The Mechanism of Mori Folium and Eucommiae Cortex against Cyclophosphamide-Induced Immunosuppression Integrating Network Pharmacology, Molecular Docking, Molecular Dynamics Simulations, and Experimental Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Database Construction of Active Ingredients and Potential Targets
2.3. Acquisition and Screening of Immunosuppression-Associated Targets
2.4. Network Construction and Topological Analysis
2.5. GO and KEGG Pathway Enrichment Analysis
2.6. Molecular Docking Verification
2.7. Molecular Dynamics Simulations
3. Results
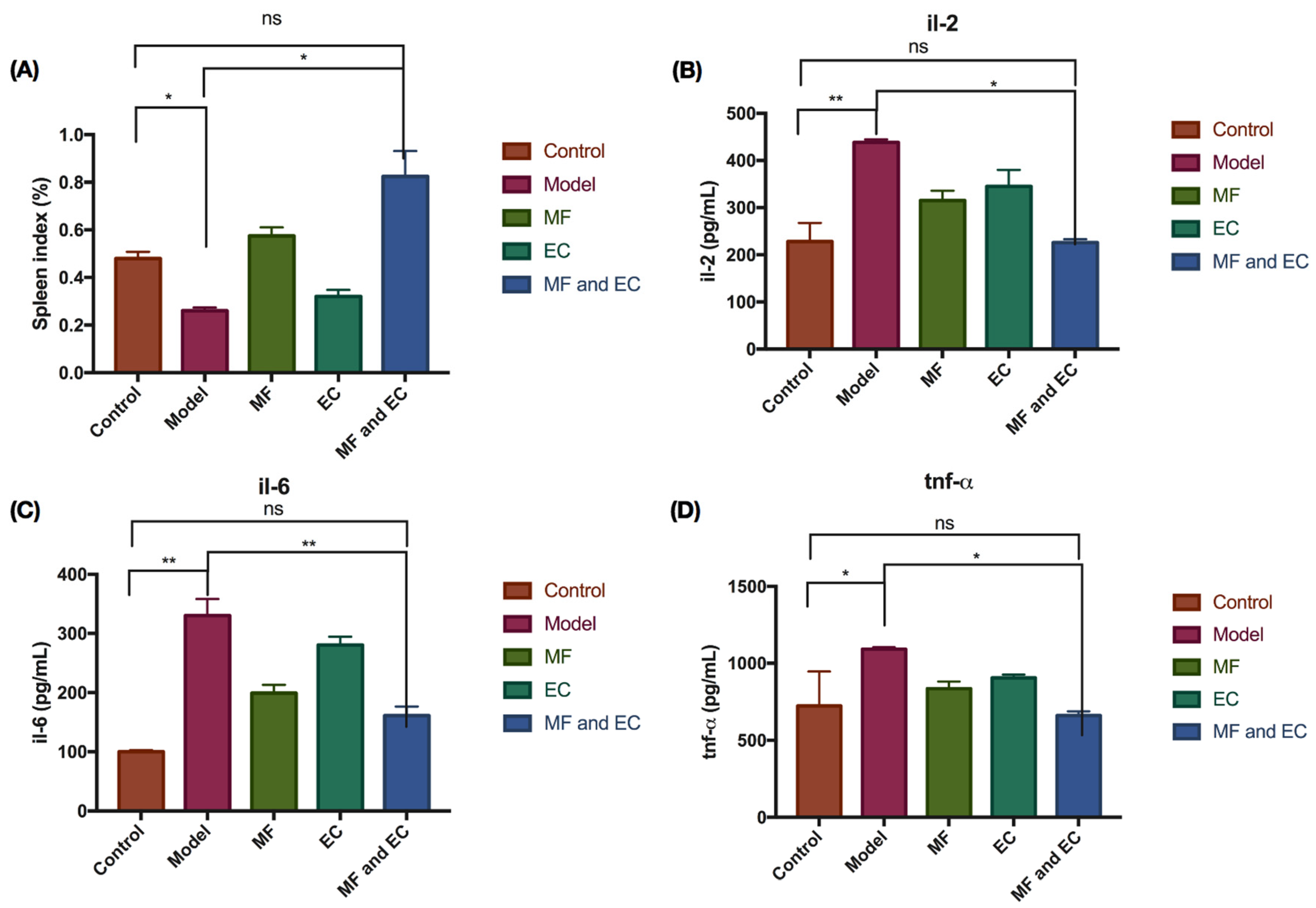
3.1. Mice Spleen Index, IL-2, IL-6, and TNF-α Level in Serum
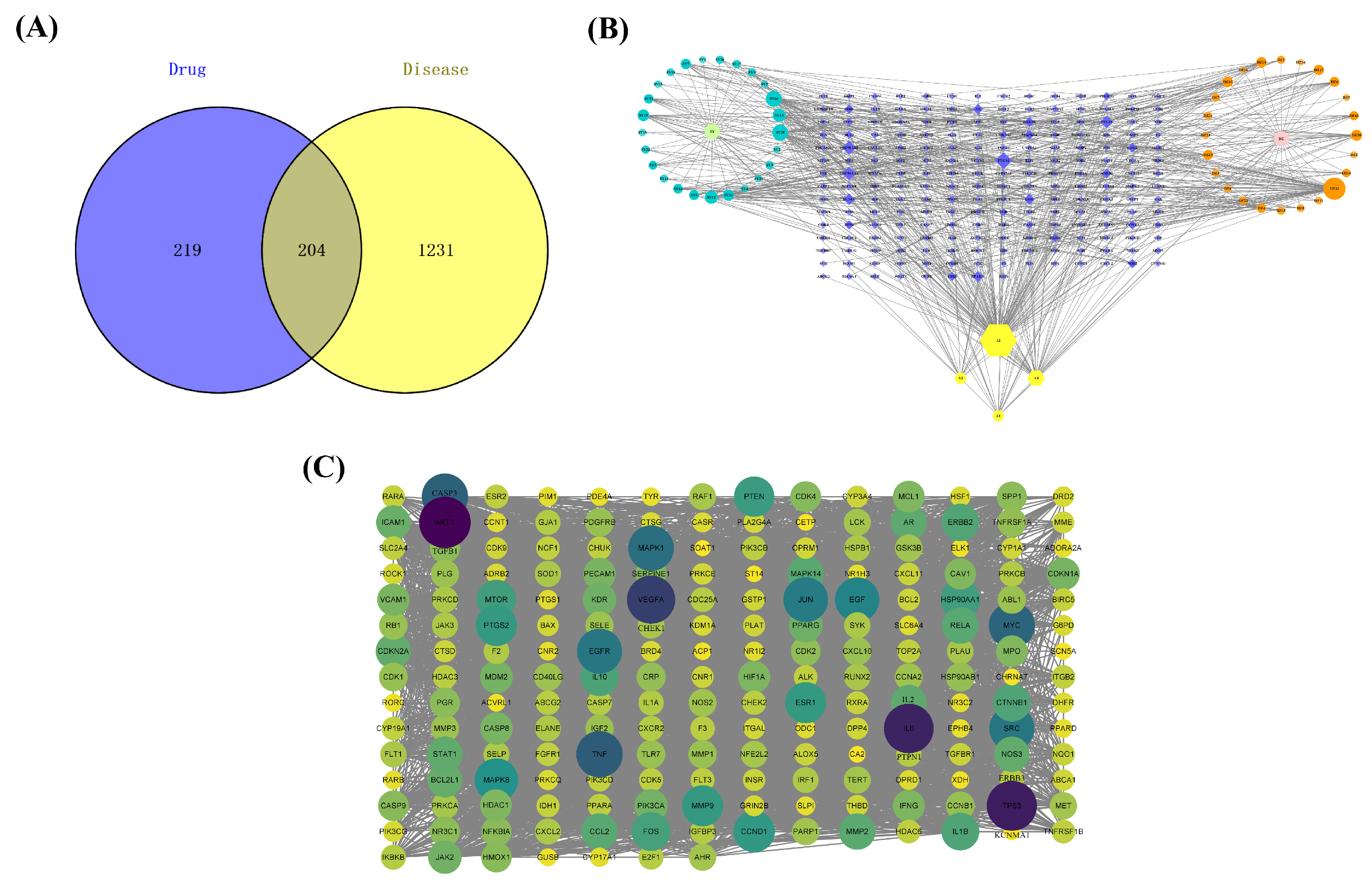
3.2. Database Construction of Active Ingredients and Potential Targets
3.3. Immunosuppression-Related Targets
3.4. Compound—Target Interaction Network
3.5. PPI Network
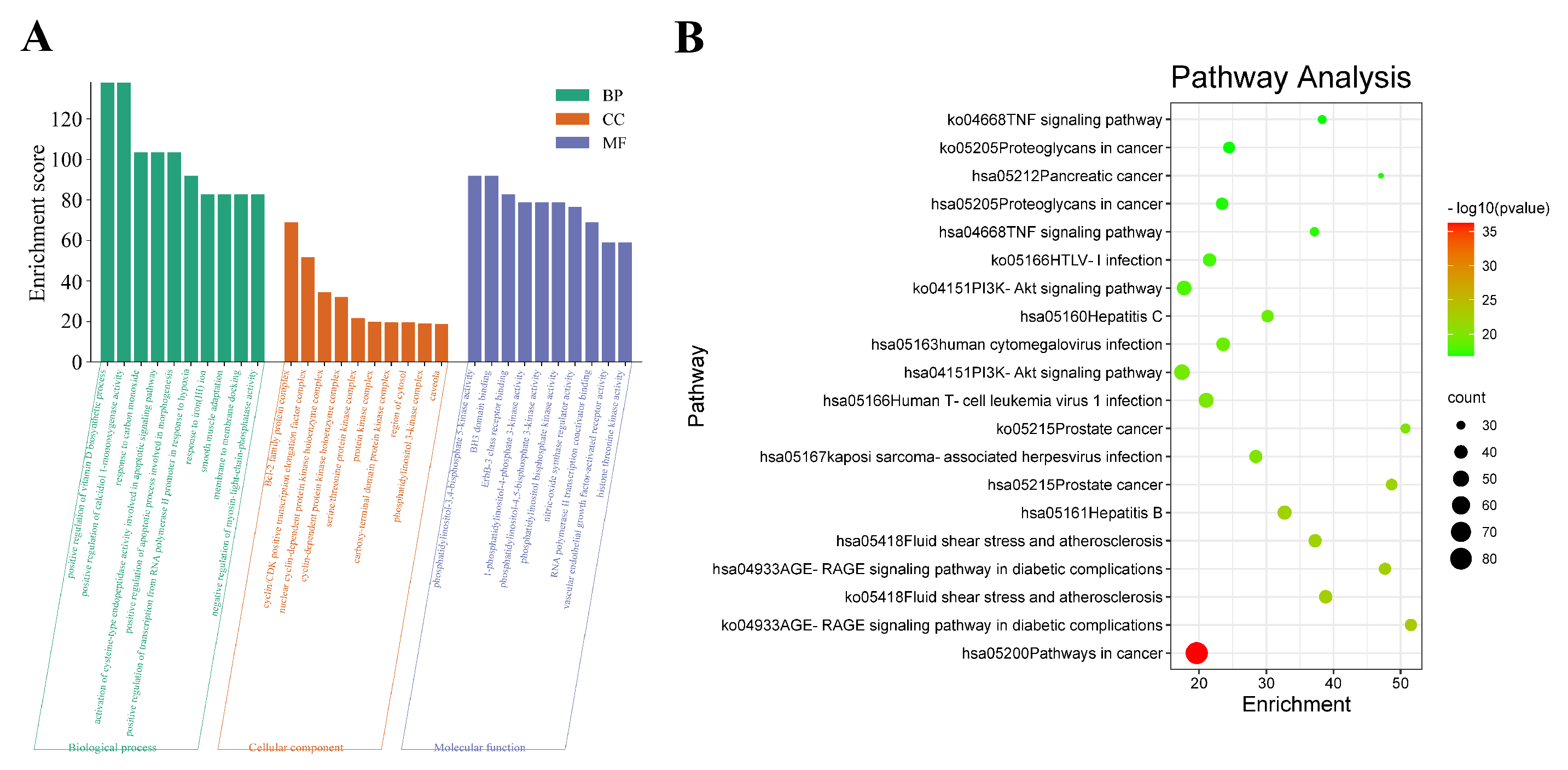
3.6. GO and KEGG Enrichment Analysis
3.6.1. GO Enrichment
3.6.2. KEGG Enrichment
3.7. Molecular Docking
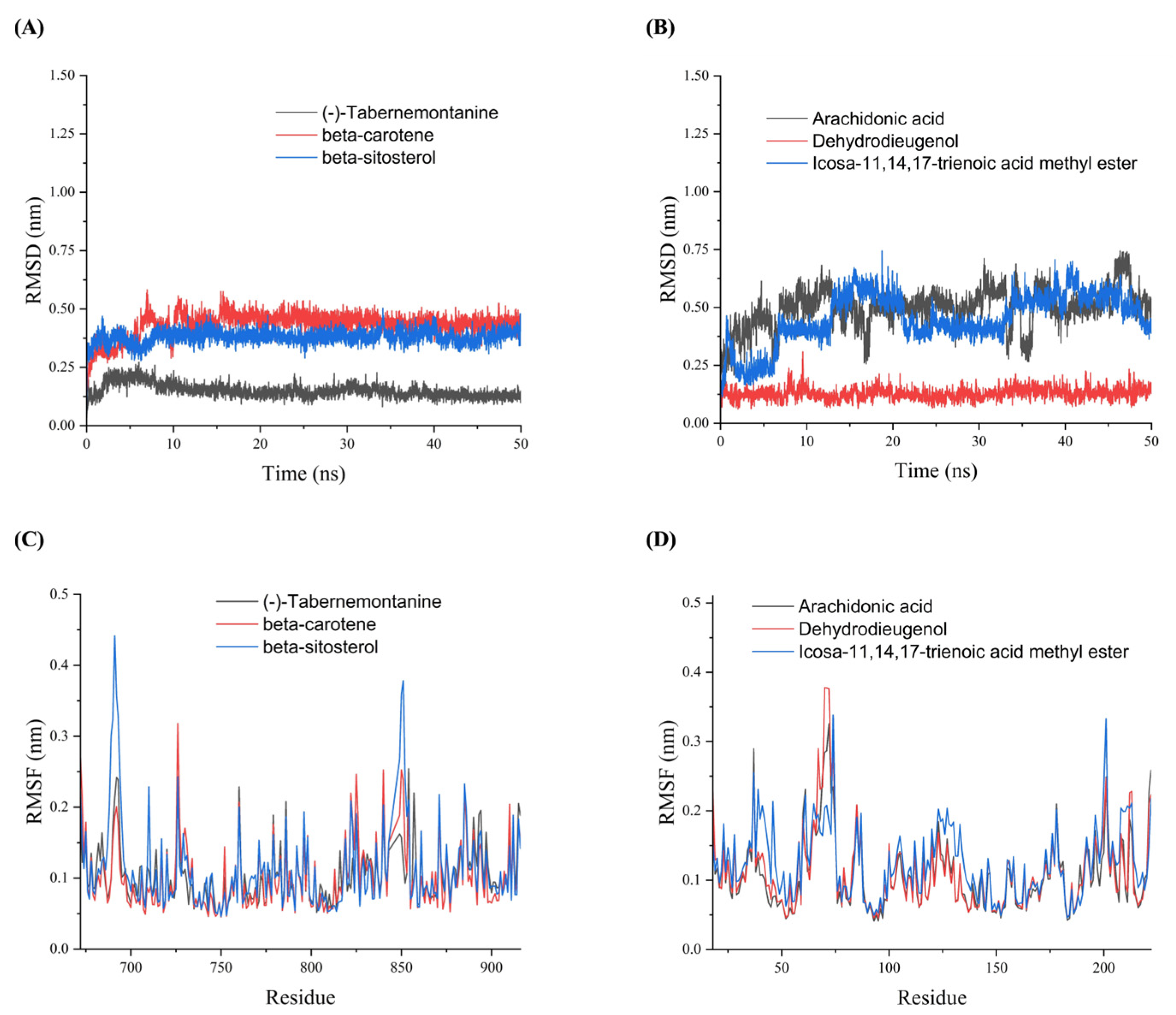
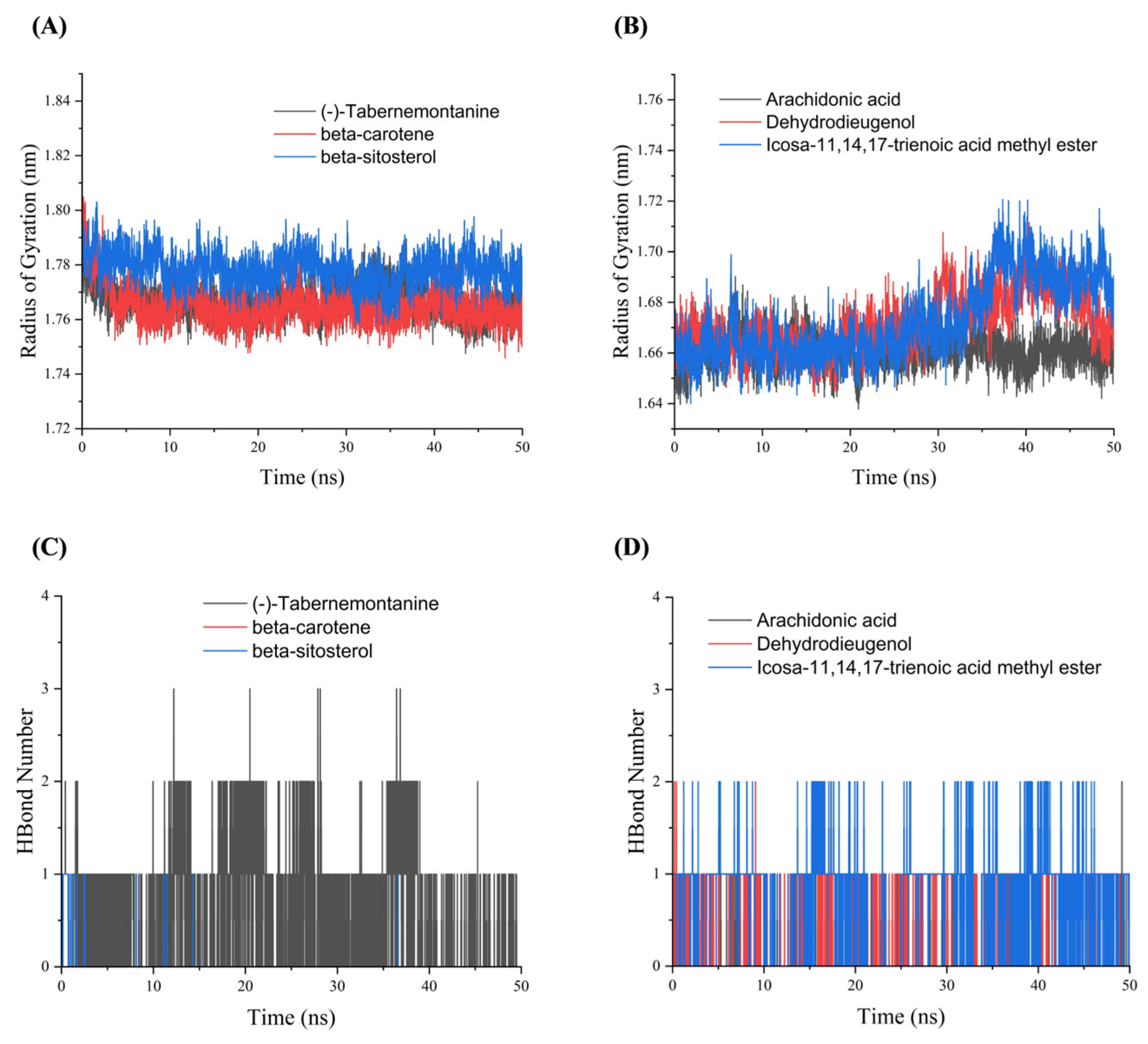
3.8. Molecular Dynamics Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.J.; Li, L.; Zhen, W.Y.; Wang, L.F.; Pan, M.; Lv, J.Q.; Wang, F.; Yao, Y.F.; Nie, S.P.; Xie, M.Y. Ganoderma atrum polysaccharide ameliorates ROS generation and apoptosis in spleen and thymus of immunosuppressed mice. Food Chem. Toxicol. 2017, 99, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.B. The immune system. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Gothandam, K.M. Immunomodulatory activity of methanolic extract of Amorphophallus commutatus var. wayanadensis under normal and cyclophosphamide induced immunosuppressive conditions in mice models. Food Chem. Toxicol. 2015, 81, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Liang, C.; Hu, J.; Yu, Z. Safety evaluation of mulberry leaf extract: Acute, subacute toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol. 2018, 95, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Gryn-Rynko, A.; Bazylak, G.; Olszewska-Slonina, D. New potential phytotherapeutics obtained from white mulberry (Morus alba L.) leaves. Biomed. Pharmacother. 2016, 84, 628–636. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Xu, S.-Q.; Xing, Y.; Hu, X.-M.; Pan, H.-Y. Effects of flavonoids in Morus indica on blood lipids and glucose in hyperlipidemia-diabetic rats. J. Chin. Herb. Med. 2012, 4, 314–318. [Google Scholar]
- Hu, X.Q.; Thakur, K.; Chen, G.H.; Hu, F.; Zhang, J.G.; Zhang, H.B.; Wei, Z.J. Metabolic effect of 1-Deoxynojirimycin from Mulberry Leaves on db/db Diabetic mice using liquid chromatography-mass spectrometry based metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef]
- Ji, T.; Li, J.; Su, S.L.; Zhu, Z.H.; Guo, S.; Qian, D.W.; Duan, J.A. Identification and determination of the polyhydroxylated alkaloids compounds with alpha-Glucosidase inhibitor activity in Mulberry Leaves of different origins. Molecules 2016, 21, 206. [Google Scholar] [CrossRef]
- Shipra, J.; Srivastava, A. Antibacterial, antifungal and pesticidal activity of plant Morus alba-a novel approach in post harvest technology. IJASR 2013, 3, 157–161. [Google Scholar]
- He, X.; Wang, J.; Li, M.; Hao, D.; Yang, Y.; Zhang, C.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [PubMed]
- Li, S. Exploring traditional chinese medicine by a novel therapeutic concept of network target. Chin. J. Integr. Med. 2016, 22, 647–652. [Google Scholar] [PubMed]
- Cerón-Carrasco, J.P. When Virtual Screening Yields Inactive Drugs: Dealing with False Theoretical Friends. ChemMedChem 2022, 17, e202200278. [Google Scholar] [PubMed]
- Habeeb, M.M.; Al-Attas, A.S.; Al-Raimi, D.S. Spectroscopic studies and molecular orbital calculations of charge transfer complexation between 3,5-dimethylpyrazole with DDQ in acetonitrile. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 196–203. [Google Scholar]
- Khan, Z.F. ChemBio3D Ultra 12.0 with GAMESS Interface; The Islamic University: Gaza, Palestine, 2013. [Google Scholar]
- Sun, F.; Liu, J.; Tariq, A.; Wang, Z.; Wu, Y.; Li, L. Unraveling the mechanism of action of cepharanthine for the treatment of novel coronavirus pneumonia (COVID-19) from the perspectives of systematic pharmacology. Arab. J. Chem. 2023, 16, 104722. [Google Scholar]
- Jiao, Y.; Shi, C.; Sun, Y. Unraveling the role of Scutellaria baicalensis for the treatment of Breast Cancer using network pharmacology, molecular docking, and molecular dynamics simulation. Int. J. Mol. Sci. 2023, 24, 3594. [Google Scholar]
- Sham, T.T.; Chan, C.O.; Wang, Y.H.; Yang, J.M.; Mok, D.K.; Chan, S.W. A review on the traditional Chinese medicinal herbs and formulae with hypolipidemic effect. BioMed Res. Int. 2014, 2014, 925302. [Google Scholar]
- Luo, T.T.; Lu, Y.; Yan, S.K.; Xiao, X.; Rong, X.L.; Guo, J. Network pharmacology in research of Chinese medicine formula: Methodology, application and prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar]
- Xiao, X.; Wang, W.; Li, Y.; Yang, D.; Li, X.; Shen, C.; Liu, Y.; Ke, X.; Guo, S.; Guo, Z. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 201. [Google Scholar]
- Yang, N.; Li, C.; Tian, G.; Zhu, M.; Bu, W.; Chen, J.; Hou, X.; Di, L.; Jia, X.; Dong, Z.; et al. Organic acid component from Taraxacum mongolicum Hand.-Mazz alleviates inflammatory injury in lipopolysaccharide-induced acute tracheobronchitis of ICR mice through TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 34, 92–100. [Google Scholar]
- Abdelmoaty, M.A.; Ibrahim, M.A.; Ahmed, N.S.; Abdelaziz, M.A. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J. Clin. Biochem. 2010, 25, 188–192. [Google Scholar]
- Mu, J.J. The Regulation of Kaemperol on Immunological Function In Vitro and Epilepsy of Mouse. Master’s Thesis, Jinan University, Guangzhou, China, 2010. [Google Scholar]
- Ti, H.; Zhuang, Z.; Yu, Q.; Wang, S. Progress of plant medicine derived extracts and alkaloids on modulating viral infections and inflammation. Drug Des. Devel. Ther. 2021, 15, 1385–1408. [Google Scholar]
- Mingrou, L.; Guo, S.; Ho, C.T.; Bai, N. Review on chemical compositions and biological activities of peanut (Arachis hypogeae L.). J. Food Biochem. 2022, 46, e14119. [Google Scholar]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [PubMed]
- Chen, J.; Chen, J.; Zhang, Y.; Lv, Y.; Qiao, H.; Tian, M.; Cheng, L.; Chen, F.; Zhang, S.; Guan, W. Effects of maternal supplementation with fully oxidised β-carotene on the reproductive performance and immune response of sows, as well as the growth performance of nursing piglets. Br. J. Nutr. 2021, 125, 62–70. [Google Scholar]
- Soman, S.; Raju, R.; Sandhya, V.K.; Advani, J.; Khan, A.A.; Harsha, H.C.; Prasad, T.S.; Sudhakaran, P.R.; Pandey, A.; Adishesha, P.K. A multicellular signal transduction network of AGE/RAGE signaling. J. Cell Commun. Signal. 2013, 7, 19–23. [Google Scholar] [PubMed]
- Al-Hussaini, H.; Kilarkaje, N. Trans-resveratrol mitigates type 1 diabetes-induced oxidative DNA damage and accumulation of advanced glycation end products in glomeruli and tubules of rat kidneys. Toxicol. Appl. Pharmacol. 2018, 339, 97–109. [Google Scholar] [PubMed]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/mTOR pathway by different flavonoids: A cancer chemopreventive approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar]
- Tang, F.; Wang, Y.; Hemmings, B.A.; Ruegg, C.; Xue, G. PKB/Akt-dependent regulation of inflammation in cancer. Semin. Cancer Biol. 2018, 48, 62–69. [Google Scholar]
- Dummler, B.; Hemmings, B.A. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007, 35, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, Z.; Wu, Y.; Yu, W.Y.; Zhang, J.; Jiang, Z.M.; Zhang, Y.; Liang, H.; Gui, Y.T. Non-genomic action of androgens is mediated by rapid phosphorylation and regulation of androgen receptor trafficking. Cell. Physiol. Biochem. 2017, 43, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Liu, J.; Chen, L.; Zhu, B.; Jing, J. The protective effects of PI3K/Akt pathway on human nucleus pulposus mesenchymal stem cells against hypoxia and nutrition deficiency. J. Orthop. Surg. Res. 2020, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Hangzo, H.; Banerjee, B.; Saha, S.; Saha, N. Ammonia stress under high environmental ammonia induces Hsp70 and Hsp90 in the mud eel, Monopterus cuchia. Fish Physiol. Biochem. 2017, 43, 77–88. [Google Scholar] [CrossRef]
- Meng, Q.; Xia, Y. c-Jun, at the crossroad of the signaling network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Amano, S.; Furuya, M.; Namiki, K.; Sakurai, K.; Nishiyama, M.; Sudo, T.; Tatsumi, K.; Kuriyama, T.; Kimura, S.; et al. Involvement of p38alpha mitogen-activated protein kinase in lung metastasis of tumor cells. J. Biol. Chem. 2006, 281, 36767–36775. [Google Scholar] [CrossRef]
- Kim, C.; Cathey, A.L.; Watkins, D.J.; Mukherjee, B.; Rosario-Pabón, Z.Y.; Vélez-Vega, C.M.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Maternal blood metal concentrations are associated with matrix metalloproteinases (MMPs) among pregnant women in Puerto Rico. Environ. Res. 2022, 209, 112874. [Google Scholar] [CrossRef]
- Cosemans, J. Platelet-derived MMP-2 in the prevention of plaque formation: How many strokes is par? Eur. Heart J. 2022, 43, 515–517. [Google Scholar] [CrossRef]
- Kang, D.S.; Lee, N.; Shin, D.Y.; Jang, Y.J.; Lee, S.H.; Lim, K.M.; Ahn, Y.S.; Lee, C.M.; Seo, Y.R. Network-based integrated analysis for toxic effects of high-concentration formaldehyde inhalation exposure through the toxicogenomic approach. Sci. Rep. 2022, 12, 5645. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Tian, X.Y.; Chen, Y.; Leung, F.P.; Liu, L.; Lee, H.K.; Ng, C.F.; Xu, A.; Yao, X.; Vanhoutte, P.M.; et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: Implications on hypertension. Circ. Res. 2010, 107, 984–991. [Google Scholar] [CrossRef] [PubMed]
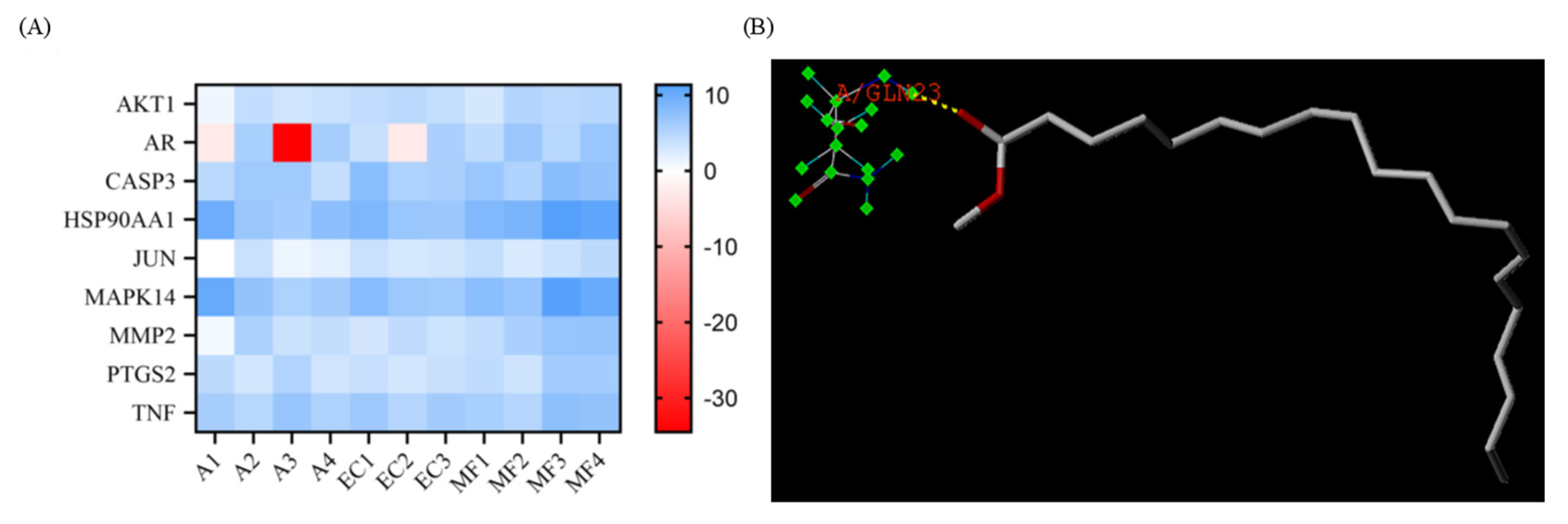
| Protein | Compound | Binding Site | Total Score |
|---|---|---|---|
| AKT1 | iristectorigenin A | A/SER56, A/LEU110, A/GLN59 | 5.0365 |
| AR | iristectorigenin A | A/ASN705, A/GLN711, A/MET745 | 6.5950 |
| CASP3 | quercetin | A/ARG64, A/SER205, A/GLY165, A/ARG164, A/GLU123 | 6.2159 |
| HSP90AA1 | icosa-11,14,17-trienoic acid methyl ester | A/GLN23 | 11.3399 |
| JUN | arachidonic acid | B/MET253 | 4.3453 |
| MAPK14 | arachidonic acid | A/HIS148 | 10.0545 |
| MMP2 | arachidonic acid | A/ASN573 | 7.0050 |
| PTGS2 | arachidonic acid | B/GLU486, B/ARG438 | 6.0676 |
| TNF | icosa-11,14,17-trienoic acid methyl ester | D/TYR151 | 7.4725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Rong, Q.; Zhang, C.; Tariq, A.; Li, L.; Wu, Y.; Sun, F. The Mechanism of Mori Folium and Eucommiae Cortex against Cyclophosphamide-Induced Immunosuppression Integrating Network Pharmacology, Molecular Docking, Molecular Dynamics Simulations, and Experimental Validation. Metabolites 2023, 13, 1151. https://doi.org/10.3390/metabo13111151
Liu J, Rong Q, Zhang C, Tariq A, Li L, Wu Y, Sun F. The Mechanism of Mori Folium and Eucommiae Cortex against Cyclophosphamide-Induced Immunosuppression Integrating Network Pharmacology, Molecular Docking, Molecular Dynamics Simulations, and Experimental Validation. Metabolites. 2023; 13(11):1151. https://doi.org/10.3390/metabo13111151
Chicago/Turabian StyleLiu, Jinde, Qiao Rong, Chunxiao Zhang, Ali Tariq, Lin Li, Yongning Wu, and Feifei Sun. 2023. "The Mechanism of Mori Folium and Eucommiae Cortex against Cyclophosphamide-Induced Immunosuppression Integrating Network Pharmacology, Molecular Docking, Molecular Dynamics Simulations, and Experimental Validation" Metabolites 13, no. 11: 1151. https://doi.org/10.3390/metabo13111151
APA StyleLiu, J., Rong, Q., Zhang, C., Tariq, A., Li, L., Wu, Y., & Sun, F. (2023). The Mechanism of Mori Folium and Eucommiae Cortex against Cyclophosphamide-Induced Immunosuppression Integrating Network Pharmacology, Molecular Docking, Molecular Dynamics Simulations, and Experimental Validation. Metabolites, 13(11), 1151. https://doi.org/10.3390/metabo13111151







