GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro
Abstract
1. Introduction
2. Materials and Methods
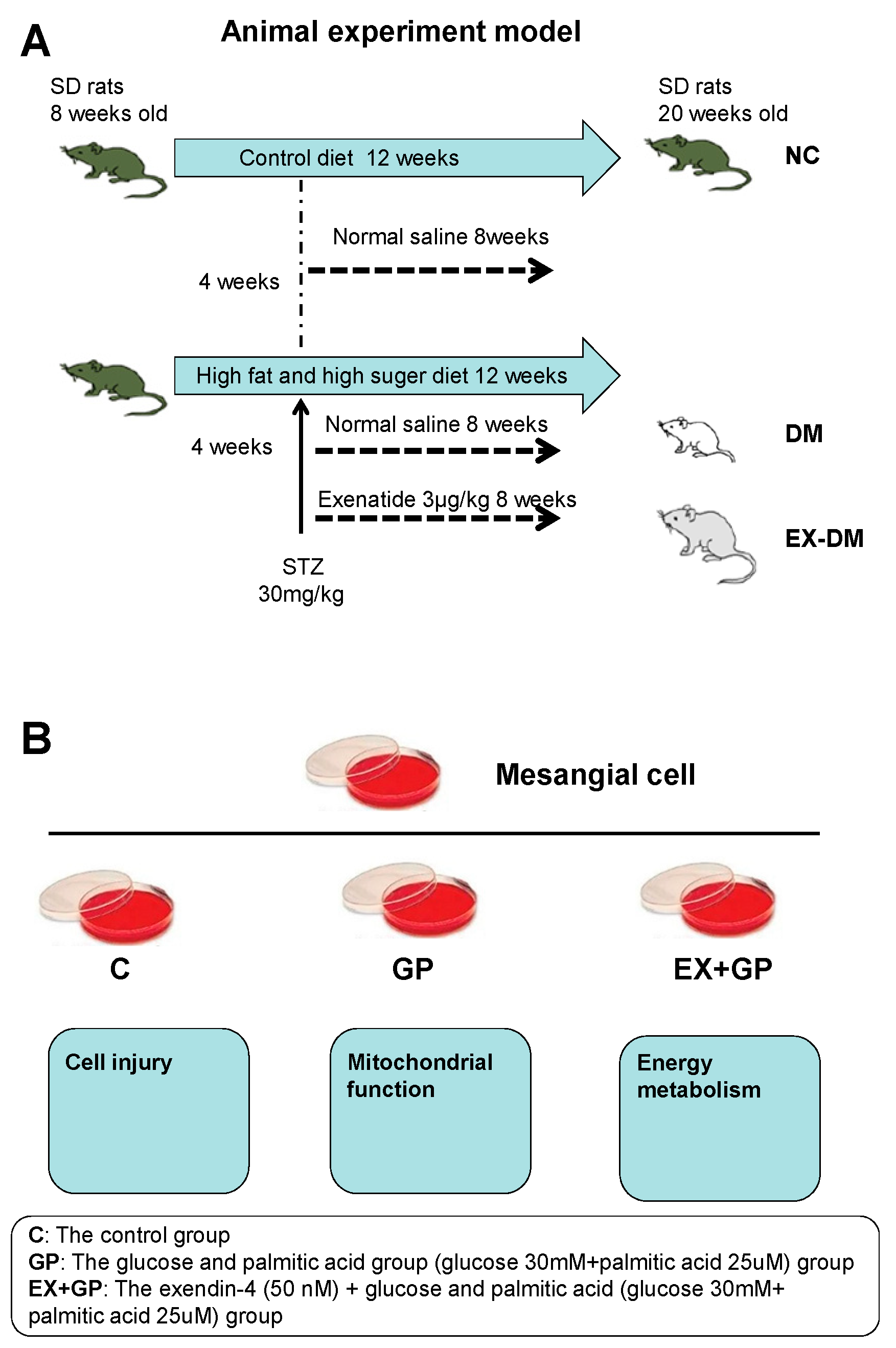
2.1. Animals and Treatments
2.2. Cell Culture and Treatments
2.3. Biological Parameters
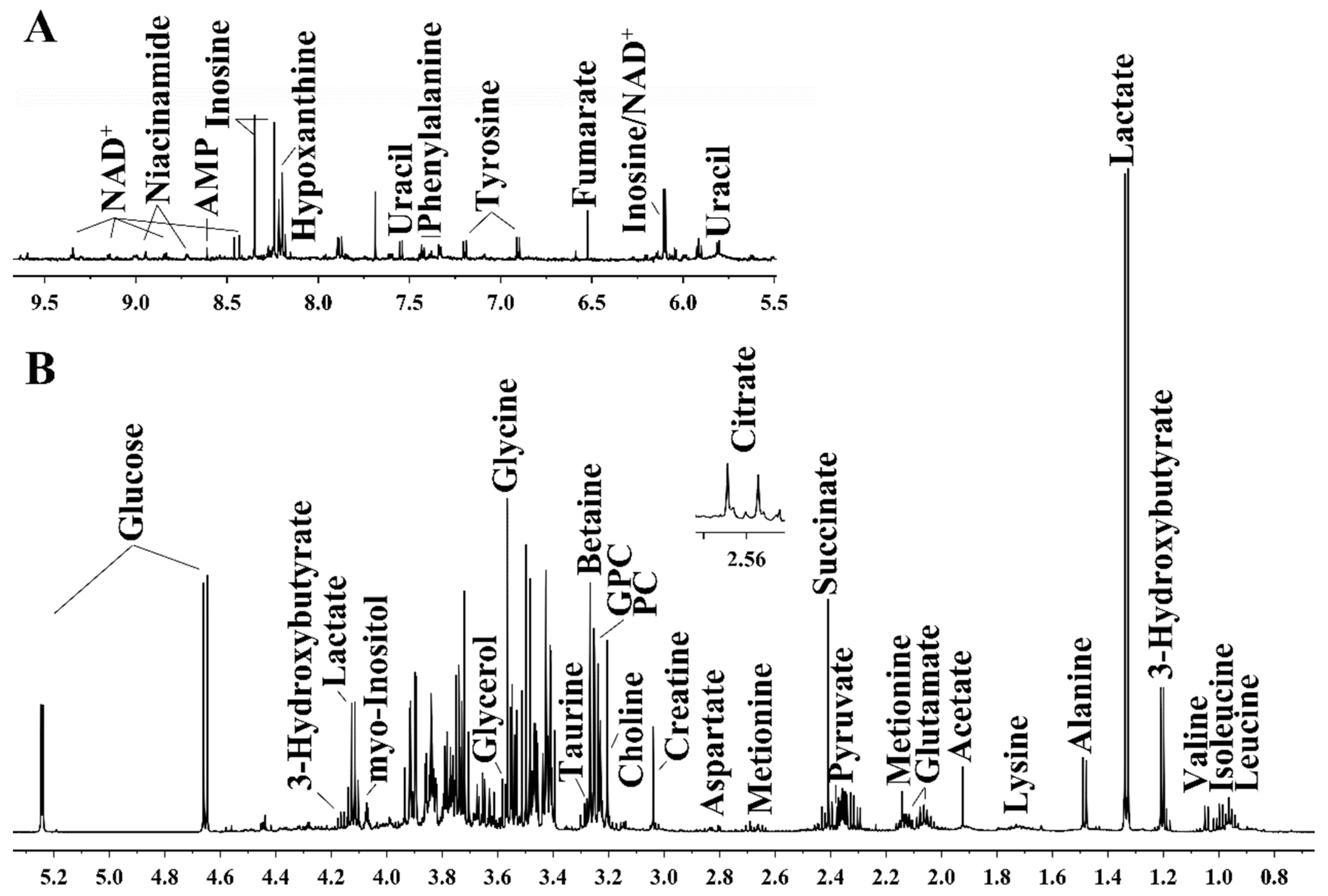
2.4. Preparation of Aqueous Kidney Extracts and Acquisition of 1H NMR Spectra
2.5. Cell Proliferation Assay
2.6. Hoechst 33342 Staining
2.7. ELISA
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
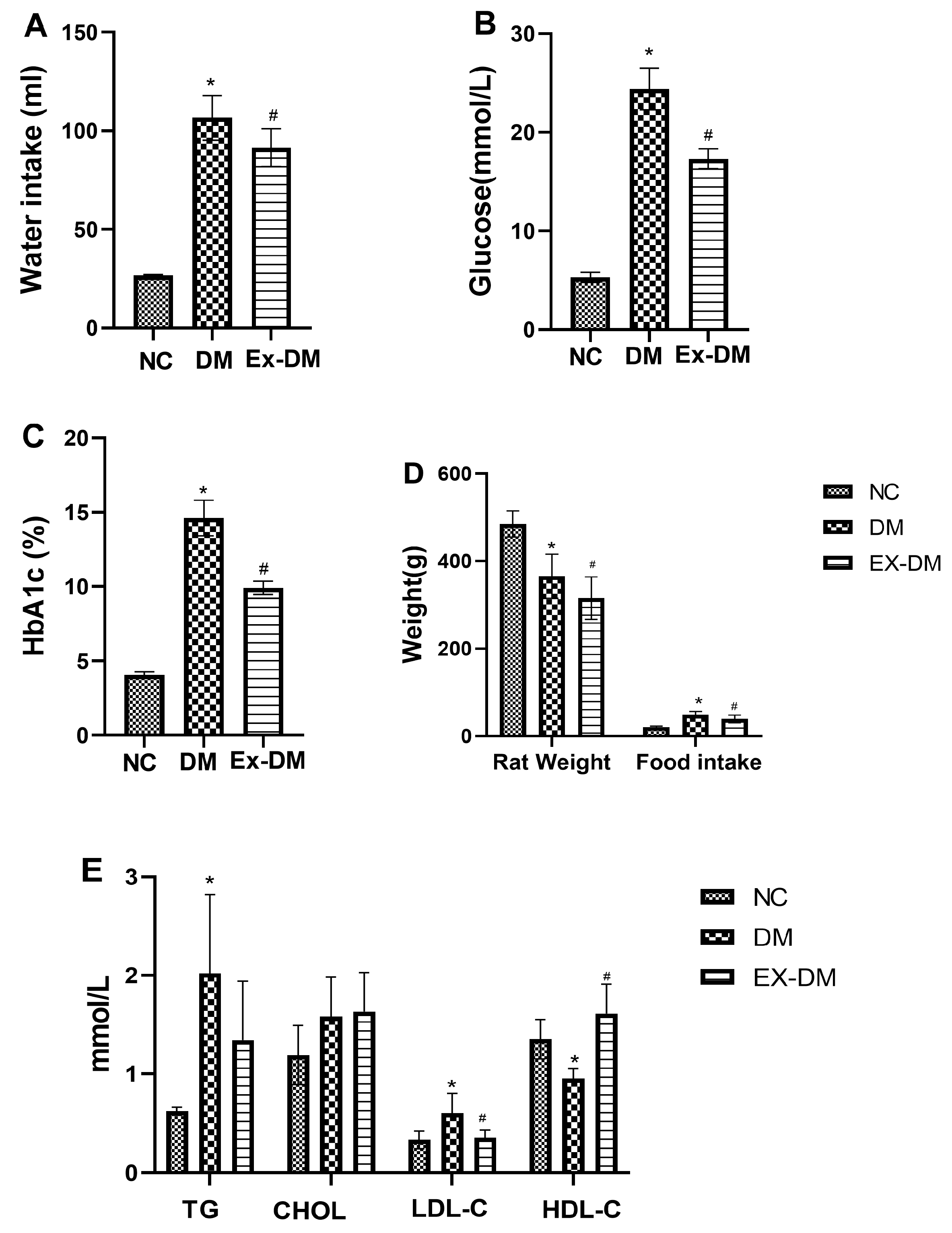
3.1. Effects of Exenatide on Plasma HbA1c Levels, Serum Fasting Blood Glucose, and Lipid Profiles in Rats with DM
3.2. Effects of Exenatide on Kidney Functions in DM-Model Rats
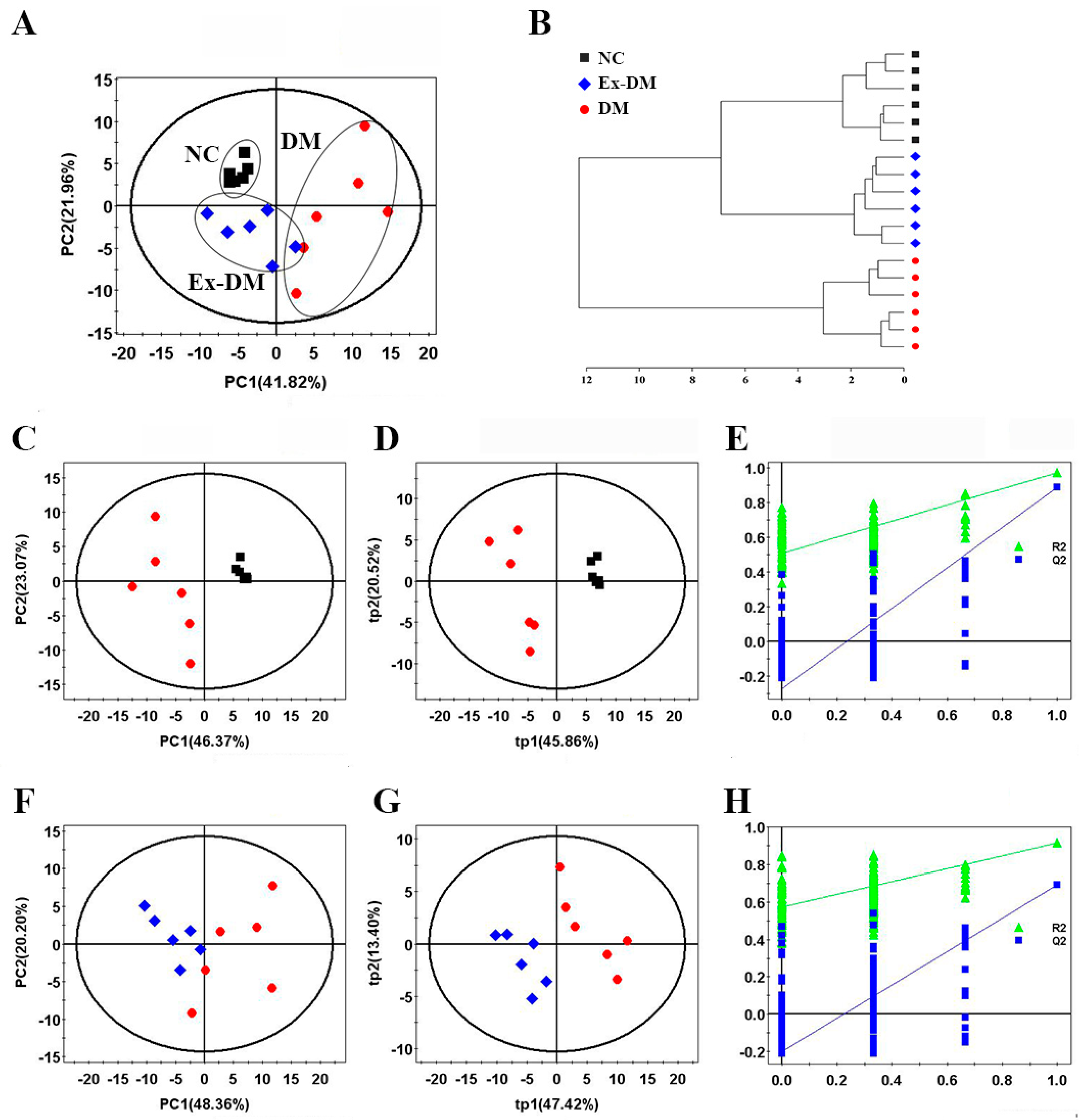
3.3. Exenatide Ameliorates Renal Metabolic Disorders in DM-Model Rats
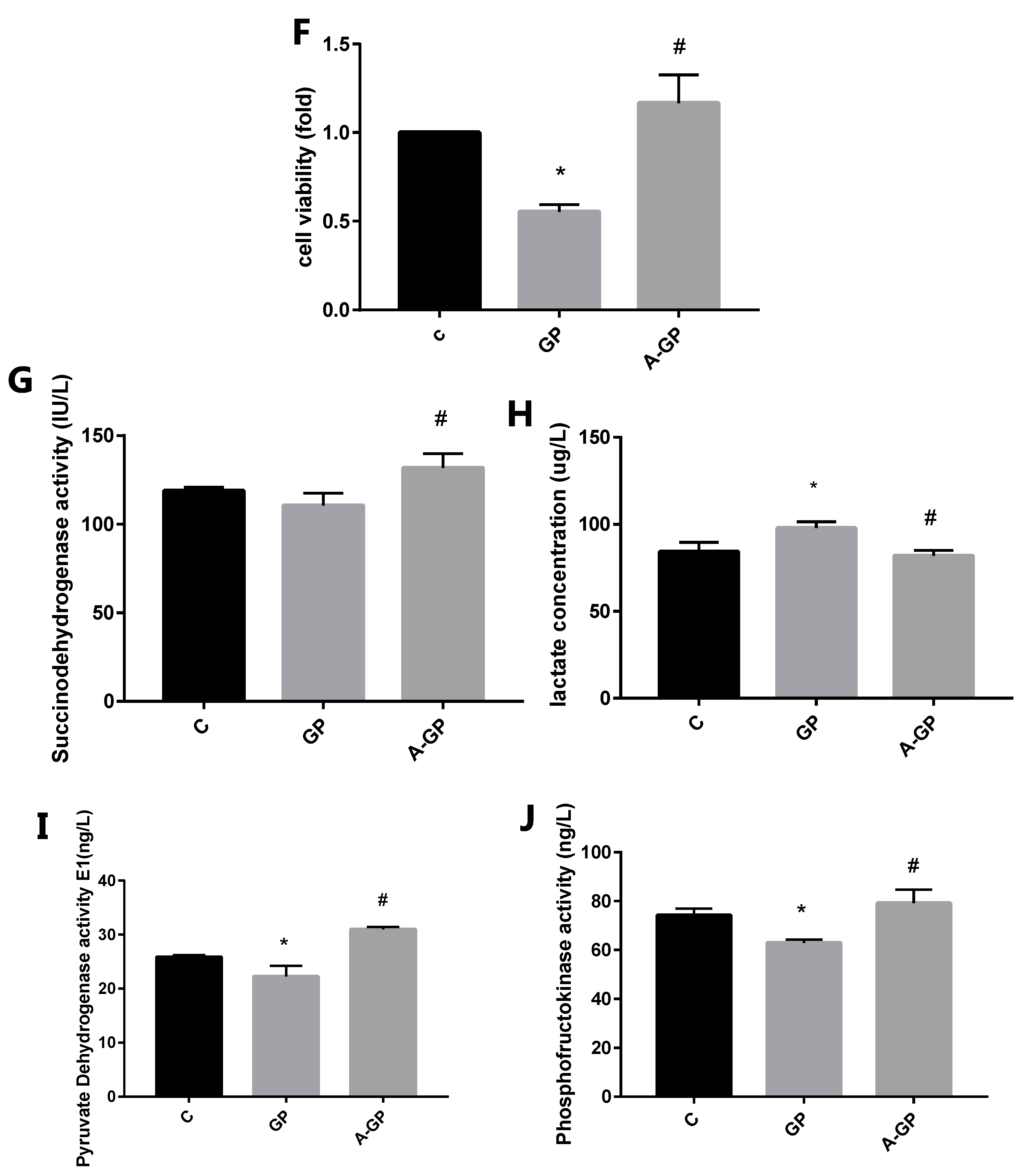
3.4. Exendin-4 Inhibits HFHG-Induced Injury in Mesangial Cells
3.5. Effect of Exendin-4 on Mitochondrial Function in Mesangial Cells
3.6. Exendin-4 Improves Glucose Metabolism and the TCA Cycle in Mesangial Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D.; et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA 2017, 317, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, S.; Cariou, B.; Fumeron, F.; Gand, E.; Charpentier, G.; Roussel, R.; Kasmi, A.-A.; Gautier, J.-F.; Mohammedi, K.; Gourdy, P.; et al. Death, end-stage renal disease and renal function decline in patients with diabetic nephropathy in French cohorts of type 1 and type 2 diabetes. Diabetologia 2016, 59, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Dom, Z.I.M.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.-J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Loretelli, C.; Rocchio, F.; D’Addio, F.; Nasr, M.B.; Castillo-Leon, E.; Dellepiane, S.; Vergani, A.; Abdelsalam, A.; Assi, E.; Maestroni, A.; et al. The IL-8-CXCR1/2 axis contributes to diabetic kidney disease. Metabolism 2021, 121, 154804. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.J.N.; Østergaard, J.; Flyvbjerg, A. AGE-RAGE and AGE Cross-link Interaction: Important Players in the Pathogenesis of Diabetic Kidney Disease. Horm. Metab. Res. 2005, 37, 26–34. [Google Scholar] [CrossRef]
- Fiorina, P.; Vergani, A.; Bassi, R.; Niewczas, M.A.; Altintas, M.M.; Pezzolesi, M.G.; D’Addio, F.; Chin, M.; Tezza, S.; Nasr, M.B.; et al. Role of podocyte B7-1 in diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1415–1429. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahsan, H.; Khan, M.R.; Siddiqui, W.A. Protective effects of tocotrienols against lipid-induced nephropathy in experimental type-2 diabetic rats by modulation in TGF-β expression. Toxicol. Appl. Pharmacol. 2013, 273, 314–324. [Google Scholar] [CrossRef]
- Scholz, H.; Boivin, F.J.; Schmidt-Ott, K.M.; Bachmann, S.; Eckardt, K.-U.; Scholl, U.I.; Persson, P.B. Kidney physiology and susceptibility to acute kidney injury: Implications for renoprotection. Nat. Rev. Nephrol. 2021, 17, 335–349. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Tang, C.; Cai, J.; Yin, X.-M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 299–318. [Google Scholar] [CrossRef]
- Liu, L.; Bai, F.; Song, H.; Xiao, R.; Wang, Y.; Yang, H.; Ren, X.; Li, S.; Gao, L.; Ma, C.; et al. Upregulation of TIPE1 in tubular epithelial cell aggravates diabetic nephropathy by disrupting PHB2 mediated mitophagy. Redox Biol. 2022, 50, 102260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Coughlan, M.T.; Cooper, M.E. The postprandial actions of GLP-1 receptor agonists: The missing link for cardiovascular and kidney protection in type 2 diabetes. Cell Metab. 2023, 35, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, X.; Chen, Y.; Huang, Y.; Wu, L.; He, J. Exendin-4 attenuates cardiac hypertrophy via AMPK/mTOR signaling pathway activation. Biochem. Biophys. Res. Commun. 2015, 468, 394–399. [Google Scholar] [CrossRef]
- Skov, J. Effects of GLP-1 in the kidney. Rev. Endocr. Metab. Disord. 2014, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Shaman, A.M.; Bain, B.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.E.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients with Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 2022, 145, 575–585. [Google Scholar] [CrossRef]
- Xu, W.-W.; Guan, M.-P.; Zheng, Z.-J.; Gao, F.; Zeng, Y.-M.; Qin, Y.; Xue, Y.-M. Exendin-4 alleviates high glucose-induced rat mesangial cell dysfunction through the AMPK pathway. Cell. Physiol. Biochem. 2014, 33, 423–432. [Google Scholar] [CrossRef]
- Bendotti, G.; Montefusco, L.; Lunati, M.E.; Usuelli, V.; Pastore, I.; Lazzaroni, E.; Assi, E.; Seelam, A.J.; Essawy, B.E.; Jang, J.; et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022, 182, 106320. [Google Scholar] [CrossRef]
- Bassi, R.; Niewczas, M.A.; Biancone, L.; Bussolino, S.; Merugumala, S.; Tezza, S.; D’Addio, F.; Nasr, M.B.; Valderrama-Vasquez, A.; Usuelli, V.; et al. Metabolomic Profiling in Individuals with a Failing Kidney Allograft. PLoS ONE 2017, 12, e0169077. [Google Scholar] [CrossRef]
- Pereira, P.R.; Carrageta, D.F.; Oliveira, P.F.; Rodrigues, A.; Alves, M.G.; Monteiro, M.P. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease. Med. Res. Rev. 2022, 42, 1518–1544. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, H.; Zhao, Y.; Lin, D. Metabonomic analysis of the therapeutic effect of Zhibai Dihuang Pill in treatment of streptozotocin-induced diabetic nephropathy. J. Ethnopharmacol. 2012, 142, 647–656. [Google Scholar] [CrossRef]
- Czajka, A.; Malik, A.N. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016, 10, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Xu, Y.; Abraham, A.G.; Coresh, J.; Chen, J.; Grams, M.E.; Feldman, H.I.; Kimmel, P.L.; Rebholz, C.M.; Rhee, E.; et al. Metabolite Biomarkers of CKD Progression in Children. Clin. J. Am. Soc. Nephrol. 2021, 16, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Wang, L.; Qiu, S.; Yao, Y.; Yan, C.; Xiong, X.; Chen, X.; Ji, Q.; Cao, J.; et al. NAD supplement potentiates tumor-killing function by rescuing defective TUB-mediated NAMPT transcription in tumor-infiltrated T cells. Cell Rep. 2021, 36, 109516. [Google Scholar] [CrossRef] [PubMed]
- Shayman, J.A. Targeting Glycosphingolipid Metabolism to Treat Kidney Disease. Nephron 2016, 134, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Mi, N.; Zhang, X.J.; Ding, Y.; Li, G.H.; Wang, W.D.; Xian, H.X.; Xu, J. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochem. Biophys. Res. Commun. 2015, 466, 240–246. [Google Scholar] [CrossRef]
- Yip, J.; Geng, X.; Shen, J.; Ding, Y. Cerebral Gluconeogenesis and Diseases. Front Pharmacol. 2016, 7, 521. [Google Scholar] [CrossRef]
- Perseghin, G.; Fiorina, P.; De Cobelli, F.; Scifo, P.; Esposito, A.; Canu, T.; Danna, M.; Gremizzi, C.; Secchi, A.; Luzi, L.; et al. Cross-sectional assessment of the effect of kidney and kidney-pancreas transplantation on resting left ventricular energy metabolism in type 1 diabetic-uremic patients: A phosphorous-31 magnetic resonance spectroscopy study. J. Am. Coll. Cardiol. 2005, 46, 1085–1092. [Google Scholar] [CrossRef][Green Version]
- Caamaño, G.J.; Sanchez-Del-Castiool, M.A.; Linares, A.; Garcia-Peregrin, E. In vivo lipid and amino acid synthesis from 3-hydroxybutyrate in 15-day-old chick. Arch. Int. Physiol.Biochim. 1990, 98, 217–224. [Google Scholar]
- Reusch, R.N.; Bryant, E.M.; Henry, D.N. Increased poly-(R)-3-hydroxybutyrate concentrations in streptozotocin (STZ) diabetic rats. Acta Diabetol. 2003, 40, 91–94. [Google Scholar] [CrossRef]
- Richter, M.; Moroniak, S.J.; Michel, H. Identification of competitive inhibitors of the human taurine transporter TauT in a human kidney cell line. Pharmacol. Rep. 2019, 71, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Ito, T.; Prentice, H.; Wu, J.-Y.; Schaffer, S.W. Role of Mitochondria and Endoplasmic Reticulum in Taurine-Deficiency-Mediated Apoptosis. Nutrients 2017, 9, 795. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Guo, J.; Zhang, Y.; Zhang, J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016, 7, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Basak, P.; Ghosh, S.; Kundu, M.; Sil, P.C. Prophylactic role of taurine and its derivatives against diabetes mellitus and its related complications. Food Chem. Toxicol. 2017, 110, 109–121. [Google Scholar] [CrossRef]
- Hirata, K.; Kume, S.; Araki, S.; Sakaguchi, M.; Chin-Kanasaki, M.; Isshiki, K.; Sugimoto, T.; Nishiyama, A.; Koya, D.; Haneda, M.; et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem. Biophys. Res. Commun. 2009, 380, 44–49. [Google Scholar] [CrossRef]
- Liu, Q.; Adams, L.; Broyde, A.; Fernandez, R.; Baron, A.D.; Parkes, D.G. The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc. Diabetol. 2010, 9, 32. [Google Scholar] [CrossRef]
- Girardi Adriana, C.C.; Di Sole, F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am. J. Physiol. Cell Physiol. 2012, 302, C1569–C1587. [Google Scholar] [CrossRef]
- Rieg, T.; Gerasimova, M.; Murray, F.; Masuda, T.; Tang, T.; Rose, M.; Drucker, D.J.; Vallon, V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Renal. Physiol. 2012, 303, F963–F971. [Google Scholar] [CrossRef]
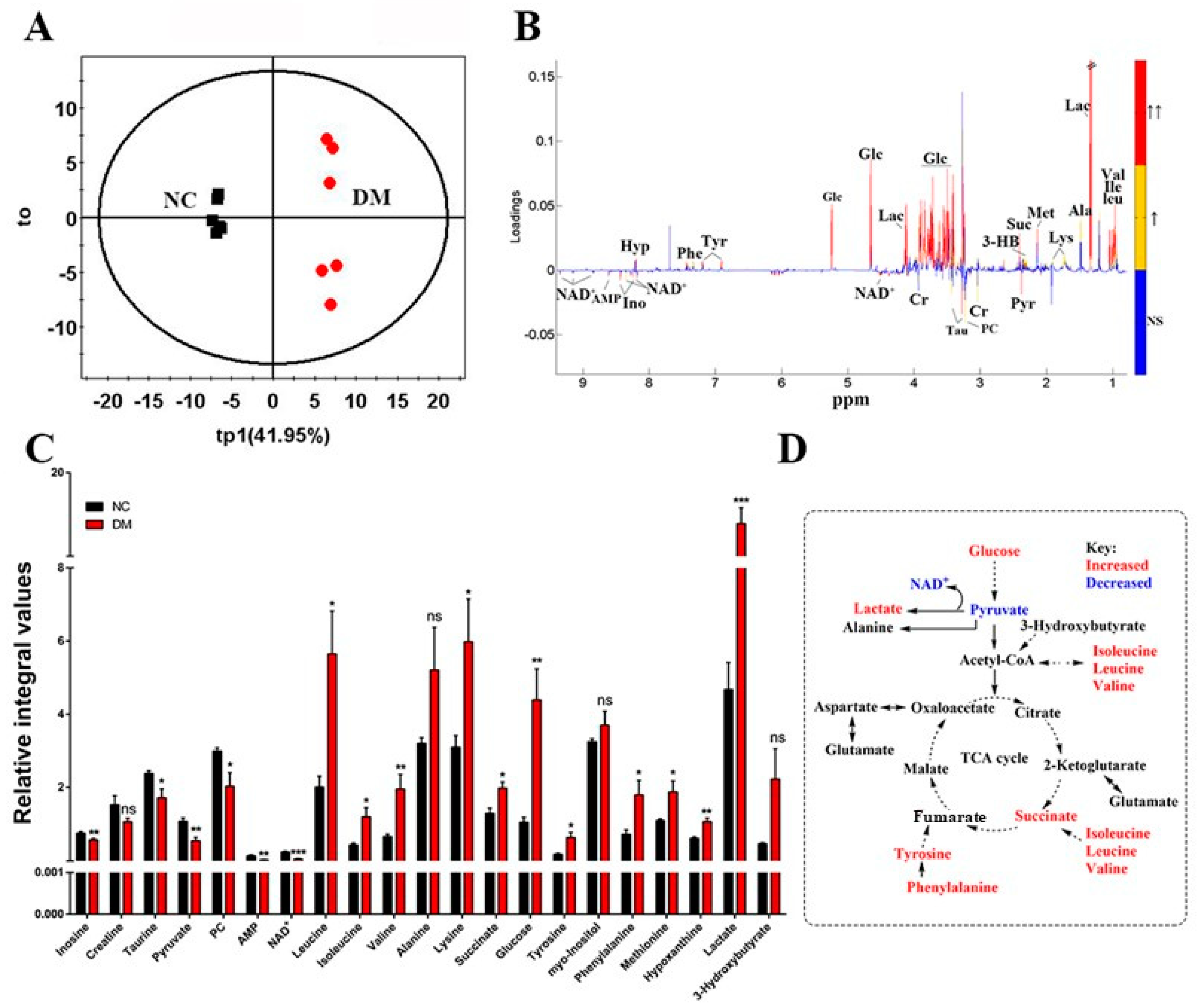
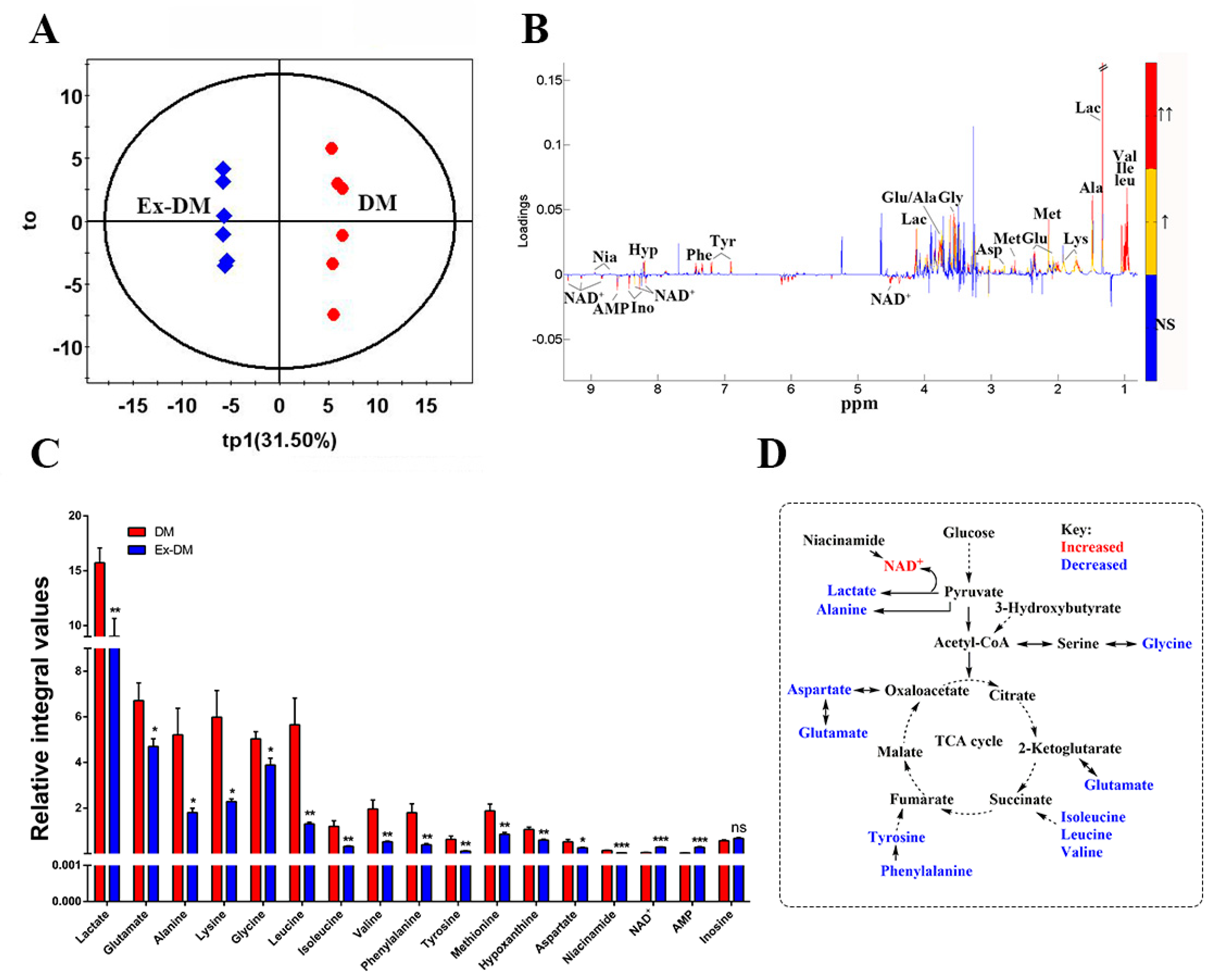
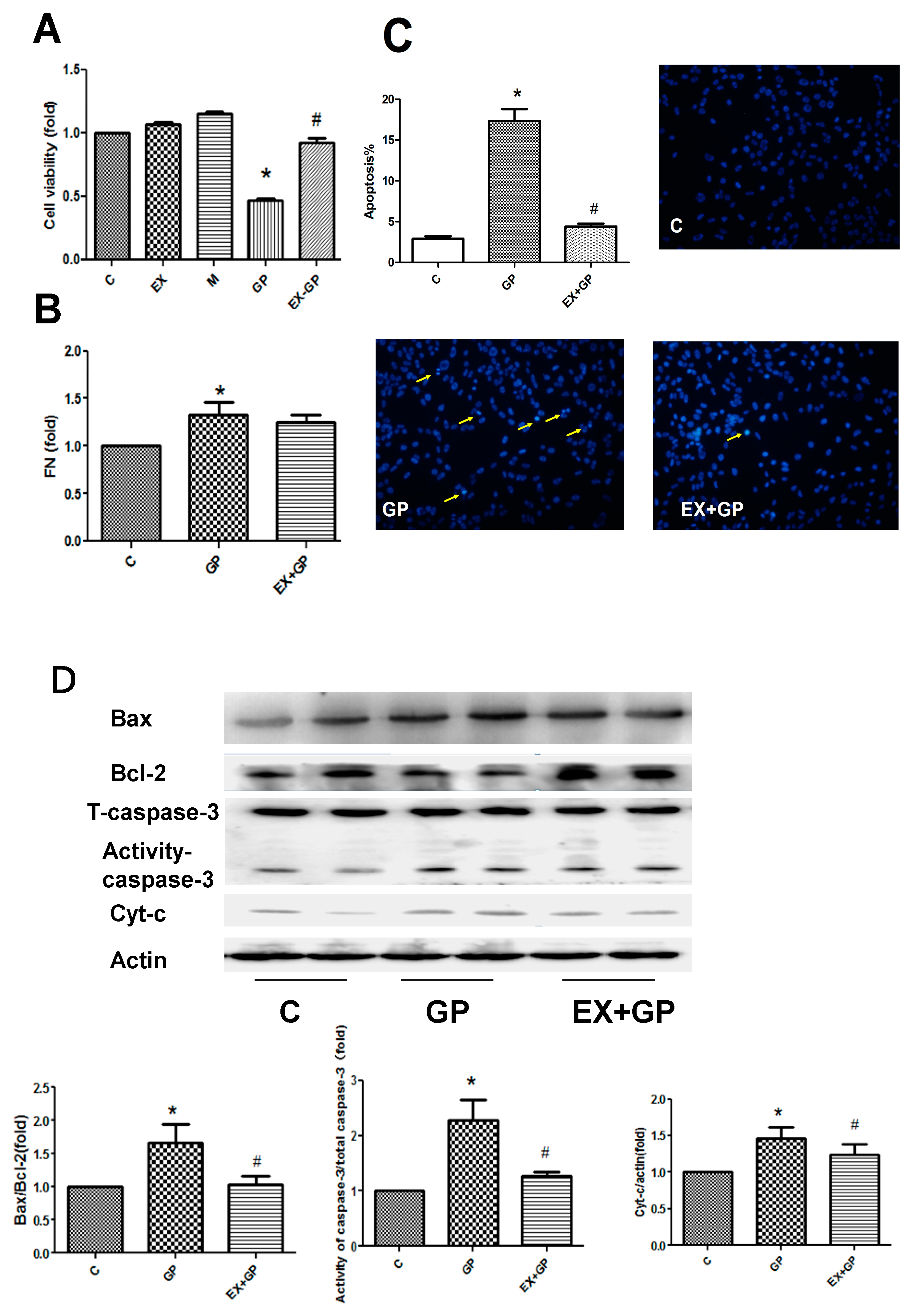
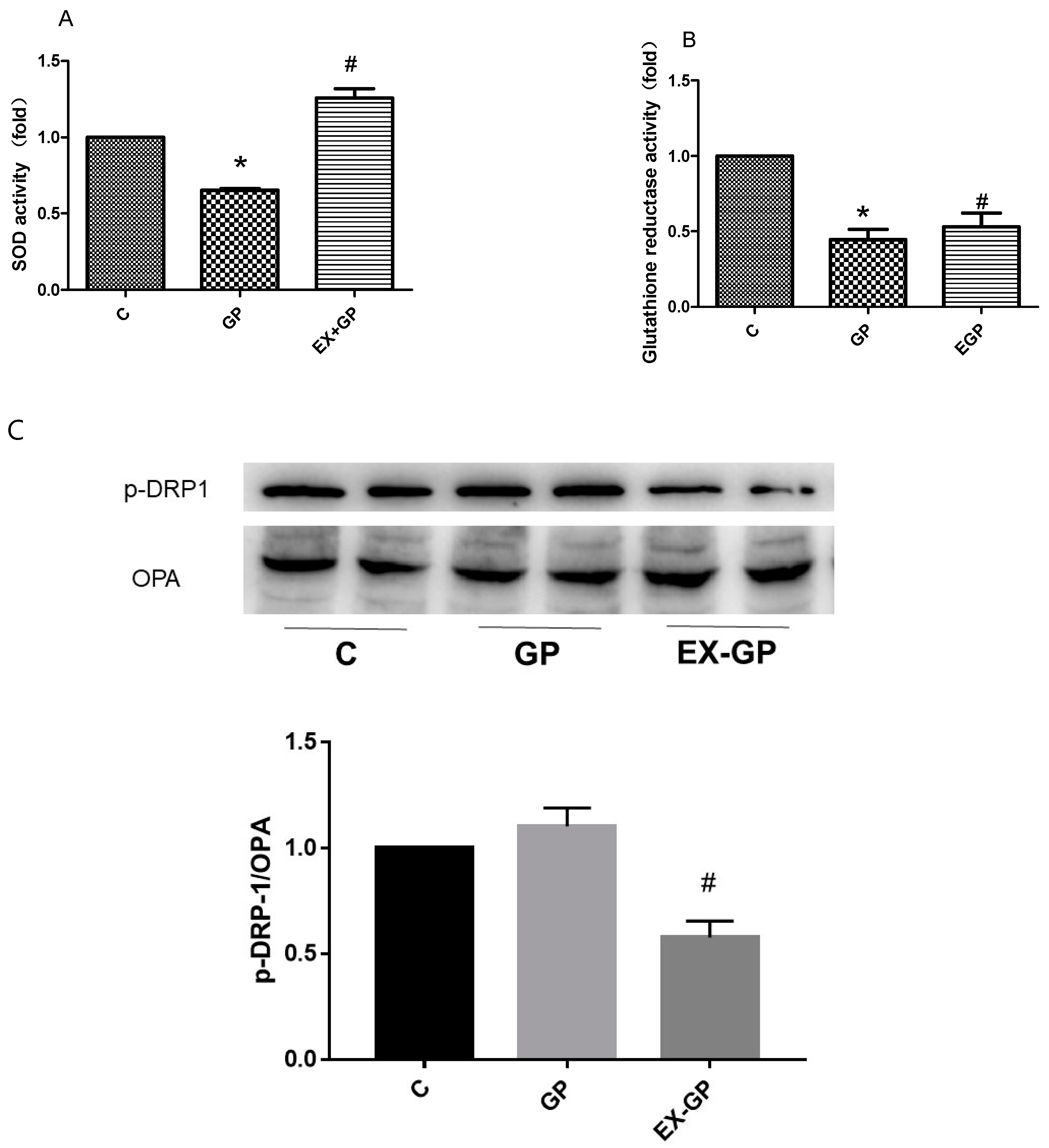

| NC | DM | Ex-DM | |
|---|---|---|---|
| Renal index | 3.34 ± 0.4 | 6.63 ± 0.7 * | 4.69 ± 0.9 # |
| BUN (mmol/L) | 6.6 ± 1.4 | 7.7 ± 2.1 | 7.1 ± 2.9 |
| CREA (µmol/L) | 50.36 ± 9.0 | 61.25 ± 8.5 | 51.23 ± 4.1 |
| 24 h UMA (mg) | 69.7 ± 8.5 | 494.7.2 ± 101.6 * | 425.3 + 95.2 # |
| NO. | Metabolites | δ 1H (ppm) and Multiplicity | Moieties |
|---|---|---|---|
| 1 | Leucine | 0.95 (d), 0.96 (d), 1.70 (m), 1.70 (m), 1.73 (m), 3.73 (m) | α-CH3, α-CH3, γ-CH, β-CH2, α-CH |
| 2 | Isoleucine | 0.92 (t), 1.00 (d), 1.24 (m), 1.45 (m), 1.97 (m), 3.66 (d) | δ-CH3, γ-CH3, half γ-CH2, half γ-CH2, β-CH, α-CH |
| 3 | Valine | 0.98 (d), 1.03 (d), 2.26 (m), 3.61 (d) | γ-CH3, γ-CH3, β-CH, α-CH |
| 4 | Alanine | 1.48 (d), 3.77 (dd) | β-CH3, α-CH |
| 5 | Acetate | 1.92 (s) | CH3 |
| 6 | Glutamate | 2.04 (m), 2.12 (m), 2.33 (m), 2.37 (m), 3.75 (dd) | half β-CH2, half β-CH2, half γ-CH2, half γ-CH2, α-CH |
| 7 | Aspartate | 2.68 (dd), 2.80 (dd), 3.89 (dd) | β-CH2; α-CH |
| 8 | Creatine | 3.02 (s), 3.92 (s) | N-CH3, α-CH2 |
| 9 | Glycine | 3.57 (s) | α-CH2 |
| 10 | Glucose | β (3.24 (dd), 3.48 (t), 3.90 (dd)), α (3.54 (dd), 3.71 (t), 3.72 (dd), 3.83 (m)) | β(H2, H3, H5), α(H2, H3, H6) |
| 11 | Myo-inositol | 3.28 (t), 3.53 (dd), 3.63 (t), 4.07 (t) | 2CH, 4,6CH, 1,3CH, 5CH |
| 12 | Lactate | 1.32 (d), 4.10 (q) | β-CH3, α-CH |
| 13 | Fumarate | 6.51 (s) | CH |
| 14 | Tyrosine | 3.05 (dd), 3.19 (dd), 6.90 (d), 7.19 (d) | half β-CH2, half β-CH2, β-CH, α-CH |
| 15 | Phenylalanine | 3.12 (dd), 3.30 (dd), 3.99 (dd), 7.32 (d), 7.37 (t), 7.42 (t) | α-CH, half β-CH2, half β-CH2, α-CH, β-CH, γ-CH |
| 16 | NAD+ | 6.03 (d), 6.08 (s), 8.18 (s), 8.21 (m), 8.43 (s), 8.82 (d), 9.15 (d), 9.34 (s) | NH2, NH2(CO), δ-CH, β-CH, 2CH, γ-CH, α-CH |
| 17 | AMP | 6.14 (d), 8.25 (s), 8.57 (s) | NH2, δ-CH, 2CH |
| 18 | Methionine | 2.01 (m), 2.14 (s), 2.16 (m), 2.64 (t), 3.86 (m) | δ-CH3, γ-CH2, β-CH2 |
| 19 | Pyruvate | 2.37 (s) | CH3 |
| 20 | Succinate | 2.41 (s) | 2CH |
| 21 | Glycerol | 3.57 (dd), 3.62 (dd), 3.79 (m) | half 1CH2, half 3CH2, 2CH |
| 22 | Lysine | 1.43 (m), 1.49 (m), 1.70 (m), 1.90 (m), 3.02 (t), 3.75 (t) | half γ-CH2, half γ-CH2, δ-CH2, β-CH2, ε-CH2, α-CH |
| 23 | Citrate | 2.54 (d), 2.69 (d) | half-CH2, half-CH2 |
| 24 | 3-Hydroxybutyrate | 1.19 (d), 2.30 (dd), 2.39 (dd), 4.14 (m) | CH3, half α-CH2, half α-CH2, γ-CH |
| 25 | Betaine | 3.31 (s), 3.91 (s) | 3CH3, α-CH2 |
| 26 | Hypoxanthine | 8.19 (s), 8.21 (s) | CH, CH |
| 27 | Inosine | 3.79 (dd), 3.89 (dd), 4.28 (dd), 4.44 (dd), 4.78 (t), 6.09 (d), 8.22 (s), 8.34 (s) | half-CH2, half-CH2, CH, CH, CH, CH, CH |
| 28 | Taurine | 3.27 (t), 3.43 (t) | 1CH2, 2CH2 |
| 29 | Phosphocholine | 3.22 (s), 3.60 (t), 4.18 (m) | N-(CH3)3, N-CH2, CH2OH |
| 30 | GPC | 3.23 (s), 3.60 (dd), 3.68 (dd), 3.87 (m), 3.94 (m), 4.33 (m) | N-(CH3)3, half 1CH2, 2CH2, half 1CH2, half 3CH2, half 3CH2, 1CH2 |
| 31 | Choline | 3.21 (s), 3.51 (dd), 4.04 (t) | N-(CH3)3, N-CH2, CH2OH |
| 32 | Uracil | 7.56 (d), 5.81 (d) | α-CH, β-CH |
| 33 | Niacinamide | 8.9 (dd), 8.7 (dd), 8.2 (m), 7.6 (m) | N(CH), δ-CH, β-CH, γ-CH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Chen, Z.; Liu, X.; Wang, L.; Zhou, Y.; Huang, J.; Liu, Z.; Lin, D.; Liu, L. GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro. Metabolites 2023, 13, 1121. https://doi.org/10.3390/metabo13111121
Wang L, Chen Z, Liu X, Wang L, Zhou Y, Huang J, Liu Z, Lin D, Liu L. GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro. Metabolites. 2023; 13(11):1121. https://doi.org/10.3390/metabo13111121
Chicago/Turabian StyleWang, Linxi, Zhou Chen, Xiaoying Liu, Lijing Wang, Yu Zhou, Jingze Huang, Zhiqing Liu, Donghai Lin, and Libin Liu. 2023. "GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro" Metabolites 13, no. 11: 1121. https://doi.org/10.3390/metabo13111121
APA StyleWang, L., Chen, Z., Liu, X., Wang, L., Zhou, Y., Huang, J., Liu, Z., Lin, D., & Liu, L. (2023). GLP-1 Receptor Agonist Improves Mitochondrial Energy Status and Attenuates Nephrotoxicity In Vivo and In Vitro. Metabolites, 13(11), 1121. https://doi.org/10.3390/metabo13111121








