Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance
Abstract
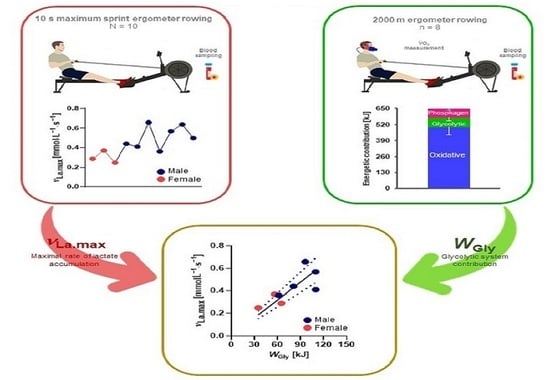
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Lactate Diagnostics
2.3. Cardio Pulmonary Exercise Testing (CPET)
2.4. Rowing Ergometers
2.5. Heart Rate
2.6. Laboratory Conditions
2.7. The 10 s RST
2.8. The 2000 m RTT
- (1)
- 2000 m RTT performance (s).
- (2)
- Average power over the first and last 300 m of 2000 m RTT (P300first and P300last in W).
- (3)
- Mechanical power output difference between 300 m first and last (∆300 first-last).
- (4)
- O2 (litres O2·min−1) before, during, and after 2000 m RTT.
- (5)
- Resting lactate and peak blood lactate concentration after 2000 m RTT.
2.9. The Incremental Step Test
2.10. Calculations of Energetic Contributions during 2000 m RTT
2.11. Statistical Analyses
3. Results
3.1. Calculation of the Three Energy System Contributions (PCr-La−-O2 Method) during 2000 m RTT
3.2. νLa.max and tPCr of 10 s RST, P300first, and P300last of 2000 m RTT
3.3. The Relationship between νLa.max and WGly over 2000 m RTT
3.4. The Relationship between νLa.max, WGly, P4, Absolute O2peak, and 2000 m RTT Performance
3.5. The Influence of νLa.max on Specific Sections of 2000 m RTT
3.6. Separate Classification of νLa.max, O2peak, and Performance over P300first and ∆300first−last for Male Athletes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleshnev, V. Biomechanics of Rowing: A Unique Insight into the Technical and Tactical Aspects of Elite Rowing; The Crowood Press: Marlborough, UK, 2020. [Google Scholar]
- Ingham, S.A.; Whyte, G.P.; Jones, K.; Nevill, A.M. Determinants of 2,000 m rowing ergometer performance in elite rowers. Eur. J. Appl. Physiol. 2002, 88, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Yoshiga, C.C.; Higuchi, M. Rowing performance of female and male rowers. Scand J. Med. Sci. Sports 2003, 13, 317–321. [Google Scholar] [CrossRef]
- Larsson, L.; Forsberg, A. Morphological muscle characteristics in rowers. Can. J. Appl. Sport Sci 1980, 5, 239–244. [Google Scholar]
- Hagerman, F.C.; Connors, M.C.; Gault, J.A.; Hagerman, G.R.; Polinski, W.J. Energy expenditure during simulated rowing. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 87–93. [Google Scholar] [CrossRef]
- Mikulic, P.; Bralic, N. Elite status maintained: A 12-year physiological and performance follow-up of two Olympic champion rowers. J. Sports Sci. 2018, 36, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Treff, G.; Schmidt, W.; Wachsmuth, N.; Völzke, C.; Steinacker, J.M. Total haemoglobin mass, maximal and submaximal power in elite rowers. Int. J. Sports Med. 2014, 35, 571–574. [Google Scholar] [CrossRef]
- Cosgrove, M.J.; Wilson, J.; Watt, D.; Grant, S.F. The relationship between selected physiological variables of rowers and rowing performance as determined by a 2000 m ergometer test. J. Sports Sci. 1999, 17, 845–852. [Google Scholar] [CrossRef]
- Secher, N.H. The physiology of rowing. J. Sport. Sci. 1983, 1, 23–53. [Google Scholar] [CrossRef]
- Gillies, E.; Bell, G. The relationship of physical and physiological parameters to 2000 m simulated rowing performance. Res. Sport. Med. Int. J. 2000, 9, 277–288. [Google Scholar] [CrossRef]
- Kramer, J.F.; Leger, A.; Paterson, D.H.; Morrow, A. Rowing performance and selected descriptive, field, and laboratory variables. Can. J. Appl. Physiol. 1994, 19, 174–184. [Google Scholar] [CrossRef]
- de Campos Mello, F.; de Moraes Bertuzzi, R.C.; Grangeiro, P.M.; Franchini, E. Energy systems contributions in 2,000 m race simulation: A comparison among rowing ergometers and water. Eur. J. Appl. Physiol. 2009, 107, 615–619. [Google Scholar] [CrossRef]
- Nielsen, H.B. pH after competitive rowing: The lower physiological range? Acta Physiol. Scand 1999, 165, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, H.; Bourdin, M.; Lacour, J.R.; Denis, C.; Moyen, B.; Messonnier, L. Lactate accumulation in response to supramaximal exercise in rowers. Scand J. Med. Sci. Sports 2013, 23, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, H.; Bourdin, M.; Féasson, L.; Dubouchaud, H.; Messonnier, L.A. Non-oxidative Energy Supply Correlates with Lactate Transport and Removal in Trained Rowers. Int. J. Sports Med. 2020, 41, 936–943. [Google Scholar] [CrossRef]
- Pripstein, L.P.; Rhodes, E.C.; McKenzie, D.C.; Coutts, K.D. Aerobic and anaerobic energy during a 2-km race simulation in female rowers. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Diry, A.; Ratel, S.; Nevill, A.; Maciejewski, H. What is the physiological impact of reducing the 2000 m Olympic distance in rowing to 1500 m and 1000 m for French young competitive rowers? Insights from the energy system contribution. Front. Physiol. 2022, 13, 896975. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef]
- Mader, A. Glycolysis and oxidative phosphorylation as a function of cytosolic phosphorylation state and power output of the muscle cell. Eur. J. Appl. Physiol. 2003, 88, 317–338. [Google Scholar] [CrossRef]
- Heck, H.; Schulz, H.; Bartmus, U. Diagnostics of anaerobic power and capacity. Eur. J. Sport Sci. 2003, 3, 1–23. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [Green Version]
- Mader, A. Zur beurteilung der sportartspezifischen ausdauerleistungsfahigkeit im labor. Sportarzt. Sportmed. 1976, 27, 80–88. [Google Scholar]
- Poole, D.C.; Rossiter, H.B.; Brooks, G.A.; Gladden, L.B. The anaerobic threshold: 50+ years of controversy. J. Physiol. 2021, 599, 737–767. [Google Scholar] [CrossRef]
- Wackerhage, H.; Gehlert, S.; Schulz, H.; Weber, S.; Ring-Dimitriou, S.; Heine, O. Lactate Thresholds and the Simulation of Human Energy Metabolism: Contributions by the Cologne Sports Medicine Group in the 1970s and 1980s. Front. Physiol. 2022, 13, 899670. [Google Scholar] [CrossRef]
- Roth, W.; Schwanitz, P.; Pas, P.; Bauer, P. Force-time characteristics of the rowing stroke and corresponding physiological muscle adaptations. Int. J. Sports Med. 1993, 14, S32–S34. [Google Scholar] [CrossRef]
- Akça, F. Prediction of rowing ergometer performance from functional anaerobic power, strength and anthropometric components. J. Hum. Kinet. 2014, 41, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Gabarren, M.; Expósito, R.G.; de Villarreal, E.S.; Izquierdo, M. Physiological factors to predict on traditional rowing performance. Eur. J. Appl. Physiol. 2010, 108, 83–92. [Google Scholar] [CrossRef]
- Cataldo, A.; Cerasola, D.; Russo, G.; Zangla, D.; Traina, M. Mean power during 20 sec all-out test to predict 2000 m rowing ergometer performance in national level young rowers. J. Sports Med. Phys. Fit. 2015, 55, 872–877. [Google Scholar]
- Heck, H.; Mader, A.; Hess, G.; Mucke, S.; Muller, R.; Hollmann, W. Justification of the 4-mmol/l lactate threshold. Int. J. Sports Med. 1985, 6, 117–130. [Google Scholar] [CrossRef]
- Mader, A. Eine Theorie zur Berechnung der Dynamik und des steady state von Phosphorylierungszustand und Stoffwechselaktivität der Muskelzelle als Folge des Energiebedarfs; German Sport University Cologne: Cologne, Germany, 1984. [Google Scholar]
- Julio, U.F.; Panissa, V.L.G.; Esteves, J.V.; Cury, R.L.; Agostinho, M.F.; Franchini, E. Energy-System Contributions to Simulated Judo Matches. Int. J. Sports Physiol. Perform. 2017, 12, 676–683. [Google Scholar] [CrossRef]
- Yang, W.H.; Park, J.H.; Shin, Y.C.; Kim, J. Physiological Profiling and Energy System Contributions During Simulated Epée Matches in Elite Fencers. Int. J. Sports Physiol. Perform. 2022, 17, 943–950. [Google Scholar] [CrossRef]
- Yang, W.-H.; Heine, O.; Grau, M. Rapid weight reduction does not impair athletic performance of Taekwondo athletes–A pilot study. PLoS ONE 2018, 13, e0196568. [Google Scholar] [CrossRef] [Green Version]
- Treff, G.; Mentz, L.; Mayer, B.; Winkert, K.; Engleder, T.; Steinacker, J.M. Initial Evaluation of the Concept-2 Rowing Ergometer’s Accuracy Using a Motorized Test Rig. Front. Sports Act. Living 2021, 3, 801617. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Appelhans, D.; Abel, T.; Strüder, H.K. Evaluation of a sport-specific field test to determine maximal lactate accumulation rate and sprint performance parameters in running. J. Sci. Med. Sport 2020, 23, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Beneke, R.; Beyer, T.; Jachner, C.; Erasmus, J.; Hütler, M. Energetics of karate kumite. Eur. J. Appl. Physiol. 2004, 92, 518–523. [Google Scholar] [CrossRef]
- Hausen, M.; Soares, P.P.; Araújo, M.P.; Porto, F.; Franchini, E.; Bridge, C.A.; Gurgel, J. Physiological responses and external validity of a new setting for taekwondo combat simulation. PLoS ONE 2017, 12, e0171553. [Google Scholar]
- Campos, F.A.D.; Bertuzzi, R.; Dourado, A.C.; Santos, V.G.F.; Franchini, E. Energy demands in taekwondo athletes during combat simulation. Eur. J. Appl. Physiol. 2012, 112, 1221–1228. [Google Scholar] [CrossRef]
- Lopes-Silva, J.P.; Da Silva Santos, J.F.; Artioli, G.G.; Loturco, I.; Abbiss, C.; Franchini, E. Sodium bicarbonate ingestion increases glycolytic contribution and improves performance during simulated taekwondo combat. Eur. J. Sport Sci. 2018, 18, 431–440. [Google Scholar] [CrossRef]
- di Prampero, P.E.; Ferretti, G. The energetics of anaerobic muscle metabolism: A reappraisal of older and recent concepts. Respir. Physiol. 1999, 118, 103–115. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Andrade, V.L.; Zagatto, A.M.; Kalva-Filho, C.A.; Mendes, O.C.; Gobatto, C.A.; Campos, E.Z.; Papoti, M. Running-based Anaerobic Sprint Test as a Procedure to Evaluate Anaerobic Power. Int. J. Sports Med. 2015, 36, 1156–1162. [Google Scholar] [CrossRef] [Green Version]
- Keir, D.A.; Thériault, F.; Serresse, O. Evaluation of the running-based anaerobic sprint test as a measure of repeated sprint ability in collegiate-level soccer players. J. Strength Cond. Res. 2013, 27, 1671–1678. [Google Scholar] [CrossRef]
- Meckel, Y.; Gottlieb, R.; Eliakim, A. Repeated sprint tests in young basketball players at different game stages. Eur. J. Appl. Physiol. 2009, 107, 273–279. [Google Scholar] [CrossRef]
- Quittmann, O.J.; Abel, T.; Vafa, R.; Mester, J.; Schwarz, Y.M.; Strüder, H.K. Maximal lactate accumulation rate and post-exercise lactate kinetics in handcycling and cycling. Eur. J. Sport Sci. 2021, 21, 539–551. [Google Scholar] [CrossRef]
- van Loon, L.J.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.; Wagenmakers, A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef]
- Astridge, D.J.; Peeling, P.; Goods, P.S.R.; Girard, O.; Hewlett, J.; Rice, A.J.; Binnie, M.J. Rowing in Los Angeles: Performance Considerations for the Change to 1500 m at the 2028 Olympic Games. Int. J. Sports Physiol. Perform. 2022, 18, 104–107. [Google Scholar] [CrossRef]
- Medbø, J.I.; Mohn, A.C.; Tabata, I.; Bahr, R.; Vaage, O.; Sejersted, O.M. Anaerobic capacity determined by maximal accumulated O2 deficit. J. Appl. Physiol. 1988, 64, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Lacour, J.R.; Bouvat, E.; Barthélémy, J.C. Post-competition blood lactate concentrations as indicators of anaerobic energy expenditure during 400-m and 800-m races. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 172–176. [Google Scholar] [CrossRef]
- Mader, A.; Hartmann, U.; Hollmann, W. Der Einfluß der Ausdauer auf die 6minütige maximale anaerobe und aerobe Arbeitskapazität eines Eliteruderers. In Rudern; Springer: Berlin/Heidelberg, Germany, 1988; pp. 62–78. [Google Scholar]
- Lee, K.H.; Ju, H.M.; Yang, W.H. Metabolic Energy Contributions During High-Intensity Hatha Yoga and Physiological Comparisons Between Active and Passive (Savasana) Recovery. Front. Physiol. 2021, 12, 743859. [Google Scholar] [CrossRef]
- Yang, W.-H.; Park, J.-H.; Park, S.-Y.; Park, Y. Energetic Contributions Including Gender Differences and Metabolic Flexibility in the General Population and Athletes. Metabolites 2022, 12, 965. [Google Scholar] [CrossRef]
- Adelnia, F.; Ubaida-Mohien, C.; Moaddel, R.; Shardell, M.; Lyashkov, A.; Fishbein, K.W.; Aon, M.A.; Spencer, R.G.; Ferrucci, L. Proteomic signatures of in vivo muscle oxidative capacity in healthy adults. Aging Cell 2020, 19, e13124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagatto, A.M.; Beck, W.R.; Gobatto, C.A. Validity of the running anaerobic sprint test for assessing anaerobic power and predicting short-distance performances. J. Strength Cond. Res. 2009, 23, 1820–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitzsche, N.; Baumgärtel, L.; Schulz, H. Comparison of Maximum Lactate Formation Rates in Ergometer Sprint and Maximum Strength Loads. Ger. J. Sports Med. 2018, 69, 13–18. [Google Scholar] [CrossRef]
- Dobson, G.P.; Yamamoto, E.; Hochachka, P.W. Phosphofructokinase control in muscle: Nature and reversal of pH-dependent ATP inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986, 250, R71–R76. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, T.; Adam, J.; Schulz, H. Comparison of calculated and experimental power in maximal lactate-steady state during cycling. Theor. Biol. Med. Model 2014, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Weber, S. Berechnung Leistungsbestimmender Parameter der Metabolischen Aktivität auf Zellulärer Ebene Mittels Fahrradergometrischer Untersuchungen. Diploma Thesis, German Sport University Cologne, Cologne, Germany, 2003. [Google Scholar]
- Esbjörnsson-Liljedahl, M.; Sundberg, C.J.; Norman, B.; Jansson, E. Metabolic response in type I and type II muscle fibers during a 30-s cycle sprint in men and women. J. Appl. Physiol. 1999, 87, 1326–1332. [Google Scholar] [CrossRef] [Green Version]
- Henriksson, J.; Chi, M.M.; Hintz, C.S.; Young, D.A.; Kaiser, K.K.; Salmons, S.; Lowry, O.H. Chronic stimulation of mammalian muscle: Changes in enzymes of six metabolic pathways. Am. J. Physiol. 1986, 251, C614–C632. [Google Scholar] [CrossRef]
- Wende, A.R.; Schaeffer, P.J.; Parker, G.J.; Zechner, C.; Han, D.H.; Chen, M.M.; Hancock, C.R.; Lehman, J.J.; Huss, J.M.; McClain, D.A.; et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 2007, 282, 36642–36651. [Google Scholar] [CrossRef] [Green Version]
- Linossier, M.T.; Dormois, D.; Perier, C.; Frey, J.; Geyssant, A.; Denis, C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol. Scand 1997, 161, 439–445. [Google Scholar] [CrossRef]
- Tesch, P.A.; Komi, P.V.; Häkkinen, K. Enzymatic adaptations consequent to long-term strength training. Int. J. Sports Med. 1987, 8, 66–69. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Hicks, A.L.; MacDonald, J.R.; McKelvie, R.S.; Green, H.J.; Smith, K.M. Muscle performance and enzymatic adaptations to sprint interval training. J. Appl. Physiol. 1998, 84, 2138–2142. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen-Urstad, M.; Svedenhag, J.; Sahlin, K. Effect of muscle mass on lactate formation during exercise in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 189–195. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle Fiber Type Transitions with Exercise Training: Shifting Perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Weber, S.; Weidmann, B.; Gutsche, K.; Platen, P.; Graf, C.; Kappes-Horn, K.; Bloch, W. Cycling exercise-induced myofiber transitions in skeletal muscle depend on basal fiber type distribution. Eur. J. Appl. Physiol. 2012, 112, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, N.; Lenz, J.C.; Voronoi, P.; Schulz, H. Adaption of Maximal Glycolysis Rate after Resistance Exercise with Different Volume Load. Sports Med. Int. Open 2020, 4, E39–E44. [Google Scholar] [CrossRef] [Green Version]
- Parra, J.; Cadefau, J.A.; Rodas, G.; Amigó, N.; Cussó, R. The distribution of rest periods affects performance and adaptations of energy metabolism induced by high-intensity training in human muscle. Acta Physiol. Scand 2000, 169, 157–165. [Google Scholar] [CrossRef]
- Millet, G.P.; Roels, B.; Schmitt, L.; Woorons, X.; Richalet, J.P. Combining hypoxic methods for peak performance. Sports Med. 2010, 40, 1–25. [Google Scholar] [CrossRef]
- Webster, K.A. Evolution of the coordinate regulation of glycolytic enzyme genes by hypoxia. J. Exp. Biol. 2003, 206, 2911–2922. [Google Scholar] [CrossRef] [Green Version]






| Parameters | Total | Male | Female |
|---|---|---|---|
| N = 10 | n = 7 | n = 3 | |
| Age [years] | 19.80 ± 0.9 | 19.8 ± 0.9 | 19.6 ± 0.5 |
| Height [cm] | 183.20 ± 7.0 | 188.4 ± 3.2 | 175.6 ± 4.7 |
| Body mass [kg] | 79.9 ± 13.3 | 85.4 ± 11.2 | 67.2 ± 3.8 |
| Body fat [%] | 16.4 ± 5.1 | 14.8 ± 5.0 | 20.08 ± 3.1 |
| Participants (Sex) | 2000 m RTT Performance | Lapeak | O2peak | P4 |
|---|---|---|---|---|
| s | mmol·L−1 | ml·min−1 | W | |
| P1 (f) | 435 | 15.77 | 4042 | 229 |
| P2 (f) | 459 | 15.77 | 4233 | 200 |
| P3 (f) | 456 | 8.95 | 3546 | 226 |
| P4 (m) | 392 | 18.82 | 5561 | 302 |
| P5 (m) | 378 | 21.30 | 5683 | 298 |
| P6 (m) | 382 | 19.66 | 5626 | 305 |
| P7 (m) | 389 | 15.01 | 5388 | 335 |
| P8 (m) | 372 | 18.02 | 5967 | 338 |
| P9 (m) | 385 | 13.51 | 5757 | 314 |
| P10 (m) | 381 | 15.91 | - | 314 |
| Participants (Sex) | νLa.max | tPCr | P300first | P300last | ∆300 first-last |
|---|---|---|---|---|---|
| mmol·L−1·s−1 | s | W | W | W | |
| P1 (f) | 0.29 | 2.95 | 303 | 290 | 13 |
| P2 (f) | 0.37 | 2.95 | 253 | 233 | 20 |
| P3 (f) | 0.25 | 2.95 | 218 | 218 | 0 |
| P4 (m) | 0.44 | 2.95 | 395 | 361 | 34 |
| P5 (m) | 0.41 | 2.95 | 466 | 408 | 58 |
| P6 (m) | 0.66 | 2.95 | 448 | 388 | 60 |
| P7 (m) | 0.36 | 2.95 | 412 | 411 | 1 |
| P8 (m) | 0.57 | 2.95 | 472 | 433 | 39 |
| P9 (m) | 0.64 | 2.95 | 407 | 320 | 87 |
| P10 (m) | 0.50 | 2.95 | 417 | 350 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schünemann, F.; Park, S.-Y.; Wawer, C.; Theis, C.; Yang, W.-H.; Gehlert, S. Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance. Metabolites 2023, 13, 317. https://doi.org/10.3390/metabo13030317
Schünemann F, Park S-Y, Wawer C, Theis C, Yang W-H, Gehlert S. Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance. Metabolites. 2023; 13(3):317. https://doi.org/10.3390/metabo13030317
Chicago/Turabian StyleSchünemann, Frederik, So-Young Park, Corinna Wawer, Christian Theis, Woo-Hwi Yang, and Sebastian Gehlert. 2023. "Diagnostics of νLa.max and Glycolytic Energy Contribution Indicate Individual Characteristics of Anaerobic Glycolytic Energy Metabolism Contributing to Rowing Performance" Metabolites 13, no. 3: 317. https://doi.org/10.3390/metabo13030317