Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease
Abstract
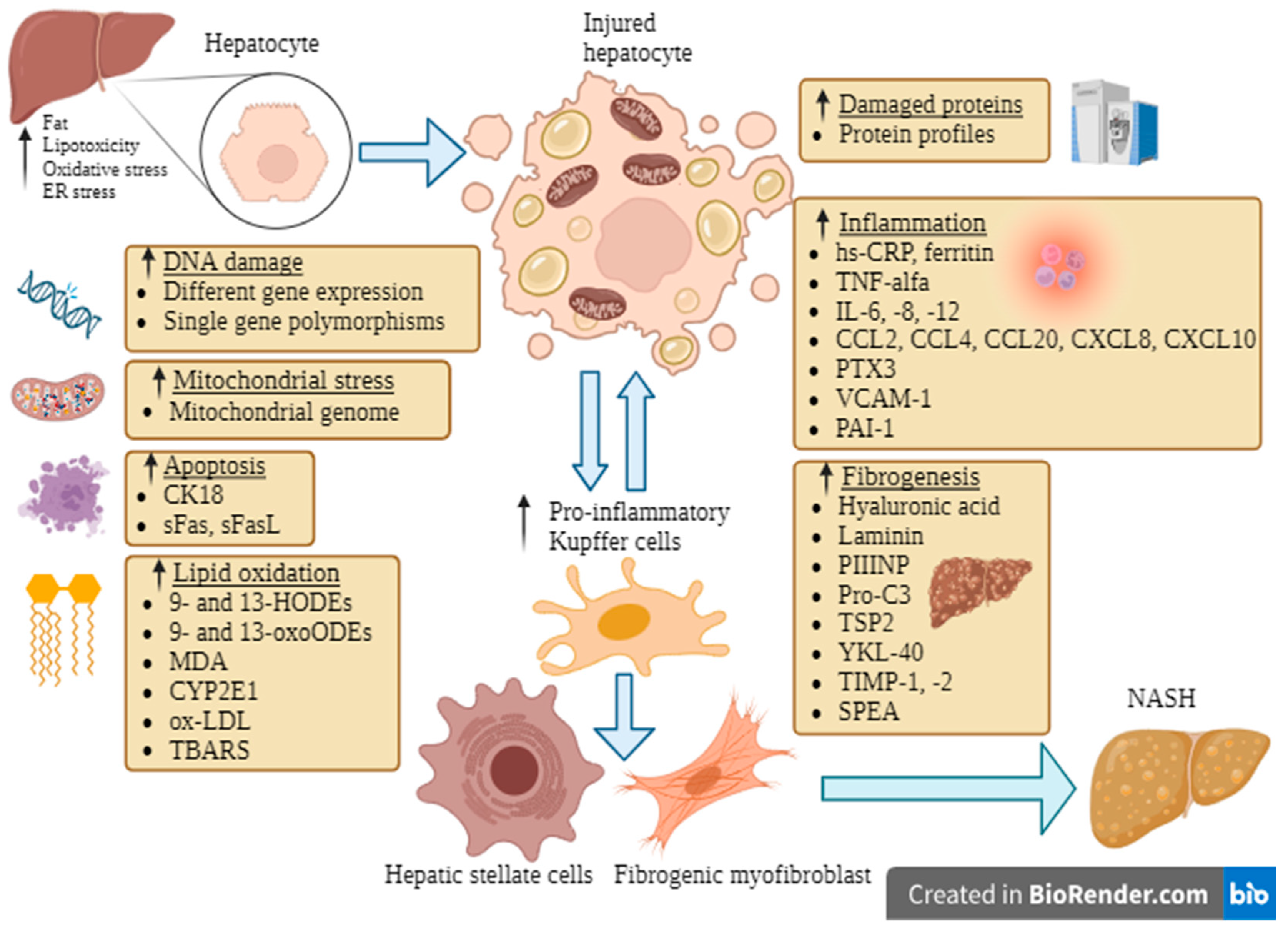
:1. Introduction
2. Currently Used Noninvasive Methods for NAFLD Evaluation
3. Emerging Biomarkers
3.1. Biomarkers of Fibrogenesis
3.2. Biomarkers of Inflammation
3.3. Apoptosis Markers
3.4. Adipokines and Hormones
3.5. Biomarkers of Lipid Oxidation
3.6. Genetic Biomarkers
3.7. Role of Epigenetics
3.8. Omics in NAFLD
3.8.1. Proteomics
3.8.2. Glycomics
3.8.3. Lipidomics
3.8.4. Metabolomics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Eltelbany, A.; Mohammed, A.; Alsabbagh Alchirazi, K.; Trakroo, S.; Asaad, I. The Epidemiology of Non-Alcoholic Steatohepatitis (NASH) in the United States between 2010–2020: A Population-Based Study. Ann. Hepatol. 2022, 27, 100727. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bril, F.; Barb, D.; Portillo-Sanchez, P.; Biernacki, D.; Lomonaco, R.; Suman, A.; Weber, M.H.; Budd, J.T.; Lupi, M.E.; Cusi, K. Metabolic and Histological Implications of Intrahepatic Triglyceride Content in Nonalcoholic Fatty Liver Disease. Hepatology 2017, 65, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis Stage but Not NASH Predicts Mortality and Time to Development of Severe Liver Disease in Biopsy-Proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Golabi, P.; Otgonsuren, M.; Cable, R.; Felix, S.; Koenig, A.; Sayiner, M.; Younossi, Z.M. Non-Alcoholic Fatty Liver Disease (NAFLD) Is Associated with Impairment of Health Related Quality of Life (HRQOL). Health Qual. Life Outcomes 2016, 14, 18. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of Liver Biopsy and Non-Invasive Diagnostic Tests for the Diagnosis of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive Biomarkers in NAFLD and NASH—Current Progress and Future Promise. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797. [Google Scholar] [CrossRef] [PubMed]
- Gomercić, M.; Duvnjak, M.; Barsić, N. Ultrasonography in the diagnosis of nonalcoholic fatty liver disease. Acta Med. Croatica 2009, 63 (Suppl. S3), 1–3. [Google Scholar] [PubMed]
- Alkhouri, N.; Feldstein, A.E. Noninvasive Diagnosis of Nonalcoholic Fatty Liver Disease: Are We There Yet? Metabolism 2016, 65, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Loomba, R. Noninvasive Imaging Biomarker Assessment of Liver Fibrosis by Elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Cassinotto, C.; Boursier, J.; de Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.-B.; Michalak, S.; Bail, B.L.; Cartier, V.; et al. Liver Stiffness in Nonalcoholic Fatty Liver Disease: A Comparison of Supersonic Shear Imaging, FibroScan, and ARFI with Liver Biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; Cai, J.-J.; Yu, Y.; She, Z.-G.; Li, H. Nonalcoholic Fatty Liver Disease: An Update on the Diagnosis. Gene Expr. 2019, 19, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Martinou, E.; Pericleous, M.; Stefanova, I.; Kaur, V.; Angelidi, A.M. Diagnostic Modalities of Non-Alcoholic Fatty Liver Disease: From Biochemical Biomarkers to Multi-Omics Non-Invasive Approaches. Diagnostics 2022, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Hepamet. Available online: https://www.hepamet-fibrosis-score.eu/ (accessed on 12 September 2023).
- Karsdal, M.A.; Daniels, S.J.; Holm Nielsen, S.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen Biology and Non-Invasive Biomarkers of Liver Fibrosis. Liver Int. 2020, 40, 736–750. [Google Scholar] [CrossRef]
- Suzuki, A.; Angulo, P.; Lymp, J.; Li, D.; Satomura, S.; Lindor, K. Hyaluronic Acid, an Accurate Serum Marker for Severe Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Liver Int. 2005, 25, 779–786. [Google Scholar] [CrossRef]
- Malik, R.; Chang, M.; Bhaskar, K.; Nasser, I.; Curry, M.; Schuppan, D.; Byrnes, V.; Afdhal, N. The Clinical Utility of Biomarkers and the Nonalcoholic Steatohepatitis CRN Liver Biopsy Scoring System in Patients with Nonalcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2009, 24, 564–568. [Google Scholar] [CrossRef]
- Lydatakis, H.; Hager, I.P.; Kostadelou, E.; Mpousmpoulas, S.; Pappas, S.; Diamantis, I. Non-Invasive Markers to Predict the Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. Liver Int. 2006, 26, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Sakugawa, H.; Nakayoshi, T.; Kobashigawa, K.; Yamashiro, T.; Maeshiro, T.; Miyagi, S.; Shiroma, J.; Toyama, A.; Nakayoshi, T.; Kinjo, F.; et al. Clinical Usefulness of Biochemical Markers of Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2005, 11, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Palekar, N.A.; Naus, R.; Larson, S.P.; Ward, J.; Harrison, S.A. Clinical Model for Distinguishing Nonalcoholic Steatohepatitis from Simple Steatosis in Patients with Nonalcoholic Fatty Liver Disease. Liver Int. 2006, 26, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.N.D.; Leite-Mór, M.M.B.; Kondo, M.; Martins, J.R.; Nader, H.; Lanzoni, V.P.; Parise, E.R. Serum Laminin, Type IV Collagen and Hyaluronan as Fibrosis Markers in Non-Alcoholic Fatty Liver Disease. Braz. J. Med. Biol. Res. 2005, 38, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Massard, J.; Charlotte, F.; Messous, D.; Imbert-Bismut, F.; Bonyhay, L.; Tahiri, M.; Munteanu, M.; Thabut, D.; Cadranel, J.F.; et al. Diagnostic Value of Biochemical Markers (FibroTest-FibroSURE) for the Prediction of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. BMC Gastroenterol. 2006, 6, 6. [Google Scholar] [CrossRef]
- Monarca, A.; Petrini, C.; Perolini, S.; Pozzi, F.; Adelasco, L.; Natangelo, R.; Croce, G. Procollagen-Type III Peptide Serum Concentrations in Alcoholic and Non-Alcoholic Liver Disease. Ric. Clin. Lab. 1985, 15, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Trembling, P.M.; Guha, I.N.; Parkes, J.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; Day, C.P.; Rosenberg, W.M. Validation of Terminal Peptide of Procollagen III for the Detection and Assessment of Nonalcoholic Steatohepatitis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2013, 57, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Suetta, C.; Henriksen, K.; Christiansen, C.; Karsdal, M.A.; et al. The Neo-Epitope Specific PRO-C3 ELISA Measures True Formation of Type III Collagen Associated with Liver and Muscle Parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar]
- Leeming, D.J.; Grove, J.; Kaye, P.; Hoad, C.; Francis, S.; Nielsen, M.; Karsdal, M.A.; Guha, I.N.; Aithal, G. Estimation of Serum “True Collagen Type III Formation” (Pro-C3) Levels as a Marker of Non-Alcoholic Steatohepatitis in a Prospective Cohort. J. Hepatol. 2017, 66, S154. [Google Scholar] [CrossRef]
- Daniels, S.J.; Leeming, D.J.; Eslam, M.; Hashem, A.M.; Nielsen, M.J.; Krag, A.; Karsdal, M.A.; Grove, J.I.; Neil Guha, I.; Kawaguchi, T.; et al. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology 2019, 69, 1075–1086. [Google Scholar] [CrossRef]
- Bel Lassen, P.; Nori, N.; Bedossa, P.; Genser, L.; Aron-Wisnewsky, J.; Poitou, C.; Surabattula, R.; Juul Nielsen, M.; Asser Karsdal, M.; Julie Leeming, D.; et al. Fibrogenesis Marker PRO-C3 Is Higher in Advanced Liver Fibrosis and Improves in Patients Undergoing Bariatric Surgery. J. Clin. Endocrinol. Metab. 2022, 107, e1356–e1366. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.L.; Lee, J.; van Dijk, A.-M.; Vali, Y.; Aithal, G.P.; Schattenberg, J.M.; Anstee, Q.M.; Brosnan, M.J.; Zafarmand, M.H.; Ramsoekh, D.; et al. Systematic Review with Meta-Analysis: Diagnostic Accuracy of Pro-C3 for Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 1920. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, E.; Mano, Y.; Yoshio, S.; Shoji, H.; Sugiyama, M.; Korenaga, M.; Ishida, T.; Arai, T.; Itokawa, N.; Atsukawa, M.; et al. Serum YKL-40 as a Marker of Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2016, 6, 35282. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tanaka, N.; Fujimori, N.; Yamazaki, T.; Katsuyama, T.; Iwashita, Y.; Pham, J.; Joshita, S.; Pydi, S.P.; Umemura, T. Serum Thrombospondin 2 Is a Novel Predictor for the Severity in the Patients with NAFLD. Liver Int. 2021, 41, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Kozumi, K.; Kodama, T.; Murai, H.; Sakane, S.; Govaere, O.; Cockell, S.; Motooka, D.; Kakita, N.; Yamada, Y.; Kondo, Y.; et al. Transcriptomics Identify Thrombospondin-2 as a Biomarker for NASH and Advanced Liver Fibrosis. Hepatology 2021, 74, 2452–2466. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, R.; Elbasel, M.; Esmat, S.; Essam, K.; Abdelaaty, S. Tissue Inhibitors of Metalloproteinase-1 and 2 and Obesity Related Non-Alcoholic Fatty Liver Disease: Is There a Relationship. Digestion 2015, 92, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Valva, P.; Rios, D.; Casciato, P.; Gadano, A.; Galdame, O.; Mullen, E.; Bertot, G.; de Matteo, E.; Preciado, M.V. Nonalcoholic Fatty Liver Disease: Biomarkers as Diagnostic Tools for Liver Damage Assessment in Adult Patients from Argentina. Eur. J. Gastroenterol. Hepatol. 2018, 30, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Feldstein, A.E. Non-Invasive Diagnosis of Nonalcoholic Fatty Liver and Nonalcoholic Steatohepatitis. J. Dig. Dis. 2011, 12, 10–16. [Google Scholar] [CrossRef]
- Kayadibi, H.; Gültepe, M.; Yasar, B.; Ince, A.T.; Ozcan, O.; Ipcioglu, O.M.; Kurdas, O.O.; Bolat, B.; Benek, Y.Z.; Guveli, H.; et al. Diagnostic Value of Serum Prolidase Enzyme Activity to Predict the Liver Histological Lesions in Non-Alcoholic Fatty Liver Disease: A Surrogate Marker to Distinguish Steatohepatitis from Simple Steatosis. Dig. Dis. Sci. 2009, 54, 1764–1771. [Google Scholar] [CrossRef]
- Haghgoo, S.M.; Sharafi, H.; Alavian, S.M. Serum Cytokines, Adipokines and Ferritin for Non-Invasive Assessment of Liver Fibrosis in Chronic Liver Disease: A Systematic Review. Clin. Chem. Lab. Med. 2019, 57, 577–610. [Google Scholar] [CrossRef]
- Shiraishi, T.; Morimoto, S.; Koh, E.; Fukuo, K.; Ogihara, T. Increased Release of Platelet-Derived Growth Factor from Platelets in Chronic Liver Disease. Eur. J. Clin. Chem. Clin. Biochem. 1994, 32, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Degertekin, B.; Ozenirler, S.; Elbeg, S.; Akyol, G. The Serum Endothelin-1 Level in Steatosis and NASH, and Its Relation with Severity of Liver Fibrosis. Dig. Dis. Sci. 2007, 52, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, J.; Verhulst, S.; Mannaerts, I.; Sowa, J.-P.; Best, J.; Canbay, A.; Reynaert, H.; van Grunsven, L.A. A PDGFRβ-Based Score Predicts Significant Liver Fibrosis in Patients with Chronic Alcohol Abuse, NAFLD and Viral Liver Disease. eBioMedicine 2019, 43, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, J.; Tacke, F. Controversies and Opportunities in the Use of Inflammatory Markers for Diagnosis or Risk Prediction in Fatty Liver Disease. Front. Immunol. 2020, 11, 634409. [Google Scholar] [CrossRef] [PubMed]
- Koruk, M.; Tayşi, S.; Savaş, M.C.; Yilmaz, O.; Akçay, F.; Karakök, M. Serum Levels of Acute Phase Proteins in Patients with Nonalcoholic Steatohepatitis. Turk. J. Gastroenterol. 2003, 14, 12–17. [Google Scholar] [PubMed]
- Yoneda, M.; Mawatari, H.; Fujita, K.; Iida, H.; Yonemitsu, K.; Kato, S.; Takahashi, H.; Kirikoshi, H.; Inamori, M.; Nozaki, Y.; et al. High-Sensitivity C-Reactive Protein Is an Independent Clinical Feature of Nonalcoholic Steatohepatitis (NASH) and Also of the Severity of Fibrosis in NASH. J. Gastroenterol. 2007, 42, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Targher, G. Relationship between High-Sensitivity C-Reactive Protein Levels and Liver Histology in Subjects with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2006, 45, 879–881. [Google Scholar] [CrossRef]
- Hui, J.M.; Farrell, G.C.; Kench, J.G.; George, J. High Sensitivity C-reactive Protein Values Do Not Reliably Predict the Severity of Histological Changes in NAFLD. Hepatology 2004, 39, 1458–1459. [Google Scholar] [CrossRef]
- Oruc, N.; Ozutemiz, O.; Yuce, G.; Akarca, U.S.; Ersoz, G.; Gunsar, F.; Batur, Y. Serum Procalcitonin and CRP Levels in Non-Alcoholic Fatty Liver Disease: A Case Control Study. BMC Gastroenterol. 2009, 9, 16. [Google Scholar] [CrossRef]
- Zimmermann, E.; Anty, R.; Anty, R.; Tordjman, J.; Verrijken, A.; Gual, P.; Tran, A.; Iannelli, A.; Gugenheim, J.; Bedossa, P.; et al. C-Reactive Protein Levels in Relation to Various Features of Non-Alcoholic Fatty Liver Disease among Obese Patients. J. Hepatol. 2011, 55, 660–665. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E. NASH Clinical Research Network Serum Ferritin Is an Independent Predictor of Histologic Severity and Advanced Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-Alpha: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.-S.; Hui, A.Y.; Tsang, S.W.-C.; Chan, J.L.-Y.; Tse, A.M.-L.; Chan, K.-F.; So, W.-Y.; Cheng, A.Y.-S.; Ng, W.-F.; Wong, G.L.-H.; et al. Metabolic and Adipokine Profile of Chinese Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.R.; Yaccha, M.; Malik, M.A.; Rabbani, M.U.; Ahmad, I.; Isalm, N.; Abdali, N. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients of Cardiovascular Diseases and Its Association with Hs-CRP and TNF-α. Indian Heart J. 2014, 66, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Jeong, G.; Kim, S.J.; Kim, M.K.; Park, S.M. Predictors Reflecting the Pathological Severity of Non-Alcoholic Fatty Liver Disease: Comprehensive Study of Clinical and Immunohistochemical Findings in Younger Asian Patients. J. Gastroenterol. Hepatol. 2007, 22, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Abiru, S.; Migita, K.; Maeda, Y.; Daikoku, M.; Ito, M.; Ohata, K.; Nagaoka, S.; Matsumoto, T.; Takii, Y.; Kusumoto, K.; et al. Serum Cytokine and Soluble Cytokine Receptor Levels in Patients with Non-Alcoholic Steatohepatitis. Liver Int. 2006, 26, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Xu, D.; Li, Q.; Xie, N.; Xia, J.; Huo, Q.; Li, P.; Chen, Q.; Huang, S. Metabonomics Screening of Serum Identifies Pyroglutamate as a Diagnostic Biomarker for Nonalcoholic Steatohepatitis. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 473, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Cai, J.-J.; She, Z.-G.; Li, H.-L. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease: Current Evidence and Practice. World J. Gastroenterol. 2019, 25, 1307–1326. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Q.; Zhou, W.; Liu, T.; Yang, L.; Zheng, P.; Zhang, L.; Ji, G. Integrated Analysis of Hepatic mRNA and miRNA Profiles Identified Molecular Networks and Potential Biomarkers of NAFLD. Sci. Rep. 2018, 8, 7628. [Google Scholar] [CrossRef]
- Wieckowska, A.; Papouchado, B.G.; Li, Z.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Increased Hepatic and Circulating Interleukin-6 Levels in Human Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2008, 103, 1372–1379. [Google Scholar] [CrossRef]
- Kar, S.; Paglialunga, S.; Jaycox, S.H.; Islam, R.; Paredes, A.H. Assay Validation and Clinical Performance of Chronic Inflammatory and Chemokine Biomarkers of NASH Fibrosis. PLoS ONE 2019, 14, e0217263. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Damås, J.K.; Konopski, Z.; Løberg, E.M.; Haaland, T.; Goverud, I.; Torjesen, P.A.; Birkeland, K.; Bjøro, K.; Aukrust, P. Systemic Inflammation in Nonalcoholic Fatty Liver Disease Is Characterized by Elevated Levels of CCL2. J. Hepatol. 2006, 44, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Coulon, S.; Francque, S.; Colle, I.; Verrijken, A.; Blomme, B.; Heindryckx, F.; De Munter, S.; Prawitt, J.; Caron, S.; Staels, B.; et al. Evaluation of Inflammatory and Angiogenic Factors in Patients with Non-Alcoholic Fatty Liver Disease. Cytokine 2012, 59, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Balakrishnan, V. Role of Cytokines in the Pathogenesis of Non-Alcoholic Fatty Liver Disease. Indian J. Clin. Biochem. 2011, 26, 202–209. [Google Scholar] [CrossRef]
- Ajmera, V.; Perito, E.R.; Bass, N.M.; Terrault, N.A.; Yates, K.P.; Gill, R.; Loomba, R.; Diehl, A.M.; Aouizerat, B.E. NASH Clinical Research Network Novel Plasma Biomarkers Associated with Liver Disease Severity in Adults with Nonalcoholic Fatty Liver Disease. Hepatology 2017, 65, 65–77. [Google Scholar] [CrossRef]
- Jamali, R.; Arj, A.; Razavizade, M.; AArabi, M.H. Prediction of Nonalcoholic Fatty Liver Disease Via a Novel Panel of Serum Adipokines. Medicine 2016, 95, e2630. [Google Scholar] [CrossRef] [PubMed]
- Darmadi, D.; Ruslie, R.H. Association between Serum Interleukin (IL)-12 Level and Severity of Non-Alcoholic Fatty Liver Disease (NAFLD). Rom. J. Intern. Med. 2021, 59, 66–72. [Google Scholar] [CrossRef]
- Janczyk, W.; Michalkiewicz, J.; Gackowska, L.; Kubiszewska, I.; Wierzbicka, A.; Litwin, M. Assessment of CC Chemokines, IL-6, TNF-α and Angiogenin Profiles in Children with Non-Alcoholic Fatty Liver Disease. Exp. Clin. Hepatol. 2009, 5, 20–21. [Google Scholar]
- Roh, Y.-S.; Seki, E. Chemokines and Chemokine Receptors in the Development of NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 45–53. [Google Scholar] [CrossRef]
- Pan, X.; Chiwanda Kaminga, A.; Liu, A.; Wen, S.W.; Chen, J.; Luo, J. Chemokines in Non-Alcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Front. Immunol. 2020, 11, 1802. [Google Scholar] [CrossRef]
- Gurel, H.; Genc, H.; Celebi, G.; Sertoglu, E.; Cicek, A.F.; Kayadibi, H.; Ercin, C.N.; Dogru, T. Plasma Pentraxin-3 Is Associated with Endothelial Dysfunction in Non-Alcoholic Fatty Liver Disease. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4305–4312. [Google Scholar] [PubMed]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in Cardiovascular Disease. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Uchiyama, T.; Kato, S.; Endo, H.; Fujita, K.; Yoneda, K.; Mawatari, H.; Iida, H.; Takahashi, H.; Kirikoshi, H.; et al. Plasma Pentraxin3 Is a Novel Marker for Nonalcoholic Steatohepatitis (NASH). BMC Gastroenterol. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Kurt, O.; Dogan, T.; Ozen, A.; Demirci, H.; Yesildal, F.; Kantarcioglu, M.; Turker, T.; Guler, A.K.; Karslioglu, Y.; et al. Pentraxin 3 Is a Predictor for Fibrosis and Arterial Stiffness in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterol. Res. Pract. 2016, 2016, 1417962. [Google Scholar] [CrossRef] [PubMed]
- Boga, S.; Koksal, A.R.; Alkim, H.; Yilmaz Ozguven, M.B.; Bayram, M.; Ergun, M.; Sisman, G.; Tekin Neijmann, S.; Alkim, C. Plasma Pentraxin 3 Differentiates Nonalcoholic Steatohepatitis (NASH) from Non-NASH. Metab. Syndr. Relat. Disord. 2015, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Maleki, I.; Rastgar, A.; Hosseini, V.; Taghvaei, T.; Rafiei, A.; Barzin, M.; Torabizadeh, Z.; Naghshvar, F.; Khalilian, A. High Sensitive CRP and Pentraxine 3 as Noninvasive Biomarkers of Nonalcoholic Fatty Liver Disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1583–1590. [Google Scholar] [PubMed]
- Ye, X.; Li, J.; Wang, H.; Wu, J. Pentraxin 3 and the TyG Index as Two Novel Markers to Diagnose NAFLD in Children. Dis. Markers 2021, 2021, 8833287. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M. VCAM-1: Closing the Gap between Lipotoxicity and Endothelial Dysfunction in Nonalcoholic Steatohepatitis. J. Clin. Investig. 2021, 131, e147556. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, M.; Mura, V.; Gracia-Sancho, J.; García-Calderó, H.; Rodriguez, A.; García-Pagán, J.; Bosch, J.; Abraldes, J. Sinusoidal Endothelial Dysfunction Precedes Inflammation and Fibrosis in a Model of NAFLD. PLoS ONE 2012, 7, e32785. [Google Scholar] [CrossRef]
- Van Oosten, M.; van de Bilt, E.; de Vries, H.E.; van Berkel, T.J.C.; Kuiper, J. Vascular Adhesion Molecule-1 and Intercellular Adhesion Molecule-1 Expression on Rat Liver Cells after Lipopolysaccharide Administration in Vivo. Hepatology 1995, 22, 1538–1546. [Google Scholar] [CrossRef]
- Furuta, K.; Guo, Q.; Pavelko, K.D.; Lee, J.-H.; Robertson, K.D.; Nakao, Y.; Melek, J.; Shah, V.H.; Hirsova, P.; Ibrahim, S.H. Lipid-Induced Endothelial Vascular Cell Adhesion Molecule 1 Promotes Nonalcoholic Steatohepatitis Pathogenesis. J. Clin. Investig. 2021, 131, e143690. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.S.; Cortez-Pinto, H.; Solá, S.; Castro, R.E.; Ramalho, R.M.; Baptista, A.; Moura, M.C.; Camilo, M.E.; Rodrigues, C.M.P. Hepatocyte Apoptosis, Expression of Death Receptors, and Activation of NF-kappaB in the Liver of Nonalcoholic and Alcoholic Steatohepatitis Patients. Am. J. Gastroenterol. 2004, 99, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Wree, A.; Feldstein, A.E. Biomarkers of Liver Cell Death. J. Hepatol. 2014, 60, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Church, R.J.; Watkins, P.B. The Transformation in Biomarker Detection and Management of Drug-Induced Liver Injury. Liver Int. 2017, 37, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Grandison, G.A.; Angulo, P. Can NASH Be Diagnosed, Graded, and Staged Noninvasively? Clin. Liver Dis. 2012, 16, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Jarrar, M.; Nugent, C.; Randhawa, M.; Afendy, M.; Stepanova, M.; Rafiq, N.; Goodman, Z.; Chandhoke, V.; Baranova, A. A Novel Diagnostic Biomarker Panel for Obesity-Related Nonalcoholic Steatohepatitis (NASH). Obes. Surg. 2008, 18, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Wieckowska, A.; Zein, N.N.; Yerian, L.M.; Lopez, A.R.; McCullough, A.J.; Feldstein, A.E. In Vivo Assessment of Liver Cell Apoptosis as a Novel Biomarker of Disease Severity in Nonalcoholic Fatty Liver Disease. Hepatology 2006, 44, 27–33. [Google Scholar] [CrossRef]
- Miller, M.H.; Ferguson, M.A.J.; Dillon, J.F. Systematic Review of Performance of Non-Invasive Biomarkers in the Evaluation of Non-Alcoholic Fatty Liver Disease. Liver Int. 2011, 31, 461–473. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Wieckowska, A.; Lopez, A.R.; Liu, Y.-C.; Zein, N.N.; McCullough, A.J. Cytokeratin-18 Fragment Levels as Noninvasive Biomarkers for Nonalcoholic Steatohepatitis: A Multicenter Validation Study. Hepatology 2009, 50, 1072–1078. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Page, S.; Rafiq, N.; Birerdinc, A.; Stepanova, M.; Hossain, N.; Afendy, A.; Younoszai, Z.; Goodman, Z.; Baranova, A. A Biomarker Panel for Non-Alcoholic Steatohepatitis (NASH) and NASH-Related Fibrosis. Obes. Surg. 2011, 21, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Diab, D.L.; Yerian, L.; Schauer, P.; Kashyap, S.R.; Lopez, R.; Hazen, S.L.; Feldstein, A.E. Cytokeratin 18 Fragment Levels as a Noninvasive Biomarker for Nonalcoholic Steatohepatitis in Bariatric Surgery Patients. Clin. Gastroenterol. Hepatol. 2008, 6, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.; Tse, Y.-K.; Wong, G.L.-H.; Ha, Y.; Lee, A.U.; Ngu, M.C.; Chan, H.L.-Y.; Wong, V.W.-S. Systematic Review with Meta-Analysis: Non-Invasive Assessment of Non-Alcoholic Fatty Liver Disease—The Role of Transient Elastography and Plasma Cytokeratin-18 Fragments. Aliment. Pharmacol. Ther. 2014, 39, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Jain, A.K.; Deppe, R.; Yates, K.; Comerford, M.; Masuoka, H.C.; Neuschwander-Tetri, B.A.; Loomba, R.; Brunt, E.M.; Kleiner, D.E.; et al. Relationship between Changes in Serum Levels of Keratin 18 and Changes in Liver Histology in Children and Adults with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2121–2130.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Chang, Z.; Harrison, S.; Lomonaco, R.; Bril, F.; Orsak, B.; Ortiz-Lopez, C.; Hecht, J.; Feldstein, A.E.; Webb, A.; et al. Limited Value of Plasma Cytokeratin-18 as a Biomarker for NASH and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2014, 60, 167–174. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Marasco, G.; Scaioli, E.; Pasqui, F.; Colecchia, A. Non-Invasive Diagnostic Approach to Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Expert. Rev. Gastroenterol. Hepatol. 2015, 9, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-K.; Sthaneshwar, P.; Nik Mustapha, N.R.; Mahadeva, S. Limited Utility of Plasma M30 in Discriminating Non-Alcoholic Steatohepatitis from Steatosis--a Comparison with Routine Biochemical Markers. PLoS ONE 2014, 9, e105903. [Google Scholar] [CrossRef] [PubMed]
- Chuah, K.; Wan Yusoff, W.N.I.; Sthaneshwar, P.; Nik Mustapha, N.R.; Mahadeva, S.; Chan, W. MACK-3 (Combination of hoMa, Ast and CK18): A Promising Novel Biomarker for Fibrotic Non-alcoholic Steatohepatitis. Liver Int. 2019, 39, 1315–1324. [Google Scholar] [CrossRef]
- Anty, R.; Iannelli, A.; Patouraux, S.; Bonnafous, S.; Lavallard, V.J.; Senni-Buratti, M.; Ben Amor, I.; Staccini-Myx, A.; Saint-Paul, M.-C.; Berthier, F.; et al. A New Composite Model Including Metabolic Syndrome, Alanine Aminotransferase and Cytokeratin-18 for the Diagnosis of Non-Alcoholic Steatohepatitis in Morbidly Obese Patients. Aliment. Pharmacol. Ther. 2010, 32, 1315–1322. [Google Scholar] [CrossRef]
- Grigorescu, M.; Crisan, D.; Radu, C.; Grigorescu, M.D.; Sparchez, Z.; Serban, A. A Novel Pathophysiological-Based Panel of Biomarkers for the Diagnosis of Nonalcoholic Steatohepatitis. J. Physiol. Pharmacol. 2012, 63, 347–353. [Google Scholar]
- Fukumoto, S. Actions and Mode of Actions of FGF19 Subfamily Members. Endocr. J. 2008, 55, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chan, H.L.-Y.; Wong, G.L.-H.; Choi, P.C.-L.; Chan, A.W.-H.; Chan, H.-Y.; Chim, A.M.-L.; Yeung, D.K.-W.; Chan, F.K.-L.; Woo, J.; et al. Non-Invasive Diagnosis of Non-Alcoholic Steatohepatitis by Combined Serum Biomarkers. J. Hepatol. 2012, 56, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Alisi, A.; Okwu, V.; Matloob, A.; Ferrari, F.; Crudele, A.; De Vito, R.; Lopez, R.; Feldstein, A.E.; Nobili, V. Circulating Soluble Fas and Fas Ligand Levels Are Elevated in Children with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, T.I.A.-R.; Elgouhari, H.M.; Alkhouri, N.; Yerian, L.M.; Berk, M.P.; Lopez, R.; Schauer, P.R.; Zein, N.N.; Feldstein, A.E. An Apoptosis Panel for Nonalcoholic Steatohepatitis Diagnosis. J. Hepatol. 2011, 54, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, C.F.M.G.; Jiang, Z.G.; Otsubo, T.; Feldbrügge, L.; Challies, T.L.; Nasser, I.; Robson, S.; Afdhal, N.; Lai, M. Poor Inter-Test Reliability Between CK18 Kits as a Biomarker of NASH. Dig. Dis. Sci. 2016, 61, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Abeygunasekera, S.; Farrell, G.C.; Holmes-Walker, J.; Hui, J.M.; Fung, C.; Karim, R.; Lin, R.; Samarasinghe, D.; Liddle, C.; et al. NASH and Insulin Resistance: Insulin Hypersecretion and Specific Association with the Insulin Resistance Syndrome. Hepatology 2002, 35, 373–379. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Toulis, K.A.; Goulis, D.G.; Zavos, C.; Kountouras, J. Serum Total Adiponectin in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Metabolism 2011, 60, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Kozakova, M.; Højlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. RISC Investigators Fatty Liver Is Associated with Insulin Resistance, Risk of Coronary Heart Disease, and Early Atherosclerosis in a Large European Population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef]
- Hui, J.M.; Hodge, A.; Farrell, G.C.; Kench, J.G.; Kriketos, A.; George, J. Beyond Insulin Resistance in NASH: TNF-Alpha or Adiponectin? Hepatology 2004, 40, 46–54. [Google Scholar] [CrossRef]
- Jarrar, M.H.; Baranova, A.; Collantes, R.; Ranard, B.; Stepanova, M.; Bennett, C.; Fang, Y.; Elariny, H.; Goodman, Z.; Chandhoke, V.; et al. Adipokines and Cytokines in Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2008, 27, 412–421. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Scala, L.; Zenari, L.; Falezza, G. Associations between Plasma Adiponectin Concentrations and Liver Histology in Patients with Nonalcoholic Fatty Liver Disease. Clin. Endocrinol. 2006, 64, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Argentou, M.; Tiniakos, D.G.; Karanikolas, M.; Melachrinou, M.; Makri, M.G.; Kittas, C.; Kalfarentzos, F. Adipokine Serum Levels Are Related to Liver Histology in Severely Obese Patients Undergoing Bariatric Surgery. Obes. Surg. 2009, 19, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Tietge, U.J.F.; Schmidt, H.H.-J.; Schütz, T.; Dippe, P.; Lochs, H.; Pirlich, M. Reduced Plasma Adiponectin in NASH: Central Obesity as an Underestimated Causative Risk Factor. Hepatology 2005, 41, 401. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Pagotto, U.; Manini, R.; Vanni, E.; Gastaldelli, A.; de Iasio, R.; Gentilcore, E.; Natale, S.; Cassader, M.; Rizzetto, M.; et al. Plasma Adiponectin in Nonalcoholic Fatty Liver Is Related to Hepatic Insulin Resistance and Hepatic Fat Content, Not to Liver Disease Severity. J. Clin. Endocrinol. Metab. 2005, 90, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Kawahara, H.; Ozaki, K.; Fukura, M.; Yano, H.; Tsuchishima, M.; Tsutsumi, M.; Takase, S. Usefulness of a Combined Evaluation of the Serum Adiponectin Level, HOMA-IR, and Serum Type IV Collagen 7S Level to Predict the Early Stage of Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2007, 102, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Milner, K.-L.; van der Poorten, D.; Xu, A.; Bugianesi, E.; Kench, J.G.; Lam, K.S.L.; Chisholm, D.J.; George, J. Adipocyte Fatty Acid Binding Protein Levels Relate to Inflammation and Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Wang, S.; Yang, Z.; He, M.; Zhang, S.; Zhang, W.; Wen, J.; Li, Q.; Huang, Y.; Wang, X.; et al. Serum Lipocalin-2, Cathepsin S and Chemerin Levels and Nonalcoholic Fatty Liver Disease. Mol. Biol. Rep. 2014, 41, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Morteza, A.; Zandieh, A.; Jafari, S.; Rezaee, M.; Nakhjavani, M.; Jamali, A.; Esteghamati, A.-R.; Khalilzadeh, O. The Value of Visfatin in the Prediction of Metabolic Syndrome: A Multi-Factorial Analysis. J. Cardiovasc. Transl. Res. 2012, 5, 541–546. [Google Scholar] [CrossRef]
- Aller, R.; de Luis, D.A.; Izaola, O.; Sagrado, M.G.; Conde, R.; Velasco, M.C.; Alvarez, T.; Pacheco, D.; González, J.M. Influence of Visfatin on Histopathological Changes of Non-Alcoholic Fatty Liver Disease. Dig. Dis. Sci. 2009, 54, 1772–1777. [Google Scholar] [CrossRef]
- Giannini, C.; Feldstein, A.E.; Santoro, N.; Kim, G.; Kursawe, R.; Pierpont, B.; Caprio, S. Circulating Levels of FGF-21 in Obese Youth: Associations With Liver Fat Content and Markers of Liver Damage. J. Clin. Endocrinol. Metab. 2013, 98, 2993–3000. [Google Scholar] [CrossRef]
- He, L.; Deng, L.; Zhang, Q.; Guo, J.; Zhou, J.; Song, W.; Yuan, F. Diagnostic Value of CK-18, FGF-21, and Related Biomarker Panel in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2017, 2017, 9729107. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, S.; Farrell, G.; Frost, L.; Kriketos, A.; Lin, R.; Fung, C.; Liddle, C.; Samarasinghe, D.; George, J. Serum Leptin in NASH Correlates with Hepatic Steatosis but Not Fibrosis: A Manifestation of Lipotoxicity? Hepatology 2002, 36, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Canbakan, B.; Tahan, V.; Balci, H.; Hatemi, I.; Erer, B.; Ozbay, G.; Sut, N.; Hacibekiroglu, M.; Imeryuz, N.; Senturk, H. Leptin in Nonalcoholic Fatty Liver Disease. Ann. Hepatol. 2008, 7, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; de Luis, D.A.; Fernandez, L.; Calle, F.; Velayos, B.; Olcoz, J.L.; Izaola, O.; Sagrado, M.G.; Conde, R.; Gonzalez, J.M. Influence of Insulin Resistance and Adipokines in the Grade of Steatosis of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2008, 53, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Soardo, G.; Pilon, C.; Milocco, C.; Basan, L.; Milan, G.; Donnini, D.; Faggian, D.; Mussap, M.; Plebani, M.; et al. Increased Serum Resistin in Nonalcoholic Fatty Liver Disease Is Related to Liver Disease Severity and Not to Insulin Resistance. J. Clin. Endocrinol. Metab. 2006, 91, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Angulo, P.; Chalasani, N.; Merriman, R.; Viker, K.; Charatcharoenwitthaya, P.; Sanderson, S.; Gawrieh, S.; Krishnan, A.; Lindor, K. Low Circulating Levels of Dehydroepiandrosterone in Histologically Advanced Nonalcoholic Fatty Liver Disease. Hepatology 2008, 47, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Stål, P.; Hultcrantz, R.; Brismar, K.; Ansurudeen, I. IGFBP-1 and IGF-I as Markers for Advanced Fibrosis in NAFLD—A Pilot Study. Scand. J. Gastroenterol. 2017, 52, 1427–1434. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. Int. J. Mol. Sci. 2021, 22, 4459. [Google Scholar] [CrossRef]
- Horoz, M.; Bolukbas, C.; Bolukbas, F.F.; Sabuncu, T.; Aslan, M.; Sarifakiogullari, S.; Gunaydin, N.; Erel, O. Measurement of the Total Antioxidant Response Using a Novel Automated Method in Subjects with Nonalcoholic Steatohepatitis. BMC Gastroenterol. 2005, 5, 35. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Lopez, R.; Tamimi, T.A.-R.; Yerian, L.; Chung, Y.-M.; Berk, M.; Zhang, R.; McIntyre, T.M.; Hazen, S.L. Mass Spectrometric Profiling of Oxidized Lipid Products in Human Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. J. Lipid Res. 2010, 51, 3046–3054. [Google Scholar] [CrossRef]
- Başkol, M.; Başkol, G.; Deniz, K.; Ozbakir, O.; Yücesoy, M. A New Marker for Lipid Peroxidation: Serum Paraoxonase Activity in Non-Alcoholic Steatohepatitis. Turk. J. Gastroenterol. 2005, 16, 119–123. [Google Scholar] [PubMed]
- Yesilova, Z.; Yaman, H.; Oktenli, C.; Ozcan, A.; Uygun, A.; Cakir, E.; Sanisoglu, S.Y.; Erdil, A.; Ates, Y.; Aslan, M.; et al. Systemic Markers of Lipid Peroxidation and Antioxidants in Patients with Nonalcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2005, 100, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Sariçam, T.; Kircali, B.; Köken, T. Assessment of Lipid Peroxidation and Antioxidant Capacity in Non-Alcoholic Fatty Liver Disease. Turk. J. Gastroenterol. 2005, 16, 65–70. [Google Scholar] [PubMed]
- Fierbinţeanu-Braticevici, C.; Bengus, A.; Neamţu, M.; Usvat, R. The Risk Factors of Fibrosis in Nonalcoholic Steatohepatitis. Rom. J. Intern. Med. 2002, 40, 81–88. [Google Scholar] [PubMed]
- Koruk, M.; Taysi, S.; Savas, M.C.; Yilmaz, O.; Akcay, F.; Karakok, M. Oxidative Stress and Enzymatic Antioxidant Status in Patients with Nonalcoholic Steatohepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar] [PubMed]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic Levels of Lipid Peroxidation and Its Metabolic and Dietary Correlates in Patients with Nonalcoholic Steatohepatitis. Am. J. Gastroenterol. 2004, 99, 1497–1502. [Google Scholar] [CrossRef]
- Alkhouri, N.; Berk, M.; Yerian, L.; Lopez, R.; Chung, Y.-M.; Zhang, R.; McIntyre, T.M.; Feldstein, A.E.; Hazen, S.L. OxNASH Score Correlates with Histologic Features and Severity of Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2014, 59, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A.; Rodrigo, R.; Orellana, M.; Fernandez, V.; Tapia, G.; Quiñones, L.; Varela, N.; Contreras, J.; Lazarte, R.; Csendes, A.; et al. Oxidative Stress-Related Parameters in the Liver of Non-Alcoholic Fatty Liver Disease Patients. Clin. Sci. Lond. Engl. 2004, 106, 261–268. [Google Scholar] [CrossRef]
- Chtioui, H.; Semela, D.; Ledermann, M.; Zimmermann, A.; Dufour, J.-F. Expression and Activity of the Cytochrome P450 2E1 in Patients with Nonalcoholic Steatosis and Steatohepatitis. Liver Int. 2007, 27, 764–771. [Google Scholar] [CrossRef]
- Orellana, M.; Rodrigo, R.; Varela, N.; Araya, J.; Poniachik, J.; Csendes, A.; Smok, G.; Videla, L.A. Relationship between in Vivo Chlorzoxazone Hydroxylation, Hepatic Cytochrome P450 2E1 Content and Liver Injury in Obese Non-Alcoholic Fatty Liver Disease Patients. Hepatol. Res. 2006, 34, 57–63. [Google Scholar] [CrossRef]
- Albano, E.; Mottaran, E.; Vidali, M.; Reale, E.; Saksena, S.; Occhino, G.; Burt, A.D.; Day, C.P. Immune Response towards Lipid Peroxidation Products as a Predictor of Progression of Non-Alcoholic Fatty Liver Disease to Advanced Fibrosis. Gut 2005, 54, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.J.; Dallinga, J.W.; Driessen, A.; de Jonge, C.; Moonen, E.J.C.; van Berkel, J.B.N.; Luijk, J.; Bouvy, N.D.; Buurman, W.A.; Rensen, S.S.; et al. Non-Alcoholic Steatohepatitis: A Non-Invasive Diagnosis by Analysis of Exhaled Breath. J. Hepatol. 2013, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Danford, C.J.; Yao, Z.; Jiang, Z.G. Non-Alcoholic Fatty Liver Disease: A Narrative Review of Genetics. J. Biomed. Res. 2018, 32, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Schork, N.; Chen, C.-H.; Bettencourt, R.; Bhatt, A.; Ang, B.; Nguyen, P.; Hernandez, C.; Richards, L.; Salotti, J.; et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology 2015, 149, 1784–1793. [Google Scholar] [CrossRef]
- Sreekumar, R.; Rosado, B.; Rasmussen, D.; Charlton, M. Hepatic Gene Expression in Histologically Progressive Nonalcoholic Steatohepatitis. Hepatology 2003, 38, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Gorreta, F.; Ong, J.P.; Schlauch, K.; Del Giacco, L.; Elariny, H.; Van Meter, A.; Younoszai, A.; Goodman, Z.; Baranova, A.; et al. Hepatic Gene Expression in Patients with Obesity-Related Non-Alcoholic Steatohepatitis. Liver Int. 2005, 25, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Bragoszewski, P.; Habior, A.; Walewska-Zielecka, B.; Ostrowski, J. Expression of Genes Encoding Mitochondrial Proteins Can Distinguish Nonalcoholic Steatosis from Steatohepatitis. Acta Biochim. Pol. 2007, 54, 341–348. [Google Scholar] [CrossRef]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene Expression in Human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef]
- Chiappini, F.; Barrier, A.; Saffroy, R.; Domart, M.-C.; Dagues, N.; Azoulay, D.; Sebagh, M.; Franc, B.; Chevalier, S.; Debuire, B.; et al. Exploration of Global Gene Expression in Human Liver Steatosis by High-Density Oligonucleotide Microarray. Lab. Investig. J. Tech. Methods Pathol. 2006, 86, 154–165. [Google Scholar] [CrossRef]
- Yoneda, M.; Endo, H.; Mawatari, H.; Nozaki, Y.; Fujita, K.; Akiyama, T.; Higurashi, T.; Uchiyama, T.; Yoneda, K.; Takahashi, H.; et al. Gene Expression Profiling of Non-Alcoholic Steatohepatitis Using Gene Set Enrichment Analysis. Hepatol. Res. 2008, 38, 1204–1212. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kolak, M.; Kiviluoto, T.; Arkkila, P.; Sirén, J.; Hamsten, A.; Fisher, R.M.; Yki-Järvinen, H. Genes Involved in Fatty Acid Partitioning and Binding, Lipolysis, Monocyte/Macrophage Recruitment, and Inflammation Are Overexpressed in the Human Fatty Liver of Insulin-Resistant Subjects. Diabetes 2007, 56, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Kamfar, S.; Alavian, S.M.; Houshmand, M.; Yadegarazari, R.; Seifi Zarei, B.; Khalaj, A.; Shabab, N.; Saidijam, M. Liver Mitochondrial DNA Copy Number and Deletion Levels May Contribute to Nonalcoholic Fatty Liver Disease Susceptibility. Hepat. Mon. 2016, 16. [Google Scholar] [CrossRef]
- Ma, C.; Liu, Y.; He, S.; Zeng, J.; Li, P.; Ma, C.; Ping, F.; Zhang, H.; Xu, L.; Li, W.; et al. Association Between Leukocyte Mitochondrial DNA Copy Number and Non-Alcoholic Fatty Liver Disease in a Chinese Population Is Mediated by 8-Oxo-2’-Deoxyguanosine. Front. Med. 2020, 7, 536. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, B.; Yilmaz, Y.; Eren, F. Potential Clinical Variants Detected in Mitochondrial DNA D-Loop Hypervariable Region I of Patients with Non-Alcoholic Steatohepatitis. Horm. Athens Greece 2019, 18, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Pirazzi, C.; Mancina, R.M.; Motta, B.M.; Indiveri, C.; Pujia, A.; Montalcini, T.; Hedfalk, K.; Romeo, S. Recombinant PNPLA3 Protein Shows Triglyceride Hydrolase Activity and Its I148M Mutation Results in Loss of Function. Biochim. Biophys. Acta 2014, 1841, 574–580. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and Epigenetics of NAFLD and NASH: Clinical Impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Krawczyk, M.; Liebe, R.; Lammert, F. Toward Genetic Prediction of Nonalcoholic Fatty Liver Disease Trajectories: PNPLA3 and Beyond. Gastroenterology 2020, 158, 1865–1880. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic Variation in PNPLA3 Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Donati, B.; Fares, R.; Lombardi, R.; Mancina, R.M.; Romeo, S.; Valenti, L. PNPLA3 I148M Polymorphism and Progressive Liver Disease. World J. Gastroenterol. 2013, 19, 6969–6978. [Google Scholar] [CrossRef]
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072. [Google Scholar] [CrossRef]
- Xu, R.; Tao, A.; Zhang, S.; Deng, Y.; Chen, G. Association between Patatin-like Phospholipase Domain Containing 3 Gene (PNPLA3) Polymorphisms and Nonalcoholic Fatty Liver Disease: A HuGE Review and Meta-Analysis. Sci. Rep. 2015, 5, 9284. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Meta-Analysis of the Influence of I148M Variant of Patatin-like Phospholipase Domain Containing 3 Gene (PNPLA3) on the Susceptibility and Histological Severity of Nonalcoholic Fatty Liver Disease. Hepatology 2011, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Jiménez-Agüero, R.; Alustiza, J.M.; Emparanza, J.I.; Perugorria, M.J.; Bujanda, L.; Lammert, F.; Banales, J.M. PNPLA3 p.I148M Variant Is Associated with Greater Reduction of Liver Fat Content after Bariatric Surgery. Surg. Obes. Relat. Dis. 2016, 12, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Sevastianova, K.; Kotronen, A.; Gastaldelli, A.; Perttilä, J.; Hakkarainen, A.; Lundbom, J.; Suojanen, L.; Orho-Melander, M.; Lundbom, N.; Ferrannini, E.; et al. Genetic Variation in PNPLA3 (Adiponutrin) Confers Sensitivity to Weight Loss–Induced Decrease in Liver Fat in Humans123. Am. J. Clin. Nutr. 2011, 94, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Orešič, M.; Leivonen, M.; Gopalacharyulu, P.; Hyysalo, J.; Arola, J.; Verrijken, A.; Francque, S.; Van Gaal, L.; Hyötyläinen, T.; et al. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin. Gastroenterol. Hepatol. 2016, 14, 1463–1472.e6. [Google Scholar] [CrossRef] [PubMed]
- Italian Association for the Study of the Liver (AISF). AISF Position Paper on Nonalcoholic Fatty Liver Disease (NAFLD): Updates and Future Directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Oldoni, F.; Das, A. TM6SF2: A Novel Genetic Player in Nonalcoholic Fatty Liver and Cardiovascular Disease. Hepatol. Commun. 2022, 6, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-Wide Association Study Identifies a TM6SF2 Variant That Confers Susceptibility to Nonalcoholic Fatty Liver Disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kahali, B.; Liu, Y.-L.; Daly, A.K.; Day, C.P.; Anstee, Q.M.; Speliotes, E.K. TM6SF2: Catch-22 in the Fight against Nonalcoholic Fatty Liver Disease and Cardiovascular Disease? Gastroenterology 2015, 148, 679–684. [Google Scholar] [CrossRef]
- Xue, W.-Y.; Zhang, L.; Liu, C.-M.; Gao, Y.; Li, S.-J.; Huai, Z.-Y.; Dai, J.; Wang, Y.-Y. Research Progress on the Relationship between TM6SF2 Rs58542926 Polymorphism and Non-Alcoholic Fatty Liver Disease. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 97–107. [Google Scholar] [CrossRef]
- Pirola, C.J.; Sookoian, S. The Dual and Opposite Role of the TM6SF2-Rs58542926 Variant in Protecting against Cardiovascular Disease and Conferring Risk for Nonalcoholic Fatty Liver: A Meta-Analysis. Hepatology 2015, 62, 1742–1756. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Mallardi, P.; Fernández Gianotti, T.; Burgueño, A.L.; San Martino, J.; Pirola, C.J. Genetic Variation in Transmembrane 6 Superfamily Member 2 and the Risk of Nonalcoholic Fatty Liver Disease and Histological Disease Severity. Hepatology 2015, 61, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mahdessian, H.; Taxiarchis, A.; Popov, S.; Silveira, A.; Franco-Cereceda, A.; Hamsten, A.; Eriksson, P.; van’t Hooft, F. TM6SF2 Is a Regulator of Liver Fat Metabolism Influencing Triglyceride Secretion and Hepatic Lipid Droplet Content. Proc. Natl. Acad. Sci. USA 2014, 111, 8913–8918. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Maglio, C.; Fracanzani, A.L.; Pipitone, R.; Mozzi, E.; Motta, B.M.; Kaminska, D.; Rametta, R.; Grimaudo, S.; et al. Transmembrane 6 Superfamily Member 2 Gene Variant Disentangles Nonalcoholic Steatohepatitis from Cardiovascular Disease. Hepatology 2015, 61, 506–514. [Google Scholar] [CrossRef]
- Beer, N.L.; Tribble, N.D.; McCulloch, L.J.; Roos, C.; Johnson, P.R.V.; Orho-Melander, M.; Gloyn, A.L. The P446L Variant in GCKR Associated with Fasting Plasma Glucose and Triglyceride Levels Exerts Its Effect through Increased Glucokinase Activity in Liver. Hum. Mol. Genet. 2009, 18, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Shima, T.; Mizuno, M.; Mitsumoto, Y.; Umemura, A.; Kanbara, Y.; Tanaka, S.; Sumida, Y.; Yasui, K.; Takahashi, M.; et al. Risk Estimation Model for Nonalcoholic Fatty Liver Disease in the Japanese Using Multiple Genetic Markers. PLoS ONE 2018, 13, e0185490. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Kozlitina, J.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Hobbs, H.H.; Cohen, J.C. Adiposity Amplifies the Genetic Risk of Fatty Liver Disease Conferred by Multiple Loci. Nat. Genet. 2017, 49, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Durán, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef]
- Mancina, R.M.; Dongiovanni, P.; Petta, S.; Pingitore, P.; Meroni, M.; Rametta, R.; Borén, J.; Montalcini, T.; Pujia, A.; Wiklund, O.; et al. The MBOAT7-TMC4 Variant Rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology 2016, 150, 1219–1230.e6. [Google Scholar] [CrossRef]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 Rs641738 Variant and Hepatocellular Carcinoma in Non-Cirrhotic Individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef]
- Lee, D.H. Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease. Endocrinol. Metab. 2020, 35, 243. [Google Scholar] [CrossRef]
- Eslam, M.; Hashem, A.M.; Romero-Gomez, M.; Berg, T.; Dore, G.J.; Mangia, A.; Chan, H.L.Y.; Irving, W.L.; Sheridan, D.; Abate, M.L.; et al. FibroGENE: A Gene-Based Model for Staging Liver Fibrosis. J. Hepatol. 2016, 64, 390–398. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Castera, L.; Wong, V.W.-S. Noninvasive Assessment of Liver Fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 2023, 21, 2026–2039. [Google Scholar] [CrossRef]
- Murphy, S.K.; Yang, H.; Moylan, C.A.; Pang, H.; Dellinger, A.; Abdelmalek, M.F.; Garrett, M.E.; Ashley–Koch, A.; Suzuki, A.; Tillmann, H.L.; et al. Relationship Between Methylome and Transcriptome in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 1076–1087. [Google Scholar] [CrossRef]
- Xu, F.; Guo, W. The Progress of Epigenetics in the Development and Progression of Non-Alcoholic Fatty Liver Disease. Liver Res. 2020, 4, 118–123. [Google Scholar] [CrossRef]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA Methylation: A Potential Biomarker for Stratification of Liver Fibrosis in Non-Alcoholic Fatty Liver Disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef]
- Johnson, N.D.; Wu, X.; Still, C.D.; Chu, X.; Petrick, A.T.; Gerhard, G.S.; Conneely, K.N.; DiStefano, J.K. Differential DNA Methylation and Changing Cell-Type Proportions as Fibrotic Stage Progresses in NAFLD. Clin. Epigenetics 2021, 13, 152. [Google Scholar] [CrossRef]
- Pirola, C.J.; Gianotti, T.F.; Burgueño, A.L.; Rey-Funes, M.; Loidl, C.F.; Mallardi, P.; Martino, J.S.; Castaño, G.O.; Sookoian, S. Epigenetic Modification of Liver Mitochondrial DNA Is Associated with Histological Severity of Nonalcoholic Fatty Liver Disease. Gut 2013, 62, 1356–1363. [Google Scholar] [CrossRef]
- Ahrens, M.; Ammerpohl, O.; von Schönfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, H.; et al. DNA Methylation Analysis in Nonalcoholic Fatty Liver Disease Suggests Distinct Disease-Specific and Remodeling Signatures after Bariatric Surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef]
- Sookoian, S.; Rosselli, M.S.; Gemma, C.; Burgueño, A.L.; Fernández Gianotti, T.; Castaño, G.O.; Pirola, C.J. Epigenetic Regulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease: Impact of Liver Methylation of the Peroxisome Proliferator-Activated Receptor γ Coactivator 1α Promoter. Hepatology 2010, 52, 1992–2000. [Google Scholar] [CrossRef]
- Ma, J.; Nano, J.; Ding, J.; Zheng, Y.; Hennein, R.; Liu, C.; Speliotes, E.K.; Huan, T.; Song, C.; Mendelson, M.M.; et al. A Peripheral Blood DNA Methylation Signature of Hepatic Fat Reveals a Potential Causal Pathway for Nonalcoholic Fatty Liver Disease. Diabetes 2019, 68, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-N.; Pan, Q.; Zheng, R.-D.; Mi, Y.-Q.; Shen, F.; Zhou, D.; Chen, G.-Y.; Zhu, C.-Y.; Fan, J.-G. Genome-Wide Analysis of DNA Methylation in Human Peripheral Leukocytes Identifies Potential Biomarkers of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Med. 2018, 42, 443–452. [Google Scholar] [CrossRef]
- Hyun, J.; Jung, Y. DNA Methylation in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 8138. [Google Scholar] [CrossRef]
- Karlas, T.; Weise, L.; Kuhn, S.; Krenzien, F.; Mehdorn, M.; Petroff, D.; Linder, N.; Schaudinn, A.; Busse, H.; Keim, V.; et al. Correlation of Cell-Free DNA Plasma Concentration with Severity of Non-Alcoholic Fatty Liver Disease. J. Transl. Med. 2017, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Stefanakis, K.; Mantzoros, C.S. The Role of Omics in the Pathophysiology, Diagnosis and Treatment of Non-Alcoholic Fatty Liver Disease. Metabolism 2020, 111S, 154320. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ge, G.; Pan, T.; Wen, D.; Gan, J. A Pilot Study of Serum MicroRNAs Panel as Potential Biomarkers for Diagnosis of Nonalcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e105192. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiong, Y.; Sheng, Q.; Zhao, S.; Wattacheril, J.; Flynn, C.R. A Micro-RNA Expression Signature for Human NAFLD Progression. J. Gastroenterol. 2016, 51, 1022–1030. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic Steatohepatitis Is Associated with Altered Hepatic MicroRNA Expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef]
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905. [Google Scholar] [CrossRef]
- Cermelli, S.; Ruggieri, A.; Marrero, J.A.; Ioannou, G.N.; Beretta, L. Circulating microRNAs in Patients with Chronic Hepatitis C and Non-Alcoholic Fatty Liver Disease. PLoS ONE 2011, 6, e23937. [Google Scholar] [CrossRef]
- Liu, X.-L.; Pan, Q.; Zhang, R.-N.; Shen, F.; Yan, S.-Y.; Sun, C.; Xu, Z.-J.; Chen, Y.-W.; Fan, J.-G. Disease-Specific miR-34a as Diagnostic Marker of Non-Alcoholic Steatohepatitis in a Chinese Population. World J. Gastroenterol. 2016, 22, 9844–9852. [Google Scholar] [CrossRef] [PubMed]
- Pirola, C.J.; Fernández Gianotti, T.; Castaño, G.O.; Mallardi, P.; San Martino, J.; Mora Gonzalez Lopez Ledesma, M.; Flichman, D.; Mirshahi, F.; Sanyal, A.J.; Sookoian, S. Circulating microRNA Signature in Non-Alcoholic Fatty Liver Disease: From Serum Non-Coding RNAs to Liver Histology and Disease Pathogenesis. Gut 2015, 64, 800–812. [Google Scholar] [CrossRef]
- Jampoka, K.; Muangpaisarn, P.; Khongnomnan, K.; Treeprasertsuk, S.; Tangkijvanich, P.; Payungporn, S. Serum miR-29a and miR-122 as Potential Biomarkers for Non-Alcoholic Fatty Liver Disease (NAFLD). MicroRNA Shariqah United Arab Emir. 2018, 7, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Suzuki, K.; Ichino, N.; Ando, Y.; Sawada, A.; Osakabe, K.; Sugimoto, K.; Ohashi, K.; Teradaira, R.; Inoue, T.; et al. Associations between Circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and Non-Alcoholic Fatty Liver. Clin. Chim. Acta 2013, 424, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hendy, O.M.; Rabie, H.; El Fouly, A.; Abdel-Samiee, M.; Abdelmotelb, N.; Elshormilisy, A.A.; Allam, M.; Ali, S.T.; Bahaa El-Deen, N.M.; Abdelsattar, S.; et al. The Circulating Micro-RNAs (-122, -34a and -99a) as Predictive Biomarkers for Non-Alcoholic Fatty Liver Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.T. Noninvasive Tests (NITs) for Hepatic Fibrosis in Fatty Liver Syndrome. Life 2020, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Kawamura, Y.; Suzuki, F.; Saitoh, S.; Arase, Y.; Fujiyama, S.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Suzuki, Y.; et al. Analysis of Association between Circulating miR-122 and Histopathological Features of Nonalcoholic Fatty Liver Disease in Patients Free of Hepatocellular Carcinoma. BMC Gastroenterol. 2016, 16, 141. [Google Scholar] [CrossRef]
- Liu, C.-H.; Ampuero, J.; Gil-Gómez, A.; Montero-Vallejo, R.; Rojas, Á.; Muñoz-Hernández, R.; Gallego-Durán, R.; Romero-Gómez, M. miRNAs in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Hepatol. 2018, 69, 1335–1348. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; An, Z. Advance of Serum Biomarkers and Combined Diagnostic Panels in Nonalcoholic Fatty Liver Disease. Dis. Markers 2022, 2022, 1254014. [Google Scholar] [CrossRef]
- Sun, C.; Huang, F.; Liu, X.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L. miR-21 Regulates Triglyceride and Cholesterol Metabolism in Non-Alcoholic Fatty Liver Disease by Targeting HMGCR. Int. J. Mol. Med. 2015, 35, 847–853. [Google Scholar] [CrossRef]
- He, Z.; Yang, J.J.; Zhang, R.; Li, H.T.; Wu, L.; Jiang, F.; Jia, W.P.; Hu, C. Circulating miR-29b Positively Correlates with Non-Alcoholic Fatty Liver Disease in a Chinese Population. J. Dig. Dis. 2019, 20, 189–195. [Google Scholar] [CrossRef]
- Zong, Y.; Yan, J.; Jin, L.; Xu, B.; He, Z.; Zhang, R.; Hu, C.; Jia, W. Relationship between Circulating miR-132 and Non-Alcoholic Fatty Liver Disease in a Chinese Population. Hereditas 2020, 157, 22. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E. Progress in the Search for Circulating Biomarkers of Nonalcoholic Fatty Liver Disease. Biomarkers 2014, 19, 541–552. [Google Scholar] [CrossRef]
- Tadokoro, T.; Morishita, A.; Masaki, T. Diagnosis and Therapeutic Management of Liver Fibrosis by MicroRNA. Int. J. Mol. Sci. 2021, 22, 8139. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Rodrigues, P.M.; Simão, A.L.; Castro, R.E. Circulating microRNAs as Potential Biomarkers in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J. Clin. Med. 2016, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Venugopal, S.K. Circulating microRNAs: Possible Role as Non-Invasive Diagnostic Biomarkers in Liver Disease. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Cao, H.X.; Fan, J.G. MicroRNAs as Biomarkers and Regulators of Nonalcoholic Fatty Liver Disease. J. Dig. Dis. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Arciello, M.; Balsano, C. MicroRNAs in Nonalcoholic Fatty Liver Disease: Novel Biomarkers and Prognostic Tools during the Transition from Steatosis to Hepatocarcinoma. Biomed. Res. Int. 2014, 2014, 741465. [Google Scholar] [CrossRef]
- Becker, P.P.; Rau, M.; Schmitt, J.; Malsch, C.; Hammer, C.; Bantel, H.; Müllhaupt, B.; Geier, A. Performance of Serum microRNAs -122, -192 and -21 as Biomarkers in Patients with Non-Alcoholic Steatohepatitis. PLoS ONE 2015, 10, e0142661. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Yi, Z.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L.; Huang, F. Genome-Wide Analysis of Long Noncoding RNA Expression Profiles in Patients with Non-Alcoholic Fatty Liver Disease. IUBMB Life 2015, 67, 847–852. [Google Scholar] [CrossRef]
- Atanasovska, B.; Rensen, S.S.; van der Sijde, M.R.; Marsman, G.; Kumar, V.; Jonkers, I.; Withoff, S.; Shiri-Sverdlov, R.; Greve, J.W.M.; Faber, K.N.; et al. A Liver-Specific Long Noncoding RNA with a Role in Cell Viability Is Elevated in Human Nonalcoholic Steatohepatitis. Hepatology 2017, 66, 794–808. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, C.-H.; Wu, D.; Jiang, W.; Zhang, N.; Tang, H. LncRNA and circRNA in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Biomolecules 2023, 13, 560. [Google Scholar] [CrossRef]
- Lădaru, A.; Bălănescu, P.; Stan, M.; Codreanu, I.; Anca, I.A. Candidate Proteomic Biomarkers for Non-Alcoholic Fatty Liver Disease (Steatosis and Non-Alcoholic Steatohepatitis) Discovered with Mass-Spectrometry: A Systematic Review. Biomarker 2016, 21, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Trak-Smayra, V.; Dargere, D.; Noun, R.; Albuquerque, M.; Yaghi, C.; Gannagé-Yared, M.-H.; Bedossa, P.; Paradis, V. Serum Proteomic Profiling of Obese Patients: Correlation with Liver Pathology and Evolution after Bariatric Surgery. Gut 2009, 58, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Theodorakis, J.L.; Vuppalanchi, R.; Saxena, R.; Bemis, K.G.; Wang, M.; Chalasani, N. Serum Proteomics and Biomarker Discovery across the Spectrum of Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Geyer, P.E.; Wewer Albrechtsen, N.J.; Gluud, L.L.; Santos, A.; Doll, S.; Treit, P.V.; Holst, J.J.; Knop, F.K.; Vilsbøll, T.; et al. Plasma Proteome Profiling Discovers Novel Proteins Associated with Non-Alcoholic Fatty Liver Disease. Mol. Syst. Biol. 2019, 15, e8793. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Viker, K.; Krishnan, A.; Sanderson, S.; Veldt, B.; Kaalsbeek, A.J.; Kendrick, M.; Thompson, G.; Que, F.; Swain, J.; et al. Differential Expression of Lumican and Fatty Acid Binding Protein-1: New Insights into the Histologic Spectrum of Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.C.; Chu, X.; Argyropoulos, G.; Benotti, P.; Rolston, D.; Mirshahi, T.; Petrick, A.; Gabrielson, J.; Carey, D.J.; DiStefano, J.K.; et al. A Multi-Component Classifier for Nonalcoholic Fatty Liver Disease (NAFLD) Based on Genomic, Proteomic, and Phenomic Data Domains. Sci. Rep. 2017, 7, 43238. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wadhawan, S.; Greenfield, A.; Decato, B.E.; Oseini, A.M.; Collen, R.; Shevell, D.E.; Thompson, J.; Jarai, G.; Charles, E.D.; et al. SOMAscan Proteomics Identifies Serum Biomarkers Associated With Liver Fibrosis in Patients With NASH. Hepatol. Commun. 2021, 5, 760–773. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Dhawan, A. Noninvasive Biomarkers in Non-Alcoholic Fatty Liver Disease: Current Status and a Glimpse of the Future. World J. Gastroenterol. 2014, 20, 10851–10863. [Google Scholar] [CrossRef]
- Chen, C.; Schmilovitz-Weiss, H.; Liu, X.; Pappo, O.; Halpern, M.; Sulkes, J.; Braun, M.; Cohen, M.; Barak, N.; Tur-Kaspa, R.; et al. Serum Protein N-Glycans Profiling for the Discovery of Potential Biomarkers for Nonalcoholic Steatohepatitis. J. Proteome Res. 2009, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, F.; Coilly, A.; Kadar, H.; Gual, P.; Tran, A.; Desterke, C.; Samuel, D.; Duclos-Vallée, J.-C.; Touboul, D.; Bertrand-Michel, J.; et al. Metabolism Dysregulation Induces a Specific Lipid Signature of Nonalcoholic Steatohepatitis in Patients. Sci. Rep. 2017, 7, 46658. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.-K.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The Plasma Lipidomic Signature of Nonalcoholic Steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Mayo, R.; Crespo, J.; Arranz, I.; Banales, J.; Arias-Loste, M.; Mincholé, I.; Aller, R.; Agüero, R.; Alonso, C.; de Luis, D.; et al. Metabolomic-Based Noninvasive Serum Test to Diagnose Nonalcoholic Steatohepatitis: Results From Discovery and Validation Cohorts. Hepatol. Commun. 2018, 2, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Hyötyläinen, T.; Kotronen, A.; Gopalacharyulu, P.; Nygren, H.; Arola, J.; Castillo, S.; Mattila, I.; Hakkarainen, A.; Borra, R.J.H.; et al. Prediction of Non-Alcoholic Fatty-Liver Disease and Liver Fat Content by Serum Molecular Lipids. Diabetologia 2013, 56, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Chuang, J.-C.; Billin, A.; Hu, T.; Wang, Y.; Subramanian, G.M.; Djedjos, C.S.; Myers, R.P.; Dennis, E.A.; Loomba, R. Plasma Eicosanoids as Noninvasive Biomarkers of Liver Fibrosis in Patients with Nonalcoholic Steatohepatitis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923904. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kobayashi, T.; Honda, Y.; Kessoku, T.; Tomeno, W.; Imajo, K.; Nakahara, T.; Oeda, S.; Nagaoki, Y.; Amano, Y.; et al. Metabolomic/Lipidomic-Based Analysis of Plasma to Diagnose Hepatocellular Ballooning in Patients with Non-Alcoholic Fatty Liver Disease: A Multicenter Study. Hepatol. Res. 2020, 50, 955–965. [Google Scholar] [CrossRef]
- Yamada, K.; Mizukoshi, E.; Seike, T.; Horii, R.; Terashima, T.; Iida, N.; Kitahara, M.; Sunagozaka, H.; Arai, K.; Yamashita, T.; et al. Serum C16:1n7/C16:0 Ratio as a Diagnostic Marker for Non-Alcoholic Steatohepatitis. J. Gastroenterol. Hepatol. 2019, 34, 1829–1835. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, M.K.; Puri, P.; Koo, B.K.; Joo, S.K.; Jang, S.Y.; Lee, D.H.; Jung, Y.J.; Kim, B.G.; Lee, K.L.; et al. Circulating Lipidomic Alterations in Obese and Non-Obese Subjects with Non-Alcoholic Fatty Liver Disease. Aliment. Pharmacol. Ther. 2020, 52, 1603–1614. [Google Scholar] [CrossRef]
- Almeda-Valdés, P.; Cuevas-Ramos, D.; Aguilar-Salinas, C.A. Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease. Ann. Hepatol. 2009, 8 (Suppl S1), S18–S24. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma Metabolomic Profile in Nonalcoholic Fatty Liver Disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Caussy, C.; Ajmera, V.H.; Puri, P.; Hsu, C.L.-S.; Bassirian, S.; Mgdsyan, M.; Singh, S.; Faulkner, C.; Valasek, M.A.; Rizo, E.; et al. Serum Metabolites Detect the Presence of Advanced Fibrosis in Derivation and Validation Cohorts of Patients with Non-Alcoholic Fatty Liver Disease. Gut 2019, 68, 1884–1892. [Google Scholar] [CrossRef]
| Parameters Involved | FibroTest [16] | FibroMeter [10] | Hepascore [16] | NAFDL Fibrosis Score [16] | BARD Score [16] | AST/Platelet Ratio Index (APRI) * [17] | Hepamet Fibrosis Score [18] |
|---|---|---|---|---|---|---|---|
| Liver function tests | Bilirubin GGT | Serum levels of ALT, AST | GGT Bilirubin | AST/ALT ratio Serum albumin level | AST/ALT ratio | AST elevation | AST (UI/L) Albumin (g/dL) |
| Anthropometric data | Age | Body weight | Age Sex | Age BMI | BMI | Age Sex | |
| Parameters of metabolic comorbidity | Fasting glucose | Diabetes mellitus status Fasting glucose level | Diabetes mellitus status | Insulin (μU/mL) HOMA Diabetes mellitus status Glucose (mg/dL) | |||
| Other parameters | Apolipoprotein A1 α2-macroglobulin | Ferritin Prothrombin index | HA a2-macroglobulin | PLT count | PLT count | PLT (×109) |
| Gene | Role of Encoded Protein(s) | Variant | Effect of Altered Gene Variant |
|---|---|---|---|
| PNPLA3 [182] | Patatin-like phospholipase-domain-containing protein 3 (PNPLA3) = adiponutrin → involved in TG and retinoid metabolism | rs738409 | Abnormal storage of triglycerides |
| Transmembrane 6 superfamily member 2 (TM6SF2) [182] | TM6SF2 protein → involved in secretion of apolipoprotein B | rs58542926 C > T→ a loss-of-function mutation | Higher liver TG content and lower circulating lipoproteins |
| Glucokinase regulator (GCKR) gene [182] | Glucokinase regulatory protein (GKRP) → involved in the regulation of glucose influx to hepatocytes (2) | rs780094 A > G and rs1260326 C > T variant (P446L) | Increased hepatic glucose uptake and increased de novo lipogenesis |
| Membrane-bound O-Acyltransferase domain containing 7 (MBOAT7) [182] | Lysophosphatidylinositol (LPI) acyltransferase 1 → involved in the regulation of free arachidonic acid | rs641738 C > T→ a loss-of-function mutation | Increased free polyunsaturated fatty acids and proinflammatory eicosanoid lipids |
| HSD17B13 [182] | A lipid droplet enzyme retinol dehydrogenase | rs72613567:TA | Decreased inflammation, Ballooning, and fibrosis → a protective variant |
| Apolipoprotein B (APOB) [144] | ApoB protein → involved in the formation and secretion of hepatic VLDL and chylomicrons | Loss-of-function mutations | Decreased serum cholesterol and increased intrahepatic triglycerides |
| Proprotein convertase subtilisin kexin 9 (PCSK9) [144] | Serine protease → promotes the degradation of LDL receptors | Loss-of-function mutations | Decreased serum cholesterol |
| Microsomal triglyceride-transfer protein (MTTP) [144] | A lipid transfer protein → involved in VLDL particle formation; chaperone for apoB | Different mutations | Abetalipoproteinemia and accumulation of hepatic triglycerides |
| APOC3 [144] | Apolipoprotein C3 (apoC3) → involved VLDL particles formation | Different mutations | Hypertriglyceridemia |
| LIPA [144] | Lysosomal acid lipase (LIPA) → hydrolysis cholesteryl esters, triglycerides, and LDL particles | Loss-of-function mutations | Hypercholesterolemia, cardiovascular disease, hepatic steatosis, and cirrhosis |
| FATP5 [144] | Fatty acid transport protein → increases hepatic fatty acid uptake | rs56225452 | Promotes hepatic steatosis and determines insulin resistance |
| LPIN1 [144] | A phosphatidate phosphatase transcriptional coactivator, which controls fatty acid metabolism | rs12412852 | Reduced lipolysis |
| miRNA | Expression | Role | Predictive Value |
|---|---|---|---|
| miR-122 [214] | Up-regulated | Regulates several genes involved in the lipid metabolism such as fatty acid synthetase, acetyl-coenzyme-A carboxylase-2, or HMG CoA reductase | Indicator for steatosis and fibrosis stage |
| miR-34a [214,215] | Up-regulated | Down-regulates the PPARα signaling pathway and induces lipid accumulation in hepatocytes | Indicator for fibrosis, steatosis, and inflammation |
| miR-16 [216] | Up-regulated in NASH but down-regulated in fibrosis | Inhibits the expression of several anti-apoptotic genes | Correlated with liver inflammation and useful for predicting NAFLD-HCC progression |
| miR-21 [217] | Up-regulated | Regulates hepatic fat accumulation targeting HMGCR, FABP7 [218] | Indicator for fibrosis and inflammation |
| miR-10b [219] | Up-regulated | Regulates the nuclear receptor called peroxisome proliferator-activated receptor-α (PPAR-α) | Indicator for steatosis |
| miR-192 [209] | Up-regulated | A target of TGFβ1 [216] | Discriminator between NAFLD and NASH |
| miR-29b [212] | Up-regulated | Regulates lipid metabolism | Indicator for steatosis |
| miR-132 [213] | Up-regulated | A target of Sirt1 | Indicator for steatosis |
| miR-199a [219] | Up-regulated in NASH and fibrosis but down-regulated in steatosis | A target of NCOR1 [218] | Positively correlated with fibrosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gîlcă-Blanariu, G.-E.; Budur, D.S.; Mitrică, D.E.; Gologan, E.; Timofte, O.; Bălan, G.G.; Olteanu, V.A.; Ștefănescu, G. Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease. Metabolites 2023, 13, 1115. https://doi.org/10.3390/metabo13111115
Gîlcă-Blanariu G-E, Budur DS, Mitrică DE, Gologan E, Timofte O, Bălan GG, Olteanu VA, Ștefănescu G. Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease. Metabolites. 2023; 13(11):1115. https://doi.org/10.3390/metabo13111115
Chicago/Turabian StyleGîlcă-Blanariu, Georgiana-Emmanuela, Daniela Simona Budur, Dana Elena Mitrică, Elena Gologan, Oana Timofte, Gheorghe Gh Bălan, Vasile Andrei Olteanu, and Gabriela Ștefănescu. 2023. "Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease" Metabolites 13, no. 11: 1115. https://doi.org/10.3390/metabo13111115
APA StyleGîlcă-Blanariu, G.-E., Budur, D. S., Mitrică, D. E., Gologan, E., Timofte, O., Bălan, G. G., Olteanu, V. A., & Ștefănescu, G. (2023). Advances in Noninvasive Biomarkers for Nonalcoholic Fatty Liver Disease. Metabolites, 13(11), 1115. https://doi.org/10.3390/metabo13111115







