Callyspongia spp.: Secondary Metabolites, Pharmacological Activities, and Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Antidiabetic, Antihypercholesterolemic, and Antiobesity
4.2. Antihypertensive
4.3. Anti-Inflammation
4.4. Antifungal
4.5. Cytotoxicity against Cancer Cell Lines
4.6. Antimicrobial and Antiparasitic
4.7. Antioxidant
4.8. Antiallergic
4.9. Antiviral
4.10. Immunomodulatory
4.11. Antineurodegenerative
- β-secretase 1
- Kinase inhibitor
4.12. Antiosteoporotic
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Visbeck, M. Ocean science research is key for a sustainable future. Nat. Commun. 2018, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; Chaudhary, J. Marine biodiversity, biogeography, deep-sea gradients, and conservation. Curr. Biol. 2017, 27, R511–R527. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; de Voogd, N.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Busutil, L.; García-Hernández, M.R.; Díaz, M.C.; Pomponi, S.A. Mesophotic sponges of the genus Callyspongia (Demospongiae, Haplosclerida) from Cuba, with the description of two new species. Zootaxa 2018, 4466, 78–94. [Google Scholar] [CrossRef]
- Kapojos, M.M.; Abdjul, D.B.; Yamazaki, H.; Ohshiro, T.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Tomoda, H.; Namikoshi, M.; Uchida, R. Callyspongiamides A and B, sterol O-acyltransferase inhibitors, from the Indonesian marine sponge Callyspongia sp. Bioorganic Med. Chem. Lett. 2018, 28, 1911–1914. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A–F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorganic Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive Brominated Oxindole Alkaloids from the Red Sea Sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Sobahi, T.R.; Ayyad, S.-E.N.; Algandaby, M.M.; Alorfi, H.S.; Abdel-Naim, A.B. Cytotoxic metabolites from Callyspongia siphonella display antiproliferative activity by inducing apoptosis in HCT-116 cells. Pharmacogn. Mag. 2017, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Tinto, W.F.; Layne, T.H. A Butenolide from the Marine Sponge Callyspongia vaginalis. Heterocycles 2006, 68, 2161–2164. [Google Scholar] [CrossRef]
- De Araújo, R.D.; Caridade, T.N.D.S.; Araújo, R.; Chaves, M.D.J.S.; Verbinnen, R.T.; Diniz, M.D.S.; Viana, J.L.M.; Mendonça, C.D.J.S.; Franco, T.C.R.S. Sulfated Polysaccharide from the Marine Sponge Callyspongia vaginalis. Rev. Virtual Química. 2018, 10, 1446–1454. [Google Scholar] [CrossRef]
- Gray, C.A.; de Lira, S.P.; Silva, M.; Pimenta, E.F.; Thiemann, O.H.; Oliva, G.; Hajdu, E.; Andersen, R.J.; Berlinck, R.G.S. Sulfated Meroterpenoids from the Brazilian Sponge Callyspongia sp. Are Inhibitors of the Antileishmaniasis Target Adenosine Phosphoribosyl Transferase. J. Org. Chem. 2006, 71, 8685–8690. [Google Scholar] [CrossRef]
- Calabro, K.; Chalén, B.E.; Genta-Jouve, G.; Jaramillo, K.B.; Domínguez, C.; de la Cruz, M.; Cautain, B.; Reyes, F.; Thomas, O.P.; Rodriguez, J. Callyspongidic Acids: Amphiphilic Diacids from the Tropical Eastern Pacific Sponge Callyspongia cf. californica. J. Nat. Prod. 2018, 81, 2301–2305. [Google Scholar] [CrossRef]
- Marzuki, I. Eksplorasi Spons Indonesia Seputar Kepulauan Spermonde; Nas Media Pustaka: Makassar, Indonesia, 2018. [Google Scholar]
- De Voogd, N.J. Indonesian Sponges: Biodiversity and Mariculture Potential; UvA-IBED: Amsterdam, The Netherlands, 2005; pp. 39–45. [Google Scholar]
- Santhanam, R.; Ramesh, S.; Sunilson, A.J. Biology and Ecology of Pharmaceutical Marine Sponges; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Cabaitan, P.C.; Gomez, E.D.; Yap, H.T. The spaghetti sponge Callyspongia samarensis (Wilson, 1925) provides temporary habitat for reef fish recruits. Mar. Biodivers. 2016, 46, 541–542. [Google Scholar] [CrossRef]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Kamath, K.; Manna, T.; Okouneva, T.; Miller, H.P.; Davis, C.; Littlefield, B.A.; Wilson, L. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005, 4, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Swami, U.; Shah, U.; Goel, S. Marine Sponge Derived Eribulin in Preclinical and Clinical Studies for Cancer. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 59–100. [Google Scholar] [CrossRef]
- Jänne, P.A.; Paz-Ares, L.; Oh, Y.; Eschbach, C.; Hirsh, V.; Enas, N.; Brail, L.; von Pawel, J. Randomized, Double-Blind, Phase II Trial Comparing Gemcitabine-Cisplatin plus the LTB4 Antagonist LY293111 versus Gemcitabine-Cisplatin plus Placebo in First-Line Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 126–131. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Zhu, H. An improved and efficient synthesis for IPL576,092 and its analogues. Monatsh. Chem. 2013, 144, 1081–1085. [Google Scholar] [CrossRef]
- Petek, B.J.; Jones, R.L. PM00104 (Zalypsis®): A marine derived alkylating agent. Molecules 2014, 19, 12328–12335. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, I.; Kim, S.-K. Marine Antitumor Drugs: Status, Shortfalls and Strategies. Mar. Drugs 2010, 8, 2702–2720. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine-Sourced Anti-Cancer and Cancer Pain Control Agents in Clinical and Late Preclinical Development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef]
- Jain, D.K.; Singh, A.; Patel, V.K.; Sharma, P.C.; Guota, A.K.; Sharma, A.K.; Rajak, H. Hydroxamic Acid Based Histone Deacetylase Inhibitors: Present and Future Prospectives as Anticancer Agent. Int. J. Pharm. Pharm. Sci. 2014, 6, 648–650. [Google Scholar]
- El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs 2019, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, T.; Valavanidis, A. Bioactive Natural Substances from Marine Sponges: New Developments and Prospects for Future Pharmaceuticals. Nat. Prod. Chem. Res. 2013, 1, 115. [Google Scholar] [CrossRef]
- de Sousa, L.H.N.; Araújo, R.D.; Sousa-Fontoura, D.; Menezes, F.G.; Araújo, R.M. Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data. Mar. Drugs. 2021, 19, 663. [Google Scholar] [CrossRef]
- Arya, A.; Nahar, L.; Khan, H.U.; Sarker, S.D. Anti-obesity natural products. In Annual Reports in Medicinal Chemistry; Academic Press Inc.: Cambridge, MA, USA, 2020; pp. 411–433. [Google Scholar]
- Nakao, Y.; Uehara, T.; Matunaga, S.; Fusetani, N.; van Soest, R.W.M. Callyspongynic Acid, a Polyacetylenic Acid Which Inhibits α-Glucosidase, from the Marine Sponge Callyspongia truncata. J. Nat. Prod. 2002, 65, 922–924. [Google Scholar] [CrossRef]
- Resuello, D.L.; Lirio, S.B.; Porto, A.E.; Macabeo, A.P.G.; Huang, H.-Y.; Corpuz, M.J.-A.T.; Villaflores, O.B. β-secretase 1 inhibitory activity and AMP-activated protein kinase activation of Callyspongia samarensis extracts. Nat. Prod. Res. 2020, 34, 525–529. [Google Scholar] [CrossRef]
- Zeb, A.M.; Khan, U.S.; Rahman, T.U.; Sajid, M.; Seloni, S. Isolation and Biological Activity of β-sitosterol and Stigmasterol from the Roots of Indigofera heterantha. Pharm. Pharmacol. Int. J. 2017, 5, 204–207. Available online: https://medcraveonline.com/PPIJ/PPIJ-05-00139.php (accessed on 1 December 2022).
- Chakraborty, K.; Francis, P. Callypyrones from marine Callyspongiidae sponge Callyspongia diffusa: Antihypertensive bis-γ-pyrone polypropionates attenuate angiotensin-converting enzyme. Nat. Prod. Res. 2020, 35, 5801–5812. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H.; El-Naggar, H.A.; El-Damhougy, K.A.; Bashar, M.A.E.; Abou Senna, F.M. Callyspongia crassa and C. siphonella (Porifera, Callyspongiidae) as a potential source for medical bioactive substances, Aqaba Gulf, Red Sea, Egypt. JOBAZ 2017, 78, 7. [Google Scholar] [CrossRef]
- Fristiohady, A.; Wahyuni, W.; Malik, F.; Purnama, L.O.M.J.; Sadarun, B.; Sahidin, I. Anti-Inflammatory Activity of Marine sponge callyspongia sp. and Its acute Toxicity. Asian J. Pharm. Clin. Res. 2019, 12, 97–100. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lan, X.-P.; Liu, Y.; Jia, A.-Q. The effects of diketopiperazines from Callyspongia sp. on release of cytokines and chemokines in cultured J774A.1 macrophages. Bioorganic Med. Chem. Lett. 2012, 22, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Nath, R.; Srivastava, N.; Shanker, K.; Kishor, K.; Bhargava, K. Anti-Inflammatory and Antipyretic Activities of β-Sitosterol. Planta Medica 1980, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.S.; Van Soest, R.W.M. New Antiinflammatory Sterols from the Red Sea Sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial Sterol from the Red Sea Sponge Callyspongia aff. implexa. Planta Medica 2015, 81, 382–387. [Google Scholar] [CrossRef]
- Kiprono, P.C.; Kaberia, F.; Keriko, J.M.; Karanja, J.N. The In Vitro Anti-Fungal and Anti-Bacterial Activities of Beta-Sitosterol from Senecio lyratus (Asteraceae). Z. Naturforsch. C. J. Biosci. 2000, 55, 485–488. Available online: https://pubmed.ncbi.nlm.nih.gov/10928565/ (accessed on 1 December 2022). [CrossRef]
- López, S.; Fernández-Trillo, F.; Midón, P.; Castedo, L.; Saá, C. First Stereoselective Syntheses of (-)-siphonodiol and (-)-tetrahydrosiphonodiol, Bioactive Polyacetylenes from Marine Sponges. J. Org. Chem. 2005, 70, 6346–6352. Available online: https://pubmed.ncbi.nlm.nih.gov/16050696/ (accessed on 1 December 2022). [CrossRef]
- Xiao-Jian, L.; Shi-Hai, X.; Qi-Chang, H.; Dong-Hong, H. Studies on chemical constituents from Callyspongia fibrosa. Chin. J. Spectrosc. Lab. 2005, 22, 281–283. [Google Scholar]
- Chong, K.P.; Rossall, S.; Atong, M. In Vitro Antimicrobial Activity and Fungitoxicity of Syringic Acid, Caffeic Acid and 4-hydroxybenzoic Acid against Ganoderma Boninense. J. Agric. Sci. 2009, 1, 15–20. [Google Scholar] [CrossRef]
- Ayyad, S.-E.N.; Angawi, R.F.; Saqer, E.; Abdel-Lateff, A.; Badria, F.A. Cytotoxic neviotane triterpene-type from the red sea sponge Siphonochalina siphonella. Pharmacogn. Mag. 2014, 10 (Suppl. S2), S334–S341. [Google Scholar] [CrossRef]
- Al-Massarani, S.; El-Gamal, A.; Al-Said, M.; Al-Lihaibi, S.; Basoudan, O. In vitro Cytotoxic, Antibacterial and Antiviral Activities of Triterpenes from the Red Sea Sponge, Siphonochalina siphonella. Trop. J. Pharm. Res. 2015, 14, 33. [Google Scholar] [CrossRef]
- Pham, C.-D.; Hartmann, R.; Boehler, P.; Stork, B.; Wesselborg, S.; Lin, W.; Lai, D.; Proksch, P. Callyspongiolide, a Cytotoxic Macrolide from the Marine Sponge Callyspongia sp. Org. Lett. 2014, 16, 266–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaala, L.A.; Youssef, D.T.A.; Ibrahim, S.R.M.; Mohamed, G.A. Callyptide A, a new cytotoxic peptide from the Red Sea marine sponge Callyspongia species. Nat. Prod. Res. 2016, 30, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Woo, J.K.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Callyazepin and (3R)-Methylazacyclodecane, Nitrogenous Macrocycles from a Callyspongia sp. Sponge. J. Nat. Prod. 2016, 79, 1179–1183. Available online: https://pubmed.ncbi.nlm.nih.gov/27015002/ (accessed on 1 December 2022). [CrossRef]
- Plisson, F.; Prasad, P.; Xiao, X.; Piggott, A.M.; Huang, X.C.; Khalil, Z.; Capon, R. Callyspongisines A-D: Bromopyrrole alkaloids from an Australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Abdillah, S.; Nurhayati, A.P.D.; Nurhatika, S.; Setiawan, E.; Heffen, W.L. Cytotoxic and antioxidant activities of marine sponge diversity at Pecaron Bay Pasir Putih Situbondo East Java, Indonesia. J. Pharm. Res. 2013, 6, 685–689. [Google Scholar] [CrossRef]
- Murakami, N.; Wang, W.; Aoki, M.; Tsutsui, Y.; Higuchi, K.; Aoki, S.; Kobayashi, M. Absolute stereostructure of callystatin A, a potent cytotoxic polyketide from the marine sponge, Callyspongia truncata. Tetrahedron Lett. 1997, 38, 5533–5536. [Google Scholar] [CrossRef]
- Shirouzu, T.; Watari, K.; Ono, M.; Koizumi, K.; Saiki, I.; Tanaka, C.; van Soest, R.; Miyamoto, T. Structure, synthesis, and biological activity of a C-20 bisacetylenic alcohol from a marine sponge Callyspongia sp. J. Nat. Prod. 2013, 76, 1337–1342. [Google Scholar] [CrossRef]
- Umeyama, A.; Matsuoka, N.; Mine, R.; Nakata, A.; Arimoto, E.; Matsui, M.; Shoji, N.; Arihara, S.; Takei, M.; Hashimoto, T. Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med. 2010, 64, 93–97. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; van Soest, R.W.M.; Fusetani, N. Callyspongenols A-C, New Cytotoxic C22-polyacetylenic Alcohols from a Red Sea Sponge, Callyspongia Species. J. Nat. Prod. 2003, 66, 679–681. Available online: https://pubmed.ncbi.nlm.nih.gov/12762806/ (accessed on 1 December 2022). [CrossRef]
- Youssef, D.T.A.; van Soest, R.W.M.; Fusetani, N. Callyspongamide A, a New Cytotoxic Polyacetylenic Amide from the Red Sea Sponge Callyspongia fistularis. J. Nat. Prod. 2003, 66, 861–862. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. Polyacetylenes from a Red Sea Sponge Callyspongia Species. J. Nat. Prod. 2000, 63, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Davies-Coleman, M.T.; Faulkner, D.J.; Dubowchik, G.M.; Roth, G.P.; Polson, C.; Fairchild, C. A new EGF-active polymeric pyridinium alkaloid from the sponge Callyspongia fibrosa. J. Org. Chem. 1993, 58, 5925–5930. [Google Scholar] [CrossRef]
- Fukami, A.; Ikeda, Y.; Kondo, S.; Naganawa, H.; Takeuchi, T.; Furuya, S.; Hirabayashi, Y.; Shimoike, K.; Hosaka, S.; Watanabe, Y.; et al. Akaterpin, a novel bioactive triterpene from the marine sponge Callyspongia sp. Tetrahedron Lett. 1997, 38, 1201–1202. [Google Scholar] [CrossRef]
- Hadisaputri, Y.E.; Andika, R.; Sopyan, I.; Zuhrotun, A.; Maharani, R.; Rachmat, R.; Abdulah, R. Caspase Cascade Activation During Apoptotic Cell Death of Human Lung Carcinoma Cells A549 Induced by Marine Sponge Callyspongia aerizusa. Drug Des. Dev. Ther. 2021, 15, 1357–1368. [Google Scholar] [CrossRef]
- Afifi, R.; Khabour, O.F. Antibacterial activity of the Saudi Red Sea sponges against Gram-positive pathogens. J. King Saud Univ. Sci. 2019, 31, 753–757. [Google Scholar] [CrossRef]
- Alam, P.; Alqahtani, A.S.; Husain, F.M.; Rehman, T.; Alajmi, M.F.; Noman, O.M.; El Gamal, A.A.; Al-Massarani, S.M.; Khan, M.S. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharm. J. 2020, 28, 1383–1391. [Google Scholar] [CrossRef]
- Soleman, P.; Yudistira, A.; Jayanti, M. Antioxidant activity test of ethanol of sponge Callyspongia aerizusa from Mantehage Islands North Minahasa regency. Pharmacon 2021, 10, 962–967. [Google Scholar]
- Yang, B.; Tao, H.; Zhou, X.; Lin, X.P.; Liu, Y. Two new alkaloids from marine sponge Callyspongia sp. Nat. Prod. Res. 2013, 27, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Andrioli, W.J.; Santos, M.S.; Silva, V.B.; Oliveira, R.B.; Chagas-Paula, D.A.; Jorge, J.A.; Furtado, N.; Pupo, M.; Silva, C.; Naal, R.; et al. δ-Lactam derivative from thermophilic soil fungus exhibits in vitro anti-allergic activity. Nat. Prod. Res. 2012, 26, 2168–2175. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Quinn, R.J. Niphatoxin C, a Cytotoxic Tripyridine Alkaloid from Callyspongia sp. J. Nat. Prod. 2007, 70, 2040–2041. Available online: https://pubmed.ncbi.nlm.nih.gov/18027906/ (accessed on 1 December 2022). [CrossRef]
- Takei, M.; Umeyama, A.; Shoji, N.; Hashimoto, T. Differential Regulation of DC Function by Siphonodiol. Immunopharmacol. Immunotoxicol. 2008, 30, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Fraile, L.; Crisci, E.; Córdoba, L.; Navarro, M.A.; Osada, J.; Montoya, M. Immunomodulatory properties of Beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Fristiohady, A.; Mesi, L.; Malaka, M.H.; Hamsidi, R.; Azizah, N.; Fransiskus, R.; Wahyuni, W.; Purnama, L.O.M.J.; Sadarun, B.; Sahidin, I. Immunomodulatory Activity of Callyspongia sp. Extract Towards Interferon-gamma (IFN-γ) and Tumor Necrosis Factor-Alpha (TNF-α) Levels in Staphylococcus aureus—Induced Wistar Male Rats. Biointerface Res. Appl. Chem. 2020, 11, 9311–9317. [Google Scholar]
- El-Beih, A.A.; El-Desoky, A.H.; Al-Hammady, M.A.; Elshamy, A.I.; Hegazy, M.-E.F.; Kato, H.; Tsukamoto, S. New inhibitors of RANKL-induced Osteoclastogenesis from the marine sponge Siphonochalina siphonella. Fitoterapia 2018, 128, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, M.-F.; Tu, Z.-C.; Zhao, Y.; Wang, H.; Li, G.-J.; Sha, X.-M. a-Glucosidase inhibition, anti-glycation and antioxidant activities of Liquidambar formosana Hance leaf, and identification of phytochemical profile. S. Afr. J. Bot. 2017, 113, 239–247. [Google Scholar] [CrossRef]
- Aoki, K.; Kamiyama, H.; Masuda, K.; Kamiko, K.; Noguchi, Y.; Tajima, K.; Terauchi, Y. Effects of miglitol, vildagliptin, or their combination on serum insulin and peptide YY levels and plasma glucose, cholecystokinin, ghrelin, and obestatin levels. Endocr. J. 2014, 61, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kaku, H.; Tajiri, Y.; Yamada, K. Anorexigenic Effects of Miglitol in Concert with the Alterations of Gut Hormone Secretion and Gastric Emptying in Healthy Subjects. Horm. Metab. Res. 2012, 44, 312–318. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Namazi, N.; Jalili, R.B.; Saeedi, M.; Imanparast, S.; Adhami, H.R.; Faramarzi, M.; Ayati, M.; Mahdavi, M.; Larijani, B. Potential Anti-obesity Effects of some Medicinal Herb: In vitro α-Amylase, α-Glucosidase and Lipase Inhibitory Activity. Int. Biol. Biomed. J. Spring. 2019, 5, 1–8. [Google Scholar]
- Domecq, J.P.; Prutsky, G.; Leppin, A.; Sonbol, M.; Altayar, O.; Undavalli, C.; Wang, Z.; Elraiyah, T.; Brito, J.P.; Mauck, K.F.; et al. Drugs Commonly Associated With Weight Change: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 363–370. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Guan, C.; Niu, Y.; Chen, S.-C.; Kang, Y.; Wu, J.-X.; Nishi, K.; Chang, C.C.Y.; Chang, T.-Y.; Luo, T.; Chen, L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat. Commun. 2020, 11, 2478. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Liu, J.; Song, B.-L.; Li, B.-L.; Chang, C.C.; Chang, T.-Y. Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators. J. Steroid Biochem. Mol. Biol. 2014, 151, 102–107. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M.; Rajagopal, P.; Periyasamy, V.; Veeraraghavan, V.; Govindan, R.; Jayaraman, S. Beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats. Eur. J. Pharmacol. 2020, 873, 173004. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Babu, S.; Rajagopal, P.; Nazar, S.P.; Chinnaiyan, M.; Jayaraman, S. Effect of β-sitosterol on insulin receptor, glucose transporter 4 protein expression and glucose oxidation in the gastrocnemius muscle of high fat diet induced type-2 diabetic experimental rats. Indian J. Pharm. Educ. Res. 2021, 55, S479–S491. [Google Scholar] [CrossRef]
- Ramalingam, S.; Packirisamy, M.; Karuppiah, M.; Vasu, G.; Gopalakrishnan, R.; Gothandam, K.; Thiruppathi, M. Effect of β-sitosterol on glucose homeostasis by sensitization of insulin resistance via enhanced protein expression of PPRγ and glucose transporter 4 in high fat diet and streptozotocin-induced diabetic rats. Cytotechnology 2020, 72, 357–366. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated Protein Kinase: Maintaining Energy Homeostasis at the Cellular and Whole-Body Levels. Annu. Rev. Nutr. 2014, 34, 31–55. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2021, 146, 112563. [Google Scholar] [CrossRef]
- Aguilar-Recarte, D.; Palomer, X.; Wahli, W.; Vázquez-Carrera, M. The PPARβ/δ-AMPK Connection in the Treatment of Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 8555. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Widiasari, S. Mekanisme Inhibisi Angiotensin Converting Enzym Oleh Flavonoid Pada Hipertensi Inhibition Angiotensin Converting Enzym Mechanism By Flavonoid in Hypertension. Collab. Med. J. 2018, 1, 30–44. [Google Scholar]
- Liao, P.-C.; Lai, M.-H.; Hsu, K.-P.; Kuo, Y.-H.; Chen, J.; Tsai, M.-C.; Li, C.-X.; Yin, X.-J.; Jeyashoke, N.; Chao, L.K.-P. Identification of β-Sitosterol as in Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Gombault, A.; Baron, L.; Couillin, I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front. Immunol. 2013, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Ti, H.; Zhuang, Z.; Yu, Q.; Wang, S. Progress of Plant Medicine Derived Extracts and Alkaloids on Modulating Viral Infections and Inflammation. Drug Des. Dev. Ther. 2021, 15, 1385–1408. [Google Scholar] [CrossRef]
- Qin, R.; You, F.-M.; Zhao, W.; Xie, X.; Peng, C.; Zhang, G.; Han, B. Naturally derived indole alkaloids targeting regulated cell death (RCD) for cancer therapy: From molecular mechanisms to potential therapeutics target. J. Hematol. Oncol. 2022, 15, 133. [Google Scholar] [CrossRef]
- Lee, H.; Baek, S.H.; Lee, J.H.; Kim, C.; Ko, J.-H.; Lee, S.-G.; Chinnathambi, A.; Alharbi, S.A.; Yang, W.M.; Um, J.-Y.; et al. Isorhynchophylline, a Potent Plant Alkaloid, Induces Apoptotic and Anti-Metastatic Effects in Human Hepatocellular Carcinoma Cells through the Modulation of Diverse Cell Signaling Cascades. Int. J. Mol. Sci. 2017, 18, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S.; Osman, W.J.; Garelnabi, E.A.; Osman, Z.; Osman, B.; Khalid, H.S.; Mohamed, M.A. Secondary metabolites as anti-inflammatory agents. J. Phytopharm. 2014, 3, 275–285. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Chen, X.; Bi, L. Research Progress on Anti-Inflammatory Effects and Mechanisms of Alkaloids from Chinese Medical Herbs. Evid.-Based Complement. Altern. Med. 2020, 2020, 1303524. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, K.; Qiao, S.-M.; Dai, Y.; Wei, Z.-F. Norisoboldine, a natural aryl hydrocarbon receptor agonist, alleviates TNBS-induced colitis in mice, by inhibiting the activation of NLRP3 inflammasome. Chin. J. Nat. Med. 2018, 16, 161–174. [Google Scholar] [CrossRef]
- Chen, Q.; Duan, X.; Fan, H.; Xu, M.; Tang, Q.; Zhang, L.; Shou, Z.; Liu, X.; Zuo, D.; Yang, J.; et al. Oxymatrine protects against DSS-induced colitis via inhibiting the PI3K/AKT signaling pathway. Int. Immunopharmacol. 2017, 53, 149–157. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Yuan, Z.-W.; Qu, C.; Yu, X.-T.; Huang, T.; Chen, P.V.; Su, Z.-R.; Dou, Y.-X.; Wu, J.-Z.; Zeng, H.-F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef]
- Fu, X.; Sun, F.; Wang, F.; Zhang, J.; Zheng, B.; Zhong, J.; Yue, T.; Zheng, X.; Xu, J.-F.; Wang, C.-Y. Aloperine Protects Mice against DSS-Induced Colitis by PP2A-Mediated PI3K/Akt/mTOR Signaling Suppression. Mediat. Inflamm. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wylie, J.L.; Hatch, G.M.; McClarty, G. Host cell phospholipids are trafficked to and then modified by Chlamydia trachomatis. J. Bacteriol. 1997, 179, 7233–7242. [Google Scholar] [CrossRef] [PubMed]
- Dhinakaran, D.I.; Manohari, V.; Atchya, B.; Tamilselvi, K.; Lipton, A.P. Antifungal and cytotoxic activities of some marine sponges collected from the South East Coast of India. J. Appl. Pharm. Sci. 2012, 2, 52–55. [Google Scholar]
- Kim, H.J.; Kang, D.W. Two new species of the genus Callyspongia (Haplosclerida:Callyspongiidae) from Korea. J. Asia Pac. Biodivers. 2017, 10, 448–452. [Google Scholar]
- Murakami, N.; Wang, W.; Aoki, M.; Tsutsui, Y.; Sugimoto, M.; Kobayashi, M. Total synthesis of callystatin A, a potent cytotoxic polyketide from the marine sponge, Callyspongia truncata. Tetrahedron Lett. 1998, 39, 2349–2352. [Google Scholar] [CrossRef]
- Sajjadi, S.E.; Ghanadian, M.; Haghighi, M.; Mouhebat, L. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. J. Herb. Med. Pharmacol. 2015, 4, 15–19. [Google Scholar]
- Hadisaputri, Y.E.; Miyazaki, T.; Suzuki, S.; Yokobori, T.; Kobayashi, T.; Tanaka, N.; Inose, T.; Sohda, M.; Kuwano, H. TNFAIP8 Overexpression: Clinical Relevance to Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2011, 19, 589–596. [Google Scholar] [CrossRef]
- Boitz, J.M.; Ullman, B. Adenine and adenosine salvage in Leishmania donovani. Mol. Biochem. Parasitol. 2013, 190, 51–55. [Google Scholar] [CrossRef]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; de Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the Marine Sponge Callyspongia aerizusa: Cyclic Peptides with Antitubercular Activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef]
- Huang, L.; Li, T.; Zhou, H.; Qiu, P.; Wu, J.; Liu, L. Sinomenine potentiates degranulation of RBL-2H3 basophils via up-regulation of phospholipase A2 phosphorylation by Annexin A1 cleavage and ERK phosphorylation without influencing on calcium mobilization. Int. Immunopharmacol. 2015, 28, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Yamanaka, N.; Matsui, N.; Fukutsuji, K.; Yamada, S.; Itoh, K.; Akagi, M. Does β-Hexosaminidase Function Only as a Degranulation Indicator in Mast Cells? The Primary Role of β-Hexosaminidase in Mast Cell Granules. J. Immunol. 2014, 193, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Dinneswara Reddy, G.; Park, S.J.; Cho, H.M.; Kim, T.J.; Lee, M.E. Antiallergic Activity Profile In Vitro RBL-2H3 and In Vivo Passive Cutaneous Anaphylaxis Mouse Model of New Sila-Substituted 1,3,4-Oxadiazoles. J. Med. Chem. 2012, 55, 6438–6444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lundqvist, A. Immunomodulatory Effects of IL-2 and IL-15; Implications for Cancer Immunotherapy. Cancers 2020, 12, 3586. [Google Scholar] [CrossRef]
- Leonard, W.J.; Lin, J.-X.; O’Shea, J.J. The γc Family of Cytokines: Basic Biology to Therapeutic Ramifications. Immunity 2019, 50, 832–850. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Peppard, J.V.; Loo, P.; Sills, M.A.; Munster, D.; Pomponi, S.A.; Wright, A.E. Characterization of an Interleukin 6 Cytokine Family Antagonist Protein from a Marine Sponge, Callyspongia sp. J. Biol. Chem. 1996, 271, 7281–7284. [Google Scholar] [CrossRef]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J.; et al. Response to IL-6 trans- A nd IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Halima, S.B.; Mishra, S.; Raja, K.M.P.; Willem, M.; Baici, A.; Simons, K.; Brüstle, O.; Koch, P.; Haass, C.; Caflisch, A.; et al. Specific Inhibition of β-Secretase Processing of the Alzheimer Disease Amyloid Precursor Protein. Cell Rep. 2016, 14, 2127–2141. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Huang, C.; Fu, X.-H.; Zhou, N.; Li, J.-M. The Role of Wnt/β-Catenin Signaling Pathway in Disrupted Hippocampal Neurogenesis of Temporal Lobe Epilepsy: A Potential Therapeutic Target? Neurochem. Res. 2015, 40, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- Valvezan, A.J.; Klein, P.S. GSK-3 and Wnt Signaling in Neurogenesis and Bipolar Disorder. Front. Mol. Neurosci. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.H.; Kim, H.-H. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem. Biophys. Res. Commun. 2003, 305, 211–214. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.H.; Tsukamoto, S. Marine natural products that inhibit osteoclastogenesis and promote osteoblast differentiation. J. Nat. Med. 2022, 76, 575–583. [Google Scholar] [CrossRef] [PubMed]
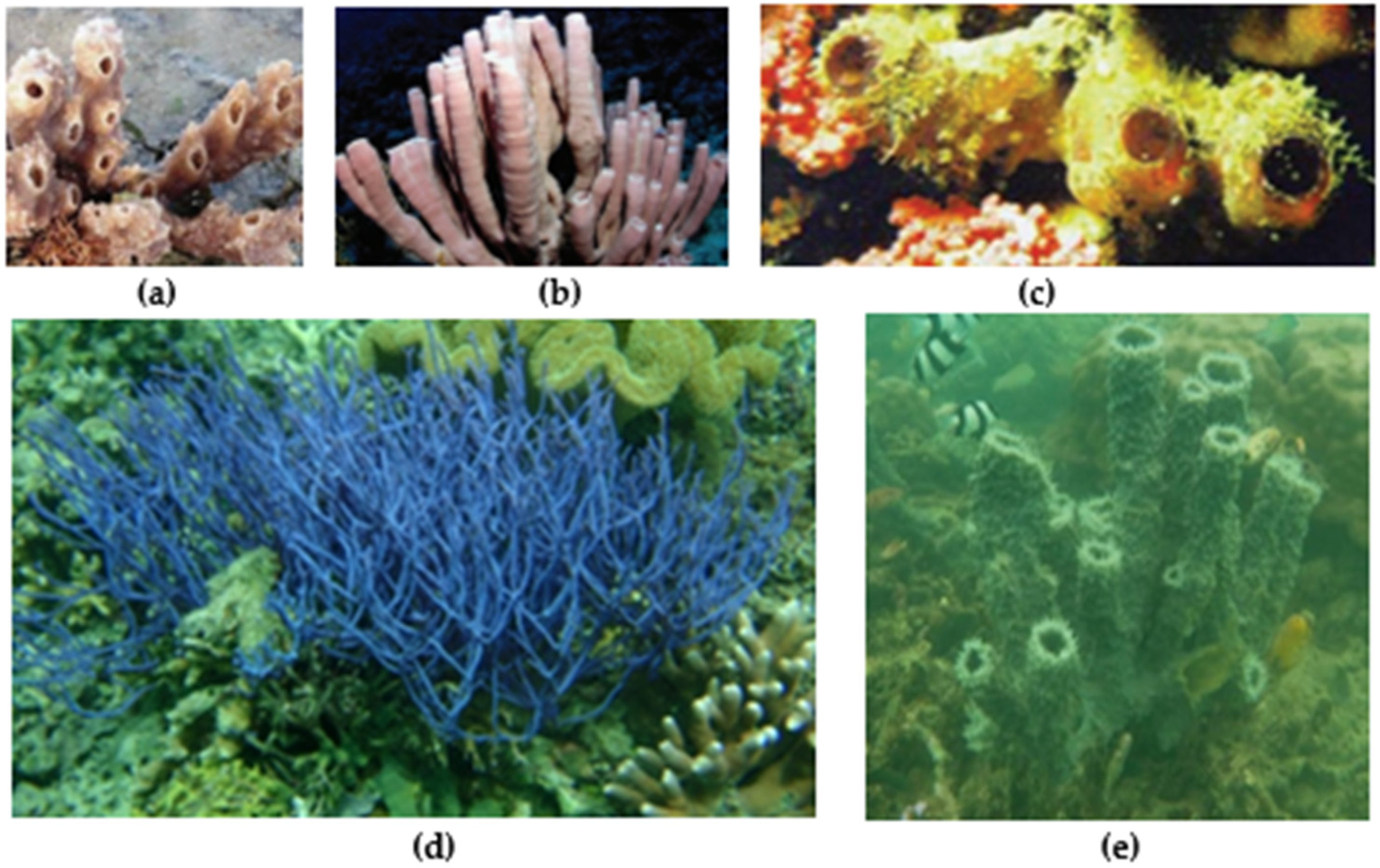

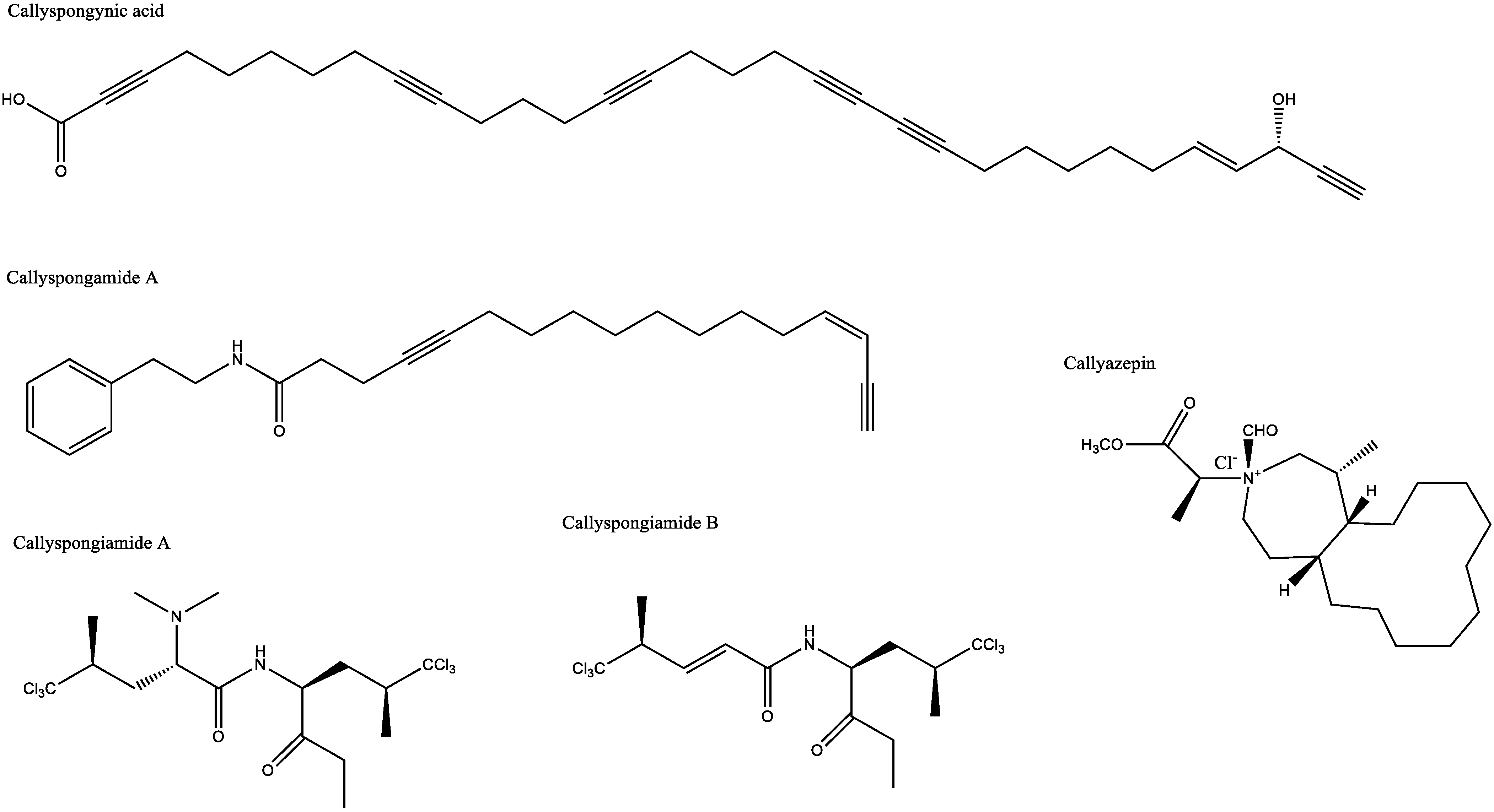
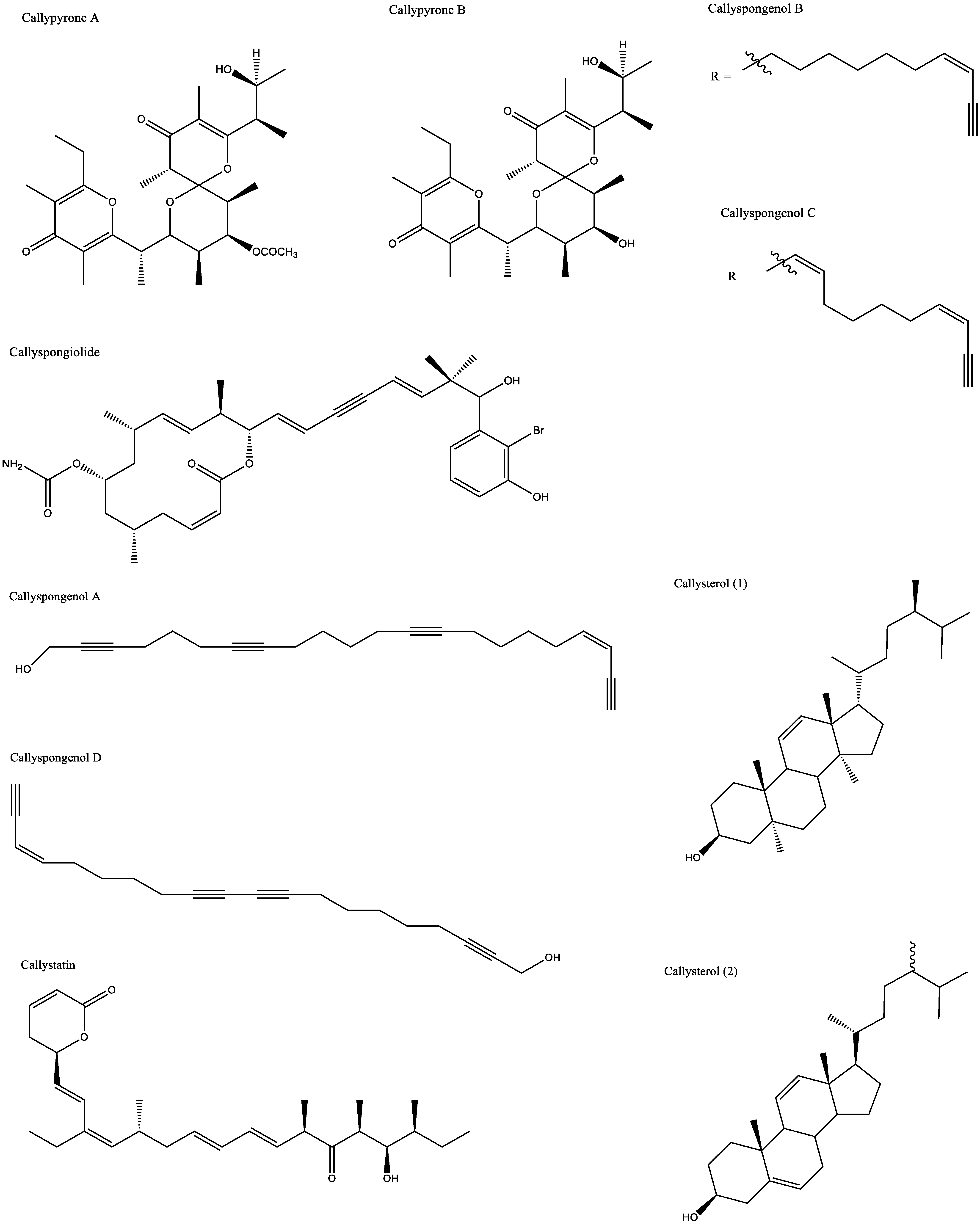
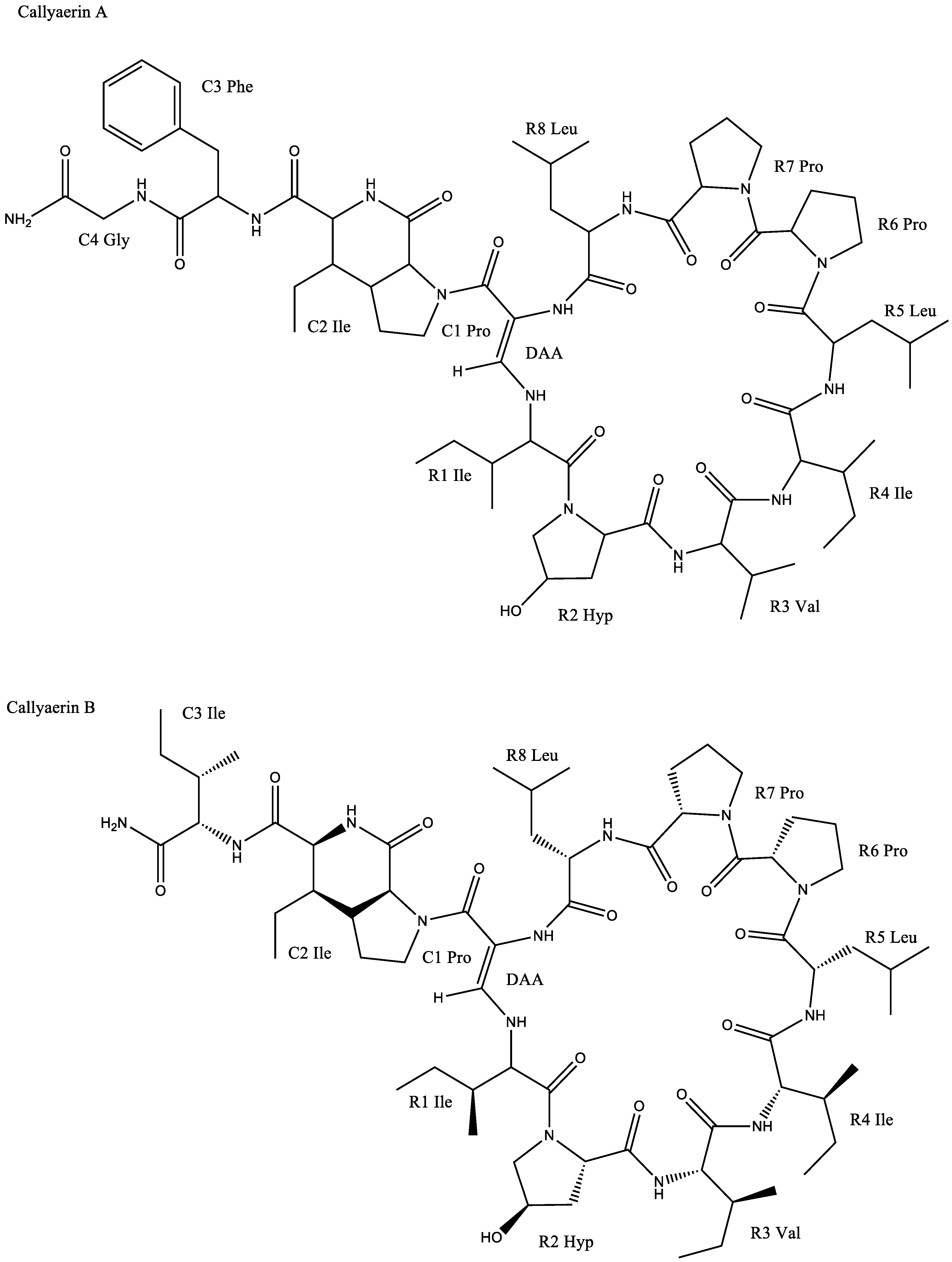
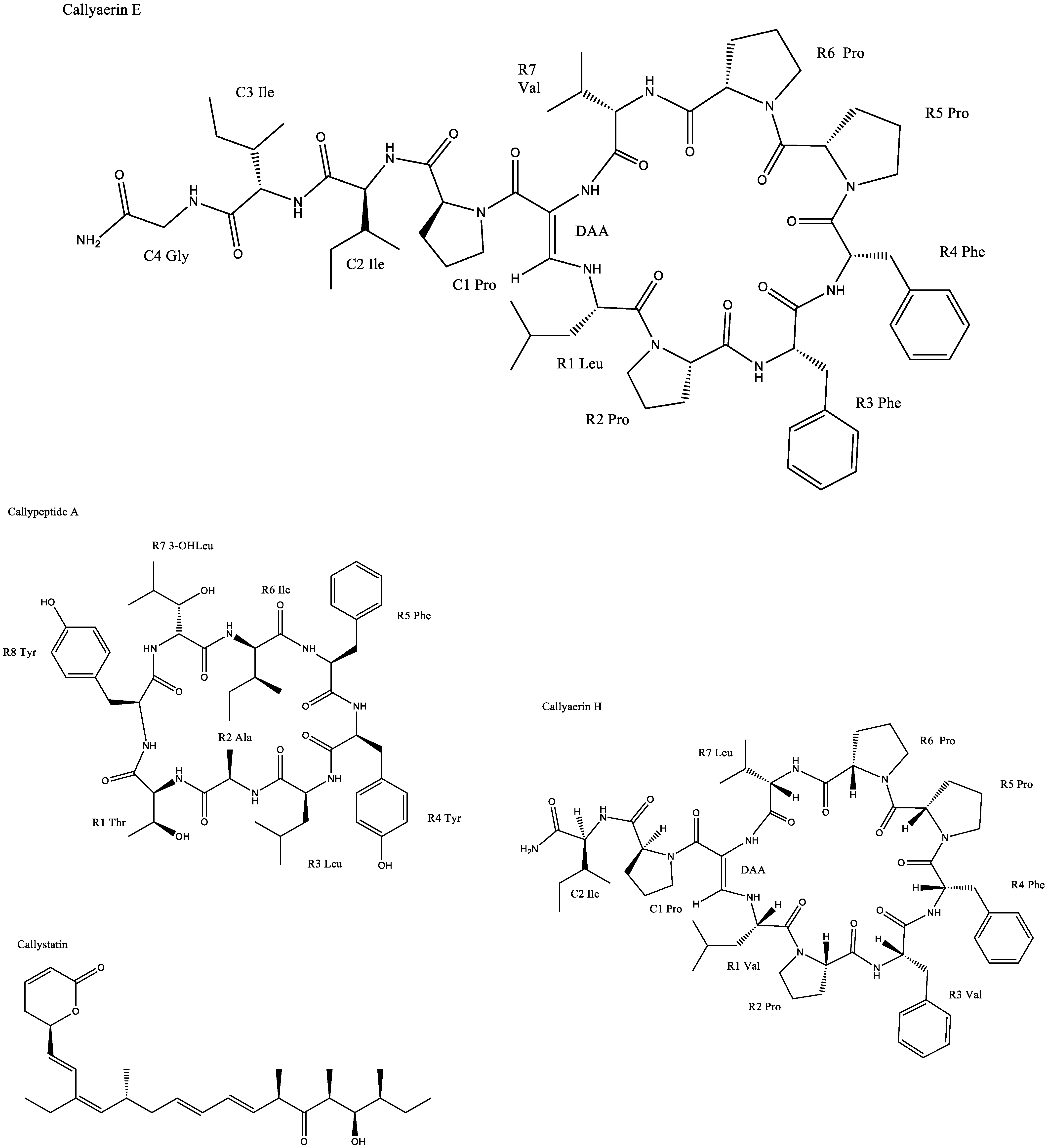




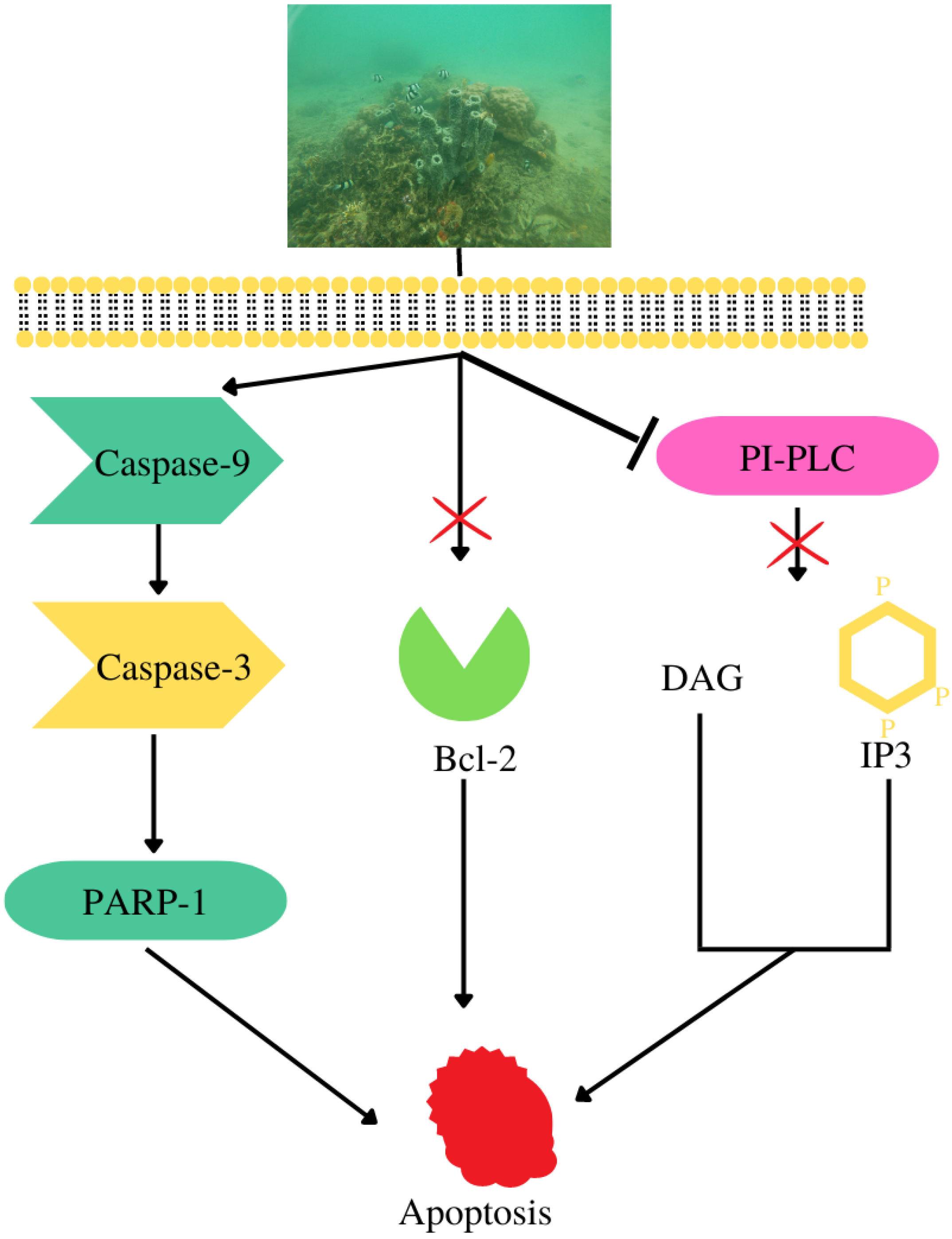
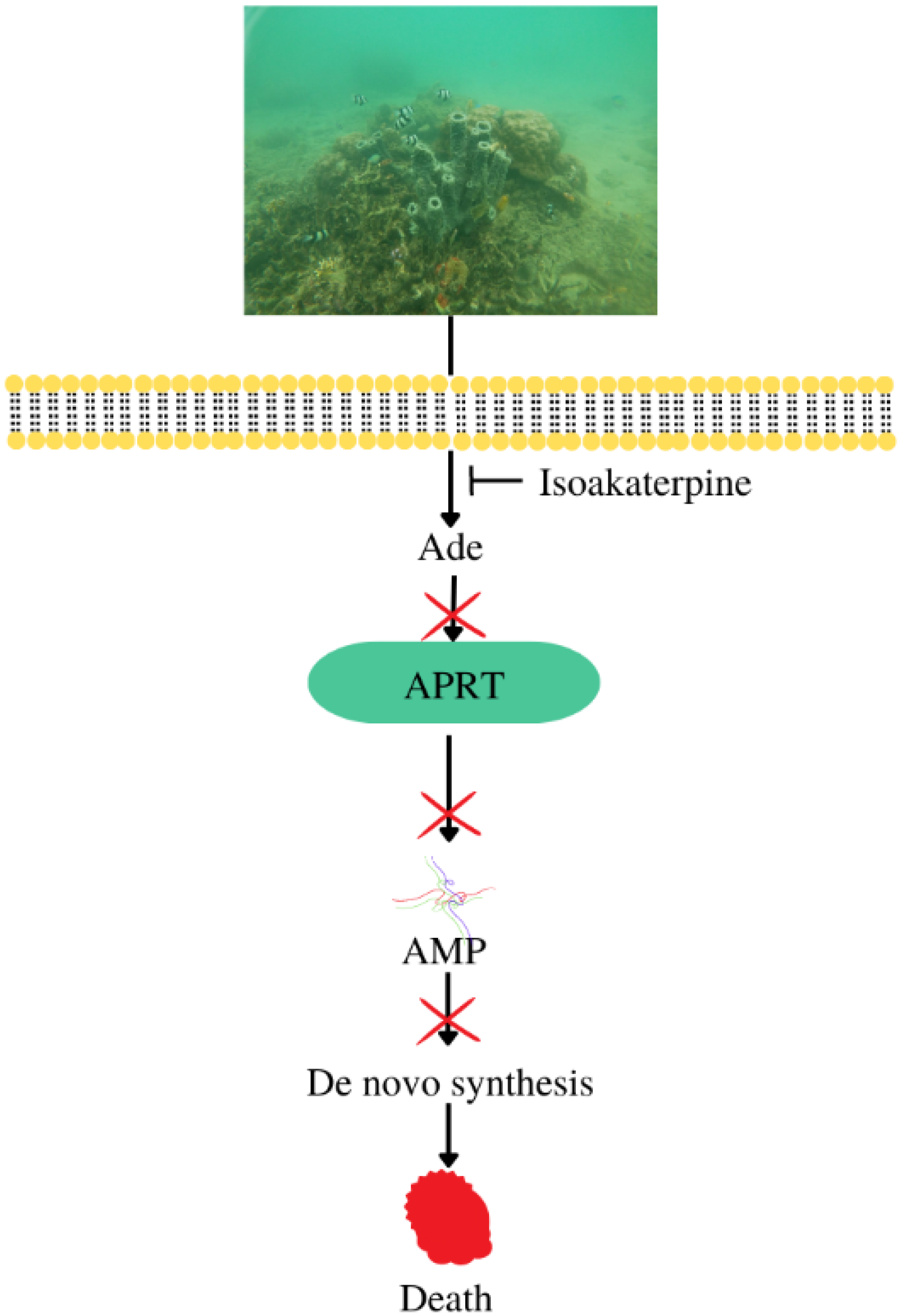
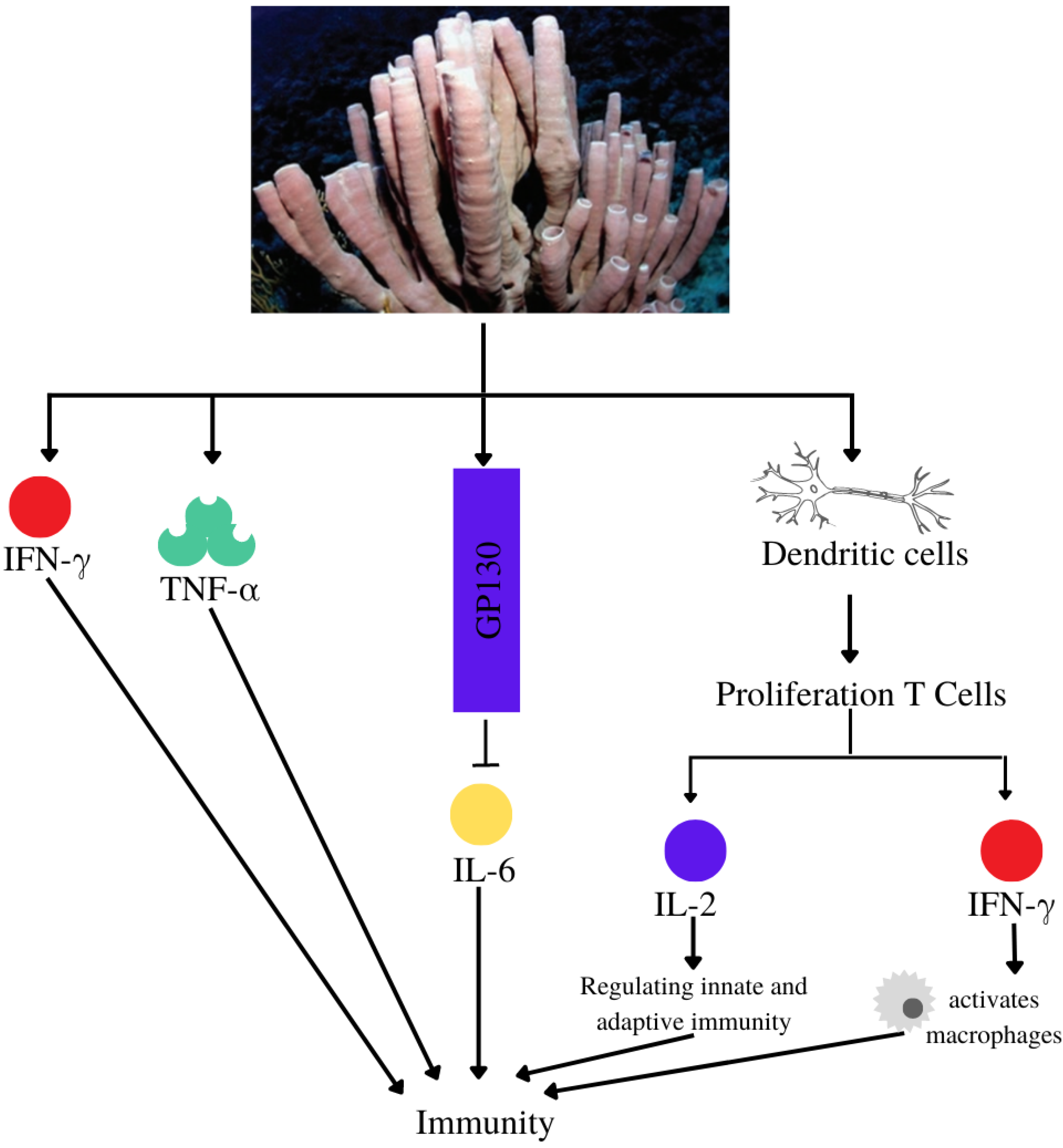



| Pharmacological Activity | Callyspongia spp. | Secondary Metabolite | Description of Activity | Ref. |
|---|---|---|---|---|
| Antidiabetic and antiobesity | Callyspongia truncata | Callyspongynic acid | IC50 against α-glucosidase: 0.25 μg/mL | [29,30] |
| Callyspongia samarensis | - | EC50 14.47 μg/mL (AMPK Activation) | [31] | |
| Callyspongia sp. | β-Sitosterol | Activation of GLUT-4 and insulin receptors | [32] | |
| Antihypercholesterolemic | Callyspongia sp. | Callyspongiamide A | IC50 against SOAT-1 and SOAT-2: 0.78 ± 0.19 and 2.8 ± 0.72 μM | [5] |
| Callyspongiamide B | IC50 against SOAT-1 and SOAT-2: 1.2 ± 0.31 and 2.4 ± 0.96 μM | |||
| Disamide A | IC50 against SOAT-1 and SOAT-2: 5.2 ± 0.93 and 4.2 ± 0.76 μM | |||
| Antihypertensive | Callyspongia diffusa | Callypyrone A | IC50 against Angiotensin I-converting enzyme (ACE): 0.48 mM | [33] |
| Callypyrone B | IC50 against ACE: 0.57 mM | |||
| Anti-inflammatory | Callyspongia crassa | - | 61.47% inhibition of protein denaturation | [34] |
| Callyspongia sp. | - | 97% inhibition of hemolysis (at a dose of 3200 ppm) | [35] | |
| Callyspongia sp. | Cyclo[L-Hyp-L-Ala] | Increase secretion of IL-10 (J774A.1 cells) by 1.65-fold | [36] | |
| Cyclo[L-Pro-Gly] | Increase secretion of IL-10 (J774A.1 cells) 1.29-fold | |||
| Cyclo[L-Pro-Phe] | Increase secretion of IL-10 (J774A.1 cells) 1.54-fold | |||
| Cyclo[L-Pro-Ala] | Increased secretion of IL-10 (J774A. 1 cells) 1.56-fold | |||
| Callyspongia sp. | β-Sitosterol |
| [37] | |
| Callyspongia siphonella | Callysterol | 19.5 ± 7.3 mL (Edema volume) | [38] | |
| Antifungal | Callyspongia aff. implexa | Gelliusterol E | IC50 against Chlamydia trachomatis: 2.34 ± 0.22 µM (No inclusion at a concentration of 40 μM) | [39] |
| Callyspongia aerizusa | Callyaerin A | Chlamydia albican inhibition with a zone of inhibition of 25–30 mm | [6] | |
| Callyaerin B | Chlamydia albican inhibition with a zone of inhibition of 15 mm | |||
| Callyaerin E | Chlamydia albican inhibition with a zone of inhibition of 20 mm | |||
| Callyspongia sp. | β-Sitosterol | Average inhibition diameter against Fusarium spp.: 10 mm | [39,40] | |
| Callyspongia sp. | (-)-Siphonodiol | MIC against Trichophyton asteroides: 25.0 μg/mL | [41] | |
| Callyspongia fibrosa | 4-hydroxybenzoic acid | Antifungal against Ganoderma boninense | [42,43] | |
| Cytotoxicity against cancer cell lines | Callyspongia siphonella | - | IC50 against: | [34] |
| ||||
| Neviotine-C | IC50 against: | [44] | ||
| ||||
| Neviotine A | IC50 against: | |||
| ||||
| IC50 against: | [45] | |||
| ||||
| Sipholenol-A | IC50 against: | [44] | ||
| ||||
| IC50 against: | [45] | |||
| ||||
| Sipholenone A | IC50 against: | [44] | ||
| ||||
| IC50 against: | [45] | |||
| ||||
| Sipholenol L | IC50 against: | |||
| ||||
| Callyspongia crassa | - | IC50 against: | [34] | |
| ||||
| Callyspongia sp. | Callyspongiolide | IC50 against: | [46] | |
| ||||
| Callyspongia sp. | Callypeptide A | GI50 against: | [47] | |
| ||||
| Callyspongia sp. | Callyazepin | IC50 against: | [48] | |
| ||||
| (3R)-methylazacyclodecane | IC50 against: | |||
| ||||
| Callyspongia sp. (CMB-01152) | Hymenialdisine | IC50 against: | [49] | |
| ||||
| Callyspongia schulzei | - | IC50 against: | [50] | |
| ||||
| Callyspongia aerizusa | Callyaerin E | IC50 against L5178Y cell line: 0.39 μM | [6] | |
| Callyaerin H | IC50 against L5178Y cell line: 0.48 μM | |||
| Callyspongia truncata | Callystatin | IC50 against KB cell line: 0.01 μg/mL | [51] | |
| - | Further research: IC50 against: | |||
| ||||
| Callyspongia sp. | (−)-(3R,18R) alcohol | IC50 against TR-LE cell line: 0.11 μM | [52] | |
| (+)-(3S,18S) | IC50 against TR-LE cell line: 0.47 μM | |||
| Callyspongia sp. | Siphonodiol | IC50 against HL-60 cell line: 6.5 μg/mL | [53] | |
| Callyspongidiol | IC50 against HL-60 cell line: 2.8 μg/mL | |||
| 14,15-dihydrosphonodiol | IC50 against HL-60 cell line: 6.5 μg/mL | |||
| Callyspongia sp. | Callyspongenols A | IC50 against: | [54] | |
| ||||
| Callyspongenols B | IC50 against: | |||
| ||||
| Callyspongenols C | IC50 against: | |||
| ||||
| Callyspongenols D | IC50 against: | |||
| ||||
| Callyspongia fistularis | Callyspongamide A | IC50 against HeLa cell line: 4.1 μg/mL | [55] | |
| Callyspongia sp. | Alkupikanye E | IC50: 5 μg/mL | [56] | |
| Alkupikanye F | IC50: 10 μg/mL | |||
| Callyspongia sp. | - | IC50: 2 μg/mL against NIH3T3 cells transfected with the human EGF receptor | [57] | |
| 8-Bromooctyl tert-butyldimethylsilyl ether (fraction, n = 3) 9-(3-Pyridyl)nonyl alcohol (fraction, n = 3) | IC50: 1.3 μg/mL against NIH3T3 cells transfected with human EGF receptor gene | |||
| Callyspongia sp. | Akaterpin | IC50 against PI-PLC: 0.5 μg/mL | [58] | |
| Callyspongia aerizusa | - | IC50 against: | [59] | |
| ||||
| Antimicrobial | Callyspongia crassa | - | LC50 against: | [60] |
| ||||
| Callyspongia siphonella | Siphonocholin | MIC against Pseudomonas aeruginosa: 64 μg/mL | [61] | |
| Sipholenol L | Inhibition against: | [45] | ||
| ||||
| Neviotine A | Inhibition against: | |||
| ||||
| Sipholenone A | Inhibition against: | |||
| ||||
| Callyspongia aerizusa | Callyaerin A | Inhibition against: | [6] | |
| ||||
| Callyaerin E | Inhibition against: | |||
| ||||
| Antioxidant | Callyspongia crassa | - | Percentage of inhibition: 58.1% at 671 μg/mL | [34] |
| Callyspongia aerizusa | - | Percentage of inhibition 56.6% at 0.5 μg/mL, 57.2% at 0.6 μg/mL, and 58.4% at 0.7 μg/mL | [62] | |
| Antiparasitic | Callyspongia sp. | Isoakaterpine | IC50 against adenosine phosphoribosyltransferase of Leishmania spp: 1.05 μM | [11] |
| Antiallergic | Callyspongia sp. | 3-(2-(4-hydroxyphenyl)-2-oxoethyl)-5,6-dihydropyridine-2(1H)-one | IC50 againts RBL-2H3: 18.7 ± 6.7 μM | [63,64] |
| Antituberculosis | Callyspongia aerizusa | Callyaerin A | MIC90 against Mycobacterium tuberculosis: 2 μM | [64] |
| Callyaerin B | MIC90 against Mycobacterium tuberculosis: 5 μM | |||
| Antiviral | Callyspongia crassa | - | 85.3% against hepatitis A virus (MIC 9.765 μg/mL) | [34] |
| Callyspongia siphonella | - | 83.7% against hepatitis A virus (MIC 0.625 μg/mL) | ||
| Immunomodulatory | Callyspongia sp. | Niphatoxin C | IC50 P2X7 antagonism: 11.5 μM | [65] |
| Callyspongia sp. | Siphonodiol |
| [66] | |
| Callyspongia sp. | Callyspongidiol | Modulates dendritic cell function for T1 cell proliferation | [53] | |
| 14,15-dihydrosphonodiol | ||||
| Callyspongia sp. | β-Sitosterol | Modulates dendritic cell activity and increases peripheral blood mononuclear cell viability | [67] | |
| Callyspongia sp. | - | Increase levels of IFN-γ and TNF-α (Wistar strain mice) at extract doses of 300 mg/kg and 400 mg/kg | [68] | |
| Antineurodegenerative | Callyspongia samarensis | - | IC50 against β-secretase: 99.82 μg/mL | [31] |
| Callyspongia sp. | Hymenialdisine | IC50 against: | [49] | |
| ||||
| Antiosteoporotic | Callyspongia siphonella | Neviotine D | IC50 against RANKL: 12.8 μM | [69] |
| Neviotine A | IC50 against RANKL: 32.8 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadisaputri, Y.E.; Nurhaniefah, A.A.; Sukmara, S.; Zuhrotun, A.; Hendriani, R.; Sopyan, I. Callyspongia spp.: Secondary Metabolites, Pharmacological Activities, and Mechanisms. Metabolites 2023, 13, 217. https://doi.org/10.3390/metabo13020217
Hadisaputri YE, Nurhaniefah AA, Sukmara S, Zuhrotun A, Hendriani R, Sopyan I. Callyspongia spp.: Secondary Metabolites, Pharmacological Activities, and Mechanisms. Metabolites. 2023; 13(2):217. https://doi.org/10.3390/metabo13020217
Chicago/Turabian StyleHadisaputri, Yuni Elsa, Annida Adha Nurhaniefah, Sendi Sukmara, Ade Zuhrotun, Rini Hendriani, and Iyan Sopyan. 2023. "Callyspongia spp.: Secondary Metabolites, Pharmacological Activities, and Mechanisms" Metabolites 13, no. 2: 217. https://doi.org/10.3390/metabo13020217
APA StyleHadisaputri, Y. E., Nurhaniefah, A. A., Sukmara, S., Zuhrotun, A., Hendriani, R., & Sopyan, I. (2023). Callyspongia spp.: Secondary Metabolites, Pharmacological Activities, and Mechanisms. Metabolites, 13(2), 217. https://doi.org/10.3390/metabo13020217











