 13(10):1104. https://doi.org/10.3390/metabo13101104
Chicago/Turabian StyleLephatsi, Motseoa Mariam, Mpho Susan Choene, Abidemi Paul Kappo, Ntakadzeni Edwin Madala, and Fidele Tugizimana. 2023. "An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials" Metabolites 13, no. 10: 1104. https://doi.org/10.3390/metabo13101104
APA StyleLephatsi, M. M., Choene, M. S., Kappo, A. P., Madala, N. E., & Tugizimana, F. (2023). An Integrated Molecular Networking and Docking Approach to Characterize the Metabolome of Helichrysum splendidum and Its Pharmaceutical Potentials. Metabolites, 13(10), 1104. https://doi.org/10.3390/metabo13101104







