Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Collection
2.3. Biochemical Analysis
2.4. Nutritional Assessment
2.5. Statistical Analysis
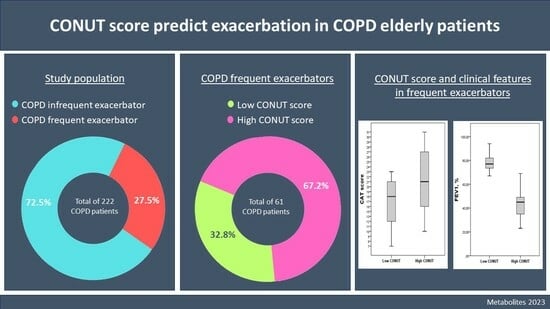
3. Results
3.1. Baseline Characteristics of Patients
3.2. Patients’ Characteristics According to CONUT Score in the Frequent Exacerbation Group
3.3. Multivariate Analysis for the Association of Risk Factors with AECOPD (Acute Exacerbations of Chronic Obstructive Pulmonary Disease)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agustí, A.; Celli, B.R.; Criner, G.J.; Halpin, D.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Han, M.K.; Martinez, F.J.; de Oca, M.M.; et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Eur. Respir. J. 2023, 61, 2300239. [Google Scholar] [CrossRef] [PubMed]
- Scioscia, G.; Blanco, I.; Arismendi, E.; Burgos, F.; Gistau, C.; Barbaro, M.P.F.; Celli, B.; O’Donnell, D.E.; Agustí, A. Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax 2016, 72, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Fei, Y.; Zhang, L.; Jie, Z.; Fan, X.; Dai, M.; Moore, M.; Willcox, M.; Hu, X.; Francis, N.; et al. Shufeng Jiedu capsule for acute exacerbation of chronic obstructive pulmonary disease: A protocol of multicentre, randomised, double-blind, placebo-controlled trial. BMJ Open 2023, 13, e070864. [Google Scholar] [CrossRef] [PubMed]
- Mete, B.; Pehlivan, E.; Gülbaş, G.; Günen, H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. SSRN Electron. J. 2018, 13, 3307–3312. [Google Scholar] [CrossRef]
- Kaluźniak-Szymanowska, A.; Krzymińska-Siemaszko, R.; Deskur-Śmielecka, E.; Lewandowicz, M.; Kaczmarek, B.; Wieczorowska-Tobis, K. Malnutrition, Sarcopenia, and Malnutrition-Sarcopenia Syndrome in Older Adults with COPD. Nutrients 2021, 14, 44. [Google Scholar] [CrossRef]
- Miano, N.; Di Marco, M.; Alaimo, S.; Coppolino, G.; L’episcopo, G.; Leggio, S.; Scicali, R.; Piro, S.; Purrello, F.; Di Pino, A. Controlling Nutritional Status (CONUT) Score as a Potential Prognostic Indicator of In-Hospital Mortality, Sepsis and Length of Stay in an Internal Medicine Department. Nutrients 2023, 15, 1554. [Google Scholar] [CrossRef]
- Buglio, A.L.; Bellanti, F.; Capurso, C.; Paglia, A.; Vendemiale, G. Adherence to Mediterranean Diet, Malnutrition, Length of Stay and Mortality in Elderly Patients Hospitalized in Internal Medicine Wards. Nutrients 2019, 11, 790. [Google Scholar] [CrossRef]
- Itoh, M.; Tsuji, T.; Nemoto, K.; Nakamura, H.; Aoshiba, K. Undernutrition in Patients with COPD and Its Treatment. Nutrients 2013, 5, 1316–1335. [Google Scholar] [CrossRef]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Buglio, A.L.; Bellanti, F.; Capurso, C.; Vendemiale, G. Controlling Nutritional Status (CONUT) Score as a Predictive Marker in Hospitalized Frail Elderly Patients. J. Pers. Med. 2023, 13, 1119. [Google Scholar] [CrossRef]
- Rinninella, E.; Borriello, R.; D’angelo, M.; Galasso, T.; Cintoni, M.; Raoul, P.; Impagnatiello, M.; Annicchiarico, B.E.; Gasbarrini, A.; Mele, M.C. COntrolling NUTritional Status (CONUT) as Predictive Score of Hospital Length of Stay (LOS) and Mortality: A Prospective Cohort Study in an Internal Medicine and Gastroenterology Unit in Italy. Nutrients 2023, 15, 1472. [Google Scholar] [CrossRef] [PubMed]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; A Wedzicha, J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Aaron, S.D. What is a COPD exacerbation? Current definitions, pitfalls, challenges and opportunities for improvement. Eur. Respir. J. 2018, 52, 1801261. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Hall, G.L.; Filipow, N.; Ruppel, G.; Okitika, T.; Thompson, B.; Kirkby, J.; Steenbruggen, I.; Cooper, B.G.; Stanojevic, S. Official ERS technical standard: Global Lung Function Initiative reference values for static lung volumes in individuals of European ancestry. Eur. Respir. J. 2021, 57, 2000289. [Google Scholar] [CrossRef]
- Peng, L.; You, H.; Xu, M.-Y.; Dong, Z.-Y.; Liu, M.; Jin, W.-J.; Zhou, C. A Novel Metabolic Score for Predicting the Acute Exacerbation in Patients with Chronic Obstructive Pulmonary Disease. SSRN Electron. J. 2023, 18, 785–795. [Google Scholar] [CrossRef]
- Müllerova, H.; Maselli, D.J.; Locantore, N.; Vestbo, J.; Hurst, J.R.; Wedzicha, J.A.; Bakke, P.; Agusti, A.; Anzueto, A. Hospitalized Exacerbations of COPD. Chest 2015, 147, 999–1007. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Jarad, N. Chronic obstructive pulmonary disease (COPD) and old age? Chronic Respir. Dis. 2011, 8, 143–151. [Google Scholar] [CrossRef]
- Montserrat-Capdevila, J.; Godoy, P.; Marsal, J.R.; Barbé, F.; Galván, L. Risk of exacerbation in chronic obstructive pulmonary disease: A primary care retrospective cohort study. BMC Fam. Pract. 2015, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; Kang, Y.; Wang, X.; Cao, W.; Li, M.; He, Y.; Pan, X.; Ye, K.; Xu, D. Frequent exacerbators of chronic obstructive pulmonary disease have distinguishable sputum microbiome signatures during clinical stability. Front. Microbiol. 2022, 13, 1037037. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Huang, Z.; Lu, J.; Yang, Y.; Zhao, X.; Tu, J.; Pan, Y.; Bao, K.; Chen, W.; et al. A Synergistic Association Between Inflammation, Malnutrition, and Mortality in Patients With Diabetics. Front. Nutr. 2022, 9, 872512. [Google Scholar] [CrossRef]
- Bellanti, F.; Buglio, A.L.; Quiete, S.; Vendemiale, G. Malnutrition in Hospitalized Old Patients: Screening and Diagnosis, Clinical Outcomes, and Management. Nutrients 2022, 14, 910. [Google Scholar] [CrossRef]
- Silvestre, C.R.; Domingues, T.D.; Mateus, L.; Cavaco, M.; Nunes, A.; Cordeiro, R.; Santos, T.S.; Falcão, T.; Domingos, A. The Nutritional Status of Chronic Obstructive Pulmonary Disease Exacerbators. Can. Respir. J. 2022, 2022, 3101486. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure. JACC Hear. Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef]
- Lee, S.-D.; Huang, M.-S.; Kang, J.; Lin, C.-H.; Park, M.J.; Oh, Y.-M.; Kwon, N.; Jones, P.W.; Sajkov, D. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir. Med. 2014, 108, 600–608. [Google Scholar] [CrossRef]
- García-Sidro, P.; Naval, E.; Rivera, C.M.; Bonnin-Vilaplana, M.; Garcia-Rivero, J.L.; Herrejón, A.; de Molina, R.M.; Marcos, P.J.; Mayoralas-Alises, S.; Ros, J.A.; et al. The CAT (COPD Assessment Test) questionnaire as a predictor of the evolution of severe COPD exacerbations. Respir. Med. 2015, 109, 1546–1552. [Google Scholar] [CrossRef]
- Bikov, A.; Lange, P.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.; Celli, B.R.; Cowans, N.J.; Crim, C.; Dixon, I.J.; Martinez, F.J.; et al. FEV1 is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. SSRN Electron. J. 2020, 15, 1135–1142. [Google Scholar] [CrossRef]




| Nutritional Status | ||||
|---|---|---|---|---|
| Variables | Normal | Light | Moderate | Severe |
| Albumin (g/dL) Score | ≥3.5 0 | 3.0–3.49 2 | 2.5–2.9 4 | <2.5 6 |
| Total lymphocyte (n/mm3) Score | >1600 0 | 1200–1599 1 | 800–1199 2 | <800 3 |
| Total cholesterol (mg/dL) Score | >180 0 | 140–180 1 | 100–139 2 | <100 3 |
| Screening total score | 0–1 | 2–4 | 5–8 | 9–12 |
| COPD IE n = 161 (72.5%) | COPD FE n = 61 (27.5%) | p Value | |
|---|---|---|---|
| Age, years | 70.9 ± 5.1 | 74.4 ± 5.0 | <0.001 |
| Sex F, n (%) | 43 (26.7) | 21 (34.4) | 0.257 |
| Hemoglobin, g/dL | 14.2 ± 1.8 | 13.8 ± 1.7 | 0.463 |
| WBC, n/mm3 | 7840 [6370 to 8670] | 12,500 [9520 to 18,050] | <0.001 |
| Lymphocytes, n/mm3 | 1656 [1287 to 2101] | 1090 [919 to 1302] | <0.001 |
| Eosinophils, n/mm3 | 112 [87 to 168] | 340 [285 to 396] | <0.001 |
| Platelets, ×109/L | 226 [189 to 256] | 257 [205 to 321] | 0.097 |
| Glucose, mg/dL | 121.5 ± 42.0 | 119.7 ± 30.6 | 0.890 |
| Creatinine, mg/dL | 0.84 [0.76 to 1.10] | 0.86 [0.79 to 1.01] | 0.889 |
| Total cholesterol, mg/dL | 164.1 ± 36.1 | 165.4 ± 34.0 | 0.906 |
| Albumin, g/dL | 3.9 ± 0.4 | 3.2 ± 0.6 | 0.005 |
| CRP, ng/mL | 5.7 [2.6 to 9.8] | 22.1 [15.6 to 26.1] | <0.001 |
| BMI, kg/m2 | 28.3 ± 4.2 | 24.0 ± 3.8 | <0.001 |
| Co-morbidities ≥ 3, n (%) | 26 (16.1) | 43 (29.5) | 0.026 |
| Current smokers, n (%) | 68 (42.2) | 21 (34.4) | 0.289 |
| Emphysema, n (%) | 39 (24.2) | 14 (23) | 0.843 |
| Bronchiectasis, n (%) | 11 (6.8) | 11 (18) | 0.013 |
| CONUT score | 1 [0 to 1] | 7 [4 to 8] | <0.001 |
| CAT score | 9 [7 to 14] | 20 [13 to 24] | <0.001 |
| mMRC score | 2 [1 to 3] | 3 [2 to 4] | 0.046 |
| FEV1, % | 78 [63 to 92] | 49 [39 to 73] | <0.001 |
| Low CONUT n = 20 (32.8%) | High CONUT n = 41 (67.2%) | p Value | |
|---|---|---|---|
| Age, years | 74.3 ± 4.98.7 | 74.4 ± 5.1 | 0.920 |
| Sex F, n (%) | 9 (45.0) | 12 (29.3) | 0.260 |
| Hemoglobin, g/dL | 13.9 ± 0.6 | 13.8 ± 1.8 | 0.974 |
| WBC, n/mm3 | 13300 [12,450 to 14,325] | 9660 [7845 to 17,380] | 0.190 |
| Lymphocytes, n/mm3 | 1311 [1112 to 1880] | 989 [721 to 1100] | <0.001 |
| Eosinophils, n/mm3 | 337 [287 to 394] | 348 [278 to 398] | 0.731 |
| Platelets, ×109/L | 276 [245 to 299] | 238 [189 to 350] | 0.269 |
| Glucose, mg/dL | 104.5 ± 17.7 | 122.1 ± 31.9 | 0.470 |
| Creatinine, mg/dL | 0.91 [0.88 to 0.96] | 0.85 [0.79 to 1.03] | 0.672 |
| Total cholesterol, mg/dL | 191.3 ± 23.9 | 139.5 ± 19.6 | <0.001 |
| Albumin, g/dL | 3.7 ± 0.5 | 2.8 ± 0.4 | <0.001 |
| CRP, ng/mL | 20.9 [13.3 to 28.9] | 22.9 [17.3 to 25.7] | 0.923 |
| BMI, kg/m2 | 24.3 ± 4.3 | 23.9 ± 3.7 | 0.729 |
| Co-morbidities ≥ 3, n (%) | 4 (20.0) | 14 (34.1) | 0.372 |
| Current smokers, n (%) | 6 (30.0) | 15 (36.6) | 0.776 |
| Emphysema, n (%) | 4 (20.0) | 10 (24.4) | 0.704 |
| Bronchiectasis, n (%) | 5 (25.0) | 6 (14.6) | 0.479 |
| CAT score | 17 [11 to 21] | 21 [15 to 27] | 0.019 |
| mMRC score | 3 [2 to 4] | 3 [2 to 4] | 0.844 |
| FEV1, % | 77 [73 to 82] | 45 [34 to 49] | <0.001 |
| GOLD stage | |||
| 1, n (%) | 6 (30) | 0 (0) | |
| 2, n (%) | 12 (60) | 10 (24.4) | <0.001 |
| 3, n (%) | 2 (10) | 23 (56.1) | |
| 4, n (%) | 0 (0) | 8 (19.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Buglio, A.; Scioscia, G.; Bellanti, F.; Tondo, P.; Soccio, P.; Natale, M.P.; Lacedonia, D.; Vendemiale, G. Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients. Metabolites 2023, 13, 1123. https://doi.org/10.3390/metabo13111123
Lo Buglio A, Scioscia G, Bellanti F, Tondo P, Soccio P, Natale MP, Lacedonia D, Vendemiale G. Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients. Metabolites. 2023; 13(11):1123. https://doi.org/10.3390/metabo13111123
Chicago/Turabian StyleLo Buglio, Aurelio, Giulia Scioscia, Francesco Bellanti, Pasquale Tondo, Piera Soccio, Matteo Pio Natale, Donato Lacedonia, and Gianluigi Vendemiale. 2023. "Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients" Metabolites 13, no. 11: 1123. https://doi.org/10.3390/metabo13111123
APA StyleLo Buglio, A., Scioscia, G., Bellanti, F., Tondo, P., Soccio, P., Natale, M. P., Lacedonia, D., & Vendemiale, G. (2023). Controlling Nutritional Status Score as a Predictor for Chronic Obstructive Pulmonary Disease Exacerbation Risk in Elderly Patients. Metabolites, 13(11), 1123. https://doi.org/10.3390/metabo13111123