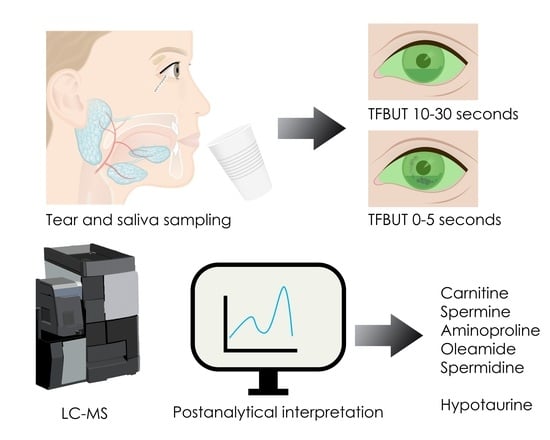
Tear and Saliva Metabolomics in Evaporative Dry Eye Disease in Females
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tear Sample Collection
2.3. Saliva Sample Collection
2.4. Materials
2.5. Sample Preparation
2.6. Metabolomics Analyses/LC-MS Analysis
2.7. Statistics and Identification
- -
- ChemSpider (http://www.chemspider.com/ (accessed on 12 July 2020)) database was used to search FullMS scans by using the molecular weight or predicted formulas when available.
- -
- mzCloud: (https://www.mzcloud.org/ (accessed on 12 July 2020)) database was used to search MSMS scans by using the fragmentation pattern.
3. Results
3.1. Tear Metabolomics
3.2. Saliva Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Sheppard, A.L.; Wolffsohn, J.S. Digital eye strain: Prevalence, measurement and amelioration. BMJ Open Ophthalmol. 2018, 3, e000146. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Romano, V.; Kaye, S.B.; Somerville, T.; Napoli, L.; Fasolo, A.; Gallon, P.; Ponzin, D.; Esposito, A.; Ferrari, S. Metagenomics in ophthalmology: Current findings and future prospectives. BMJ Open Ophthalmol. 2019, 4, e000248. [Google Scholar] [CrossRef]
- Borroni, D.; Paytuvi-Gallart, A.; Sanseverino, W.; Gomez-Huertas, C.; Bonci, P.; Romano, V.; Giannaccare, G.; Rechichi, M.; Meduri, A.; Oliverio, G.W.; et al. Exploring the Healthy Eye Microbiota Niche in a Multicenter Study. Int. J. Mol. Sci. 2022, 23, 10229. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Patel, D.A.; Keith, M.S.; Snedecor, S.J. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul. Surf. 2016, 14, 144–167. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Hammond, C.J.; Vehof, J. The work-related burden of dry eye. Ocul. Surf. 2023, 28, 30–36. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Snieder, H.; Hammond, C.J.; Vehof, J. The physical and mental burden of dry eye disease: A large population-based study investigating the relationship with health-related quality of life and its determinants. Ocul. Surf. 2021, 21, 107–117. [Google Scholar] [CrossRef]
- Morthen, M.K.; Magno, M.S.; Utheim, T.P.; Snieder, H.; Jansonius, N.; Hammond, C.J.; Vehof, J. The vision-related burden of dry eye. Ocul. Surf. 2022, 23, 207–215. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Baer, A.N.; Walitt, B. Update on Sjogren Syndrome and Other Causes of Sicca in Older Adults. Rheum. Dis. Clin. N. Am. 2018, 44, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Fraga, J.; Enríquez-de-Salamanca, A.; Calonge, M.; González-García, M.J.; López-Miguel, A.; López-de la Rosa, A.; García-Vázquez, C.; Calder, V.; Stern, M.E.; Fernández, I. Severity, therapeutic, and activity tear biomarkers in dry eye disease: An analysis from a phase III clinical trial. Ocul. Surf. 2018, 16, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr. Eye Res. 2016, 41, 1143–1149. [Google Scholar] [CrossRef]
- Thulasi, P.; Djalilian, A.R. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology 2017, 124, S27–S33. [Google Scholar] [CrossRef]
- Vehof, J.; Hysi, P.G.; Hammond, C.J. A Metabolome-Wide Study of Dry Eye Disease Reveals Serum Androgens as Biomarkers. Ophthalmology 2017, 124, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Elgstoen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, O.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef]
- Skogvold, H.B.; Sandas, E.M.; Osteby, A.; Lokken, C.; Rootwelt, H.; Ronning, P.O.; Wilson, S.R.; Elgstoen, K.B.P. Bridging the Polar and Hydrophobic Metabolome in Single-Run Untargeted Liquid Chromatography-Mass Spectrometry Dried Blood Spot Metabolomics for Clinical Purposes. J. Proteome Res. 2021, 20, 4010–4021. [Google Scholar] [CrossRef]
- National Library of Medicine. Glycyl-L-Tyrosine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glycyl-L-tyrosine (accessed on 20 August 2023).
- The Human Metabolome Database. Glycyltyrosine. Available online: https://hmdb.ca/metabolites/HMDB0028853 (accessed on 20 August 2023).
- National Library of Medicine. 1-Aminopyrrolidine-2-carboxylic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Aminopyrrolidine-2-carboxylic-acid (accessed on 11 September 2023).
- The Human Metabolome Database. Cucurbitin. Available online: https://hmdb.ca/metabolites/HMDB0302581 (accessed on 20 August 2023).
- National Library of Medicine. O-(2-Aminoethyl)-L-serine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/O-_2-Aminoethyl_-L-serine (accessed on 20 August 2023).
- Kenjic, N.; Hoag, M.R.; Moraski, G.C.; Caperelli, C.A.; Moran, G.R.; Lamb, A.L. PvdF of pyoverdin biosynthesis is a structurally unique N(10)-formyltetrahydrofolate-dependent formyltransferase. Arch. Biochem. Biophys. 2019, 664, 40–50. [Google Scholar] [CrossRef]
- Blatzer, M.; Schrettl, M.; Sarg, B.; Lindner, H.H.; Pfaller, K.; Haas, H. SidL, an Aspergillus fumigatus transacetylase involved in biosynthesis of the siderophores ferricrocin and hydroxyferricrocin. Appl. Environ. Microbiol. 2011, 77, 4959–4966. [Google Scholar] [CrossRef]
- The Human Metabolome Database. 3,4,5-Trimethoxycinnamic Acid. Available online: https://hmdb.ca/metabolites/HMDB0002511 (accessed on 20 August 2023).
- Kumar, S.; Arya, P.; Mukherjee, C.; Singh, B.K.; Singh, N.; Parmar, V.S.; Prasad, A.K.; Ghosh, B. Novel aromatic ester from Piper longum and its analogues inhibit expression of cell adhesion molecules on endothelial cells. Biochemistry 2005, 44, 15944–15952. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Panthenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4678 (accessed on 20 August 2023).
- Proksch, E.; de Bony, R.; Trapp, S.; Boudon, S. Topical use of dexpanthenol: A 70th anniversary article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- The Human Metabolome Database. Resorcinol. Available online: https://hmdb.ca/metabolites/HMDB0257165 (accessed on 21 October 2023).
- National Library of Medicine. Resorcinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Resorcinol (accessed on 21 October 2023).
- Modanloo, M.; Shokrzadeh, M. Analyzing Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis: Potential Role of L-carnitine. Iran. J. Kidney Dis. 2019, 13, 74–86. [Google Scholar]
- Hua, X.; Deng, R.; Li, J.; Chi, W.; Su, Z.; Lin, J.; Pflugfelder, S.C.; Li, D.Q. Protective Effects of L-Carnitine Against Oxidative Injury by Hyperosmolarity in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5503–5511. [Google Scholar] [CrossRef]
- Deng, R.; Su, Z.; Hua, X.; Zhang, Z.; Li, D.Q.; Pflugfelder, S.C. Osmoprotectants suppress the production and activity of matrix metalloproteinases induced by hyperosmolarity in primary human corneal epithelial cells. Mol. Vis. 2014, 20, 1243–1252. [Google Scholar]
- Chen, W.; Zhang, X.; Li, J.; Wang, Y.; Chen, Q.; Hou, C.; Garrett, Q. Efficacy of osmoprotectants on prevention and treatment of murine dry eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6287–6297. [Google Scholar] [CrossRef]
- Nebbioso, M.; Evangelista, M.; Librando, A.; Plateroti, A.M.; Pescosolido, N. Iatrogenic dry eye disease: An eledoisin/carnitine and osmolyte drops study. Biomed. Pharmacother. 2013, 67, 659–663. [Google Scholar] [CrossRef]
- Evangelista, M.; Koverech, A.; Messano, M.; Pescosolido, N. Comparison of three lubricant eye drop solutions in dry eye patients. Optom. Vis. Sci. 2011, 88, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A., Jr. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, C.; Zheng, Y.; Liu, Y.; Chen, Y. A Set of Global Metabolomic Biomarker Candidates to Predict the Risk of Dry Eye Disease. Front. Cell Dev. Biol. 2020, 8, 344. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine. Proline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/proline (accessed on 11 September 2023).
- Chen, X.; Rao, J.; Zheng, Z.; Yu, Y.; Lou, S.; Liu, L.; He, Q.; Wu, L.; Sun, X. Integrated Tear Proteome and Metabolome Reveal Panels of Inflammatory-Related Molecules via Key Regulatory Pathways in Dry Eye Syndrome. J. Proteome Res. 2019, 18, 2321–2330. [Google Scholar] [CrossRef]
- Hernandez-Zulueta, J.; Navarro-Partida, J.; Sanchez-Aguilar, O.E.; Cruz-Pavlovich, H.D.S.; Castro-Castaneda, C.R.; Gonzalez-De la Rosa, A. An insight on the eye bacterial microbiota and its role on dry eye disease. APMIS 2023, 131, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J.; Majzoub, M.E.; Coroneo, M.; Thomas, T.; Willcox, M. Ocular microbiome changes in dry eye disease and meibomian gland dysfunction. Exp. Eye Res. 2023, 235, 109615. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Tromethamine, Aminomethyl Propanediol, and Aminoethyl Propanediol as Used in Cosmetics. Int. J. Toxicol. 2018, 37, 5S–18S. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water. Available online: https://www.ncbi.nlm.nih.gov/books/NBK373177/ (accessed on 11 September 2023).
- National Library of Medicine. Diethanolamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethanolamine (accessed on 11 September 2023).
- National Library of Medicine. 2-Amino-2-methyl-1,3-propanediol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Amino-2-methyl-1_3-propanediol (accessed on 11 September 2023).
- Gao, K.; Zheng, C.; Wang, T.; Zhao, H.; Wang, J.; Wang, Z.; Zhai, X.; Jia, Z.; Chen, J.; Zhou, Y.; et al. 1-Deoxynojirimycin: Occurrence, Extraction, Chemistry, Oral Pharmacokinetics, Biological Activities and In Silico Target Fishing. Molecules 2016, 21, 1600. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.D.; Carney, A.E.; Holden, H.M. Accommodation of GDP-linked sugars in the active site of GDP-perosamine synthase. Biochemistry 2008, 47, 10685–10693. [Google Scholar] [CrossRef]
- Cook, P.D.; Holden, H.M. GDP-perosamine synthase: Structural analysis and production of a novel trideoxysugar. Biochemistry 2008, 47, 2833–2840. [Google Scholar] [CrossRef]
- Shimohata, H.; Yamashita, M.; Yamada, K.; Hirayama, K.; Kobayashi, M. Treatment of Fabry Nephropathy: A Literature Review. Medicina 2023, 59, 1478. [Google Scholar] [CrossRef]
- Butovich, I.A. The Meibomian puzzle: Combining pieces together. Prog. Retin. Eye Res. 2009, 28, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Ham, B.M.; Nichols, J.J.; Ziegler, C.; Green-Church, K.B. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Investig. Ophthalmol. Vis. Sci. 2007, 48, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, O.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Cavuoto, K.M.; Zhu, A.Y. The Role of the Ocular Surface Microbiome (OSM) in Diseases of the Anterior Segment and Ocular Surface. Curr. Ophthalmol. Rep. 2022, 10, 179–187. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Y.; Wang, W.; Lin, P.; Huang, Y. Composition and Diversity of Bacterial Community on the Ocular Surface of Patients with Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4774–4783. [Google Scholar] [CrossRef]
- Borroni, D.; Bonzano, C.; Sanchez-Gonzalez, J.M.; Rachwani-Anil, R.; Zamorano-Martin, F.; Pereza-Nieves, J.; Traverso, C.E.; Garcia Lorente, M.; Rodriguez-Calvo-de-Mora, M.; Esposito, A.; et al. Shotgun metagenomic sequencing in culture negative microbial keratitis. Eur. J. Ophthalmol. 2023, 33, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of taurine, hypotaurine and their metabolic precursors. Biochem. J. 1988, 256, 251–255. [Google Scholar] [CrossRef]
- Nishimura, T.; Duereh, M.; Sugita, Y.; Yoshida, Y.; Higuchi, K.; Tomi, M.; Nakashima, E. Protective effect of hypotaurine against oxidative stress-induced cytotoxicity in rat placental trophoblasts. Placenta 2015, 36, 693–698. [Google Scholar] [CrossRef]
- Green, T.R.; Fellman, J.H.; Eicher, A.L.; Pratt, K.L. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim. Biophys. Acta 1991, 1073, 91–97. [Google Scholar] [CrossRef]
- Bosman, P.; Pichon, V.; Acevedo, A.C.; Modesto, F.M.B.; Paula, L.M.; Le Pottier, L.; Pers, J.O.; Chardin, H.; Combes, A. Identification of potential salivary biomarkers for Sjogren’s syndrome with an untargeted metabolomic approach. Metabolomics 2023, 19, 76. [Google Scholar] [CrossRef]
- Rentka, A.; Koroskenyi, K.; Harsfalvi, J.; Szekanecz, Z.; Szucs, G.; Szodoray, P.; Kemeny-Beke, A. Evaluation of commonly used tear sampling methods and their relevance in subsequent biochemical analysis. Ann. Clin. Biochem. 2017, 54, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.; Tashbayev, B.; Utheim, Ø.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Singh, P.B.; Palm, Ø.; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjögren’s syndrome correlate with clinical ocular and oral manifestations. Sci. Rep. 2019, 9, 7319. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Patient Group (n = 19) | Control Group (n = 12) | p-Value |
|---|---|---|---|
| Age (years) | 56.9 ± 17.3 | 54.4 ± 18.8 | 0.80 |
| Female sex | 100% | 100% | |
| Schirmer value (mm/5 min) | 12.0 ± 1.7 | 12.2 ± 1.6 | 0.74 |
| TFBUT (seconds) | 3.0 ± 1.2 | 14.6 ± 5.8 | <0.0001 |
| OSDI | 35.8 ± 18.5 | 29.1 ± 20.2 | 0.37 |
| Level 1 | Validated identification using in-house library of analytical standards (MS/MS spectrum and retention time match). |
| Level 2 | Putative identification using online databases (MS/MS spectrum match). |
| Level 3 | Putative identification supported by extra information. |
| Level 4 | Tentative identification using online databases (chemical formula or molecular mass). |
| Level 5 | Unique feature with m/z value and retention time. |
| Increased in Patient Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predicted Formula | Suggested Component Name(s) | Molecular Weight (g/mol) | Patient Group (Area 106) (a.u.) | Control Group (Area 106) (a.u.) | Retention Time (minutes) | Ratio | p-Value | Level of Confidence of Identification |
| C10H26N4 | Spermine | 202.22 | 7.6 | 3.3 | 1.426 | 2.292 | 0.025 | 2 |
| C7H19N3 | Spermidine | 145.16 | 9.1 | 4.1 | 1.489 | 2.205 | 0.003 | 2 |
| C7H15NO3 | Carnitine | 161.11 | 47.9 | 2.5 | 2.954 | 18.566 | 0.020 | 4 |
| C5H12N2O3 | O-(2-aminoethyl)serine; N(5)-hydroxyornithine | 148.08 | 13.4 | 1.0 | 3.399 | 13.346 | 0.002 | 4 |
| C4H10O3 | Diethylene glycol; 1,2,3-butanetriol | 106.06 | 518 | 140 | 4.120 | 3.689 | 0.001 | 4 |
| C5H10N2O2 | (2S)-2-piperazinecarboxylic acid; aminoproline; cucurbitine; 5-imino-L-norvaline | 130.07 | 23.5 | 1.5 | 7.408 | 15.992 | 0.002 | 4 |
| C6H12N2O3 | Daminozide; dialanine; 4-acetoamido-2-aminobutanoic acid; methylglutamine | 160.08 | 10.4 | 1.0 | 8.224 | 10.688 | 0.004 | 4 |
| C11H14N2O4 | Glycyltyrosine; 5-hydroxy-N-(4-hydroxyphenyl)-5-iminonorvaline | 238.10 | 457.2 | 1.5 | 12.945 | 301.056 | 0.001 | 4 |
| C12H24O3 | 12-hydroxylauric acid; 5-hydroxydodecanoic acid | 216.17 | 21.9 | 7.4 | 14.277 | 2.948 | 0.016 | 4 |
| Decreased in Patient Group | ||||||||
| C2H7NO | Ethanolamine; N,O-dimethylhydroxylamine | 61.05 | 1.1 | 3.4 | 2.190 | 0.340 | 0.001 | 4 |
| C5H11NO4 | Triethanolamine; N,N-dihydroxy-L-valine | 149.07 | 0.33 | 1.2 | 2.291 | 0.266 | 0.001 | Triethanolamine: 2; N,N-dihydroxy-L-valine: 4 |
| C6H13NO4 | Bicine; 1-deoxynojirimycin; perosamine; migalastat | 163.08 | 0.10 | 0.75 | 2.296 | 0.133 | 0.001 | 4 |
| C4H11NO2 | Diethanolamine; aminomethyl propanediol | 105.08 | 0.48 | 3.8 | 2.405 | 0.124 | 0.007 | 4 |
| C8H9NO3 | Pyridoxal; methyl-4-aminosalicylate; 3,5,6-indolinetriol; orthocaine | 167.05 | 3.9 | 10.0 | 3.255 | 0.389 | 0.001 | 4 |
| C9H19NO4 | Panthenol | 205.13 | 0.44 | 10.1 | 10.625 | 0.044 | 0.012 | 2 |
| C6H6O2 | Hydroquinone; resorcinol; benzenediol | 110.04 | 0.64 | 6.1 | 13.706 | 0.104 | 0.039 | 4 |
| C18H35NO | Oleamide | 281.27 | 14.1 | 62.5 | 19.060 | 0.226 | 0.001 | 2 |
| C22H44O3 | Hydroxydocosanoic acid | 356.33 | 21.2 | 53.3 | 21.385 | 0.399 | 0.007 | 4 |
| Increased in Patient Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Predicted Formula | Suggested Component Name(s) | Molecular Weight (g/mol) | Patient Group (Area 106) (a.u.) | Control Group (Area 106) (a.u.) | Retention Time (minutes) | Ratio | p-Value | Level of Confidence of Identification |
| C10H10O5 | 3-(3-hydroxy-4-methoxyphenyl)-2-oxiranecarboxylic acid; 2,4-diacetylphloroglucinol; hydroxyferulic acid | 210.05 | 13.7 | 3.2 | 12.631 | 4.263 | 0.001 | 4 |
| C25H22O9 | Silandrin | 466.13 | 48.2 | 6.3 | 13.721 | 7.695 | 0.008 | 4 |
| C20H32O2 | Arachidonic acid; drosthanolone | 304.24 | 4.0 | 1.9 | 20.442 | 2.102 | 0.039 | 4 |
| C22H32O2 | Retinyl acetate; docosahexanoic acid; methylprogesterone | 328.24 | 2.0 | 0.39 | 21.014 | 5.128 | 0.012 | 4 |
| Decreased in Patient Group | ||||||||
| C9H19NO4 | Panthenol | 205.13 | 0.22 | 4.2 | 10.603 | 0.053 | 0.020 | 2 |
| C12H14O5 | 3,4,5-trimethoxycinnamic acid; 3-(6,7-dimethoxy-1,3-benzodioxol-5-yl)-2-propen-1-ol | 238.08 | 0.03 | 3.6 | 13.183 | 0.007 | 0.003 | 4 |
| C6H6O2 | Resorcinol; cathecol | 110.04 | 0.53 | 5.8 | 13.704 | 0.091 | 0.028 | 2 |
| C22H44O3 | Hydroxydocosanoic acid | 356.33 | 14.5 | 34.2 | 21.381 | 0.424 | 0.011 | 4 |
| C22H42O4 | Adipid acid di(2-ethylhexyl) ester; docosanedioic acid | 370.31 | 4.7 | 10.4 | 21.415 | 0.456 | 0.011 | 4 |
| Increased in Patient Group | |||
|---|---|---|---|
| Predicted Formula | Suggested Component Name(s) | Function | Level of Confidence of Identification |
| C11H14N2O4 | Glycyltyrosine; 5-hydroxy-N-(4-hydroxyphenyl)-5-iminonorvaline | Glycyltyrosine: belongs to peptides, might be associated with colorectal cancer [19]. Has been detected in poultry and pigs, might indicate human consumption of this meat [20]. Secondary metabolite and as such may serve a role in defense or signaling molecule [20]. 5-hydroxy-N-(4-hydroxyphenyl)-5-iminonorvaline: no relevant information. | 4 |
| C5H10N2O2 | (2S)-2-piperazinecarboxylic acid; aminoproline; cucurbitine; 5-imino-L-norvaline | (2S)-2-piperazinecarboxylic acid: no relevant information. Aminoproline: also known as 1-aminopyrrolidine-2-carboxylic acid is a proline derivative [21]. Cucurbitine: exogenous alpha amino acid found in muskmelon, cucurbita seeds, and cucumber, might indicate consumption [22]. 5-imino-L-norvaline: no relevant information. | 4 |
| C5H12N2O3 | O-(2-aminoethyl)serine; N(5)-hydroxyornithine | O-(2-aminoethyl)serine: is an alpha amino acid [23]. It is obtained from Streptomyces reseoviridofuscus and is an antimetabolic antibiotic. N(5)-hydroxyornithine: involved in siderophore synthesis in Pseudomonas aeruginosa and Aspergillus fumigatus, promoting virulence and biofilm formation [24,25]. | 4 |
| Decreased in Patient Group | |||
| C12H14O5 | 3,4,5-trimethoxycinnamic acid; 3-(6,7-dimethoxy-1,3-benzodioxol-5-yl)-2-propen-1-ol | 3,4,5-trimethoxycinnamic acid: found in normal human urine and Piper longum, has a role as an allergen, and inhibits TNF-α induced cytokine expression [26,27]. 3-(6,7-dimethoxy-1,3-benzodioxol-5-yl)-2-propen-1-ol: no relevant information. | 4 |
| C9H19NO4 | Panthenol | Is the alcohol equivalent of pantothenic acid (vitamin B5), an essential nutrient necessary for the synthesis of coenzyme A, which plays an important role in epithelial protein metabolism [28]. Topical application promotes skin regeneration and wound healing [29]. | 2 |
| C6H6O2 | Resorcinol | Not a naturally occurring metabolite exerting keratolytic activity often found in topical pharmaceutical products as an antiseptic [30,31]. | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fineide, F.A.; Tashbayev, B.; Elgstøen, K.B.P.; Sandås, E.M.; Rootwelt, H.; Hynne, H.; Chen, X.; Ræder, S.; Vehof, J.; Dartt, D.; et al. Tear and Saliva Metabolomics in Evaporative Dry Eye Disease in Females. Metabolites 2023, 13, 1125. https://doi.org/10.3390/metabo13111125
Fineide FA, Tashbayev B, Elgstøen KBP, Sandås EM, Rootwelt H, Hynne H, Chen X, Ræder S, Vehof J, Dartt D, et al. Tear and Saliva Metabolomics in Evaporative Dry Eye Disease in Females. Metabolites. 2023; 13(11):1125. https://doi.org/10.3390/metabo13111125
Chicago/Turabian StyleFineide, Fredrik A., Behzod Tashbayev, Katja B. P. Elgstøen, Elise M. Sandås, Helge Rootwelt, Håvard Hynne, Xiangjun Chen, Sten Ræder, Jelle Vehof, Darlene Dartt, and et al. 2023. "Tear and Saliva Metabolomics in Evaporative Dry Eye Disease in Females" Metabolites 13, no. 11: 1125. https://doi.org/10.3390/metabo13111125
APA StyleFineide, F. A., Tashbayev, B., Elgstøen, K. B. P., Sandås, E. M., Rootwelt, H., Hynne, H., Chen, X., Ræder, S., Vehof, J., Dartt, D., Jensen, J. L., & Utheim, T. P. (2023). Tear and Saliva Metabolomics in Evaporative Dry Eye Disease in Females. Metabolites, 13(11), 1125. https://doi.org/10.3390/metabo13111125