Thermogenic Capacity of Human Supraclavicular Brown Fat and Cold-Stimulated Brain Glucose Metabolism
Abstract
:1. Introduction
2. Materials and Methods
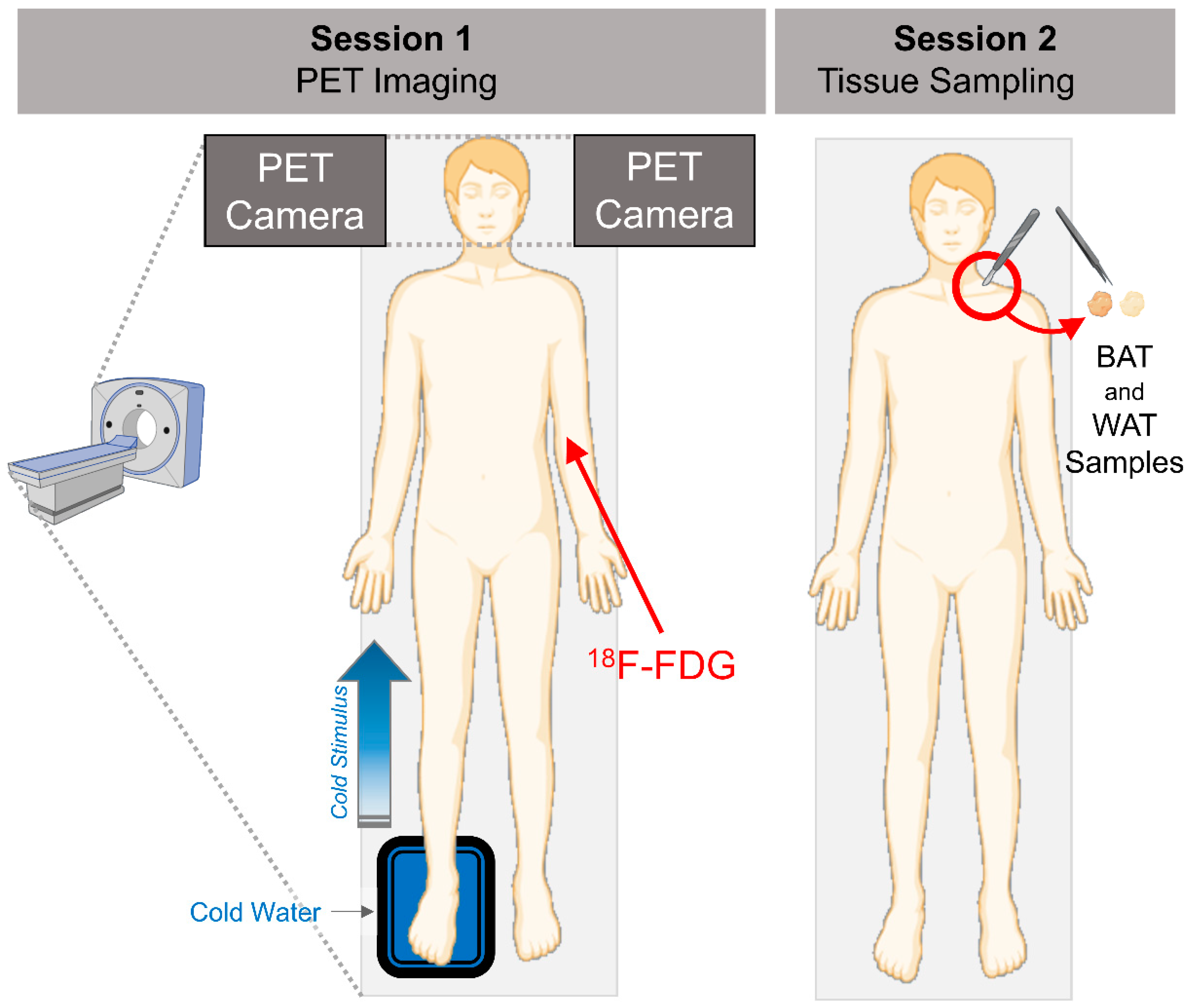
2.1. Human Study Participants and Study Design
2.2. Brain PET Imaging and Analysis
2.3. Adipose Tissue Biopsies
2.4. Statistical Analyses
3. Results
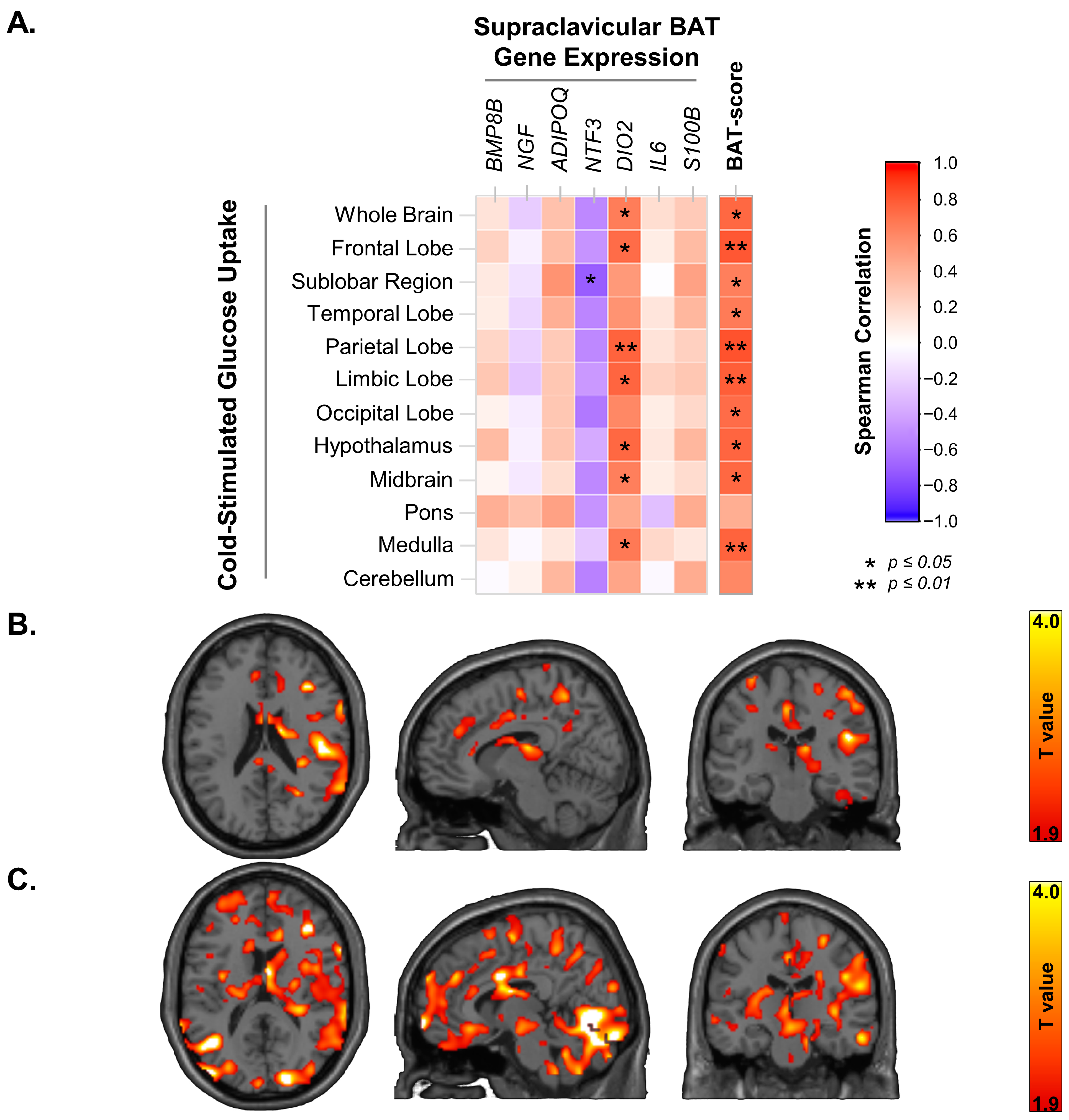
3.1. Supraclavicular BAT UCP-1 Expression and Cold-Stimulated Brain Glucose Uptake
3.2. Supraclavicular BAT Secretome Genes, BAT-Probability Score and Brain Glucose Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanier, W.L. Cerebral Metabolic Rate and Hypothermia: Their Relationship with Ischemic Neurologic Injury. J. Neurosurg. Anesthesiol. 1995, 7, 216–221. [Google Scholar] [CrossRef]
- Brooks, V.B. Study of Brain Function by Local, Reversible Cooling. Rev. Physiol. Biochem. Pharmacol. 1983, 95, 1–109. [Google Scholar] [CrossRef]
- Delgado, J.M.; Hanai, T. Intracerebral Temperatures in Free-Moving Cats. Am. J. Physiol. 1966, 211, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Serota, H.M.; Gerard, R.W. Localized Thermal Changes in the Cat’s Brain. J. Neurophysiol. 1938, 1, 115–124. [Google Scholar] [CrossRef]
- Abrams, R.; Hammel, H.T. Hypothalamic Temperature in Unanesthetized Albino Rats during Feeding and Sleeping. Am. J. Physiol. 1964, 206, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Hayward, J.N.; Baker, M.A. Role of Cerebral Arterial Blood in the Regulation of Brain Temperature in the Monkey. Am. J. Physiol. 1968, 215, 389–403. [Google Scholar] [CrossRef]
- Hayward, J.N.; Smith, E.; Stuart, D.G.; Sawyer, C.H. Temperature Gradients between Arterial Blood and Brain in the Monkey. Exp. Biol. Med. 2016, 121, 547–551. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Lidell, M.E.; Oikonen, V.; Noponen, T.; Viljanen, T.; Scheinin, M.; Taittonen, M.; Niemi, T.; Enerbäck, S.; et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metab. 2011, 14, 272–279. [Google Scholar] [CrossRef] [Green Version]
- U Din, M.; Raiko, J.; Saari, T.; Kudomi, N.; Tolvanen, T.; Oikonen, V.; Teuho, J.; Sipilä, H.T.; Savisto, N.; Parkkola, R.; et al. Human Brown Adipose Tissue [15O]O2 PET Imaging in the Presence and Absence of Cold Stimulus. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1878–1886. [Google Scholar] [CrossRef] [Green Version]
- Ricquier, D. Uncoupling Protein 1 of Brown Adipocytes, the Only Uncoupler: A Historical Perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Monfort-Pires, M.; Regeni-Silva, G.; Dadson, P.; Nogueira, G.A.; U-Din, M.; Ferreira, S.R.G.; Sapienza, M.T.; Virtanen, K.A.; Velloso, L.A. Brown Fat Triglyceride Content Is Associated with Cardiovascular Risk Markers in Adults from a Tropical Region. Front. Endocrinol. 2022, 13, 919588. [Google Scholar] [CrossRef] [PubMed]
- Saari, T.J.; Raiko, J.; U-Din, M.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.; Haaparanta-Solin, M.; Nuutila, P.; Virtanen, K.A. Basal and Cold-Induced Fatty Acid Uptake of Human Brown Adipose Tissue Is Impaired in Obesity. Sci. Rep. 2020, 10, 14373. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Nuutila, P.; Noponen, T.; Parkkola, R.; Viljanen, T.; Enerbäck, S.; Rissanen, A.; Pietiläinen, K.H.; Virtanen, K.A. Blunted Metabolic Responses to Cold and Insulin Stimulation in Brown Adipose Tissue of Obese Humans. Obesity 2013, 21, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Orava, J.; Nummenmaa, L.; Noponen, T.; Viljanen, T.; Parkkola, R.; Nuutila, P.; Virtanen, K.A. Brown Adipose Tissue Function Is Accompanied by Cerebral Activation in Lean but Not in Obese Humans. J. Cereb. Blood Flow Metab. 2014, 34, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarroya, J.; Cereijo, R.; Villarroya, F. An Endocrine Role for Brown Adipose Tissue? Am. J. Physiol. Endocrinol. Metab. 2013, 305, E567–E572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U Din, M.; Saari, T.; Raiko, J.; Kudomi, N.; Maurer, S.F.; Lahesmaa, M.; Fromme, T.; Amri, E.-Z.; Klingenspor, M.; Solin, O.; et al. Postprandial Oxidative Metabolism of Human Brown Fat Indicates Thermogenesis. Cell Metab. 2018, 28, 207–216.e3. [Google Scholar] [CrossRef]
- Cheng, Y.; Jiang, L.; Keipert, S.; Zhang, S.; Hauser, A.; Graf, E.; Strom, T.; Tschöp, M.; Jastroch, M.; Perocchi, F. Prediction of Adipose Browning Capacity by Systematic Integration of Transcriptional Profiles. Cell Rep. 2018, 23, 3112–3125. [Google Scholar] [CrossRef]
- Soukup, J.; Zauner, A.; Doppenberg, E.M.R.; Menzel, M.; Gilman, C.; Young, H.F.; Bullock, R. The Importance of Brain Temperature in Patients after Severe Head Injury: Relationship to Intracranial Pressure, Cerebral Perfusion Pressure, Cerebral Blood Flow, and Outcome. J. Neurotrauma 2004, 19, 559–571. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Normoyle, K.P.; Jackson, K.; Spitler, K.; Sharrock, M.; Miller, C.M.; Best, C.; Llano, D.; Du, R. Brain Temperature and Its Fundamental Properties: A Review for Clinical Neuroscientists. Front. Neurosci. 2014, 8, 307. [Google Scholar] [CrossRef]
- Yablonskiy, D.A.; Ackerman, J.J.H.; Raichle, M.E. Coupling between Changes in Human Brain Temperature and Oxidative Metabolism during Prolonged Visual Stimulation. Proc. Natl. Acad. Sci. USA 2000, 97, 7603–7608. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central Control of Brown Adipose Tissue Thermogenesis. Front. Endocrinol. 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Błaszczyk, J.W. Pathogenesis of Dementia. Int. J. Mol. Sci. 2022, 24, 543. [Google Scholar] [CrossRef]
- Teräs, M. Performance and Methodological Aspects in Positron Emission Tomography; Turun Yliopisto: Turku, Finland, 2008. [Google Scholar]
- Benarroch, E.E. Thermoregulation: Recent Concepts and Remaining Questions. Neurology 2007, 69, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Viitanen, R.; Misiewicz, Z.; Mennesson, M.; Saarnio, S.; Kulesskaya, N.; Kängsep, S.; Liljenbäck, H.; Marjamäki, P.; Autio, A.; et al. The Circadian Gene Cryptochrome 2 Influences Stress-Induced Brain Activity and Depressive-Like Behavior in Mice. Genes Brain Behav. 2021, 20, e12708. [Google Scholar] [CrossRef] [PubMed]
- Morte, B.; Bernal, J. Thyroid Hormone Action: Astrocyte-Neuron Communication. Front. Endocrinol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Fernandez, J.A.; Mampel, T.; Villarroya, F.; Iglesias, R. Direct Assessment of Brown Adipose Tissue as a Site of Systemic Tri-Iodothyronine Production in the Rat. Biochem. J. 1987, 243, 281–284. [Google Scholar] [CrossRef] [Green Version]
- López, M.; Varela, L.; Vázquez, M.J.; Rodríguez-Cuenca, S.; González, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and Fatty Acid Metabolism Mediate Thyroid Regulation of Energy Balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Roeder, L.M.; Williams, I.B.; Tildon, J.T. Glucose Transport in Astrocytes: Regulation by Thyroid Hormone. J. Neurochem. 1985, 45, 1653–1657. [Google Scholar] [CrossRef]
- Roeder, L.M.; Hopkins, I.B.; Kaiser, J.R.; Hanukoglu, L.; Tildon, J.T. Thyroid Hormone Action on Glucose Transporter Activity in Astrocytes. Biochem. Biophys. Res. Commun. 1988, 156, 275–281. [Google Scholar] [CrossRef]
- Zimmer, E.R.; Parent, M.J.; Souza, D.G.; Leuzy, A.; Lecrux, C.; Kim, H.-I.; Gauthier, S.; Pellerin, L.; Hamel, E.; Rosa-Neto, P. [(18)F]FDG PET Signal Is Driven by Astroglial Glutamate Transport. Nat. Neurosci. 2017, 20, 393–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus, L.A.; Carvalho, S.D.; Ribeiro, M.O.; Schneider, M.; Kim, S.-W.; Harney, J.W.; Larsen, P.R.; Bianco, A.C. The Type 2 Iodothyronine Deiodinase Is Essential for Adaptive Thermogenesis in Brown Adipose Tissue. J. Clin. Investig. 2001, 108, 1379–1385. [Google Scholar] [CrossRef]
- Bianco, A.C.; Sheng, X.; Silva, J.E. Triiodothyronine Amplifies Norepinephrine Stimulation of Uncoupling Protein Gene Transcription by a Mechanism Not Requiring Protein Synthesis. J. Biol. Chem. 1988, 263, 18168–18175. [Google Scholar] [CrossRef] [PubMed]
- Rehnmark, S.; Bianco, A.C.; Kieffer, J.D.; Silva, J.E. Transcriptional and Posttranscriptional Mechanisms in Uncoupling Protein MRNA Response to Cold. Am. J. Physiol. 1992, 262, E58–E67. [Google Scholar] [CrossRef]
- Lahesmaa, M.; Orava, J.; Schalin-Jäntti, C.; Soinio, M.; Hannukainen, J.C.; Noponen, T.; Kirjavainen, A.; Iida, H.; Kudomi, N.; Enerbäck, S.; et al. Hyperthyroidism Increases Brown Fat Metabolism in Humans. J. Clin. Endocrinol. Metab. 2014, 99, E28–E35. [Google Scholar] [CrossRef]
- Li, Y.; Braun, K.; Gabler, S.; Willershäuser, M.; Reber, J.; Karlas, A.; Laurila, S.; Lahesmaa, M.; U Din, M.; Bast, A.; et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell 2018, 175, 1561–1574.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurila, S.; Sun, L.; Lahesmaa, M.; Schnabl, K.; Laitinen, K.; Klén, R.; Li, Y.; Balaz, M.; Wolfrum, C.; Steiger, K.; et al. Secretin Activates Brown Fat and Induces Satiation. Nat. Metab. 2021, 3, 798–809. [Google Scholar] [CrossRef]
- Raiko, J.R.H.; Tuulari, J.J.; Saari, T.; Parkkola, R.; Savisto, N.; Nuutila, P.; Virtanen, K. Associations between Brain Gray Matter Volumes and Adipose Tissue Metabolism in Healthy Adults. Obesity 2021, 29, 543–549. [Google Scholar] [CrossRef]
- Cui, W.; Cao, G.; Park, J.H.; Ouyang, Q.; Zhu, Y. Influence of Indoor Air Temperature on Human Thermal Comfort, Motivation and Performance. Build. Environ. 2013, 68, 114–122. [Google Scholar] [CrossRef]


| N | 10 |
| Age, years | 39.2 ± 8.3 |
| Male/Female, n | 1/9 |
| BMI, kg/m2 | 24.4 ± 4.5 |
| Waist, cm | 81.4 ± 19.1 |
| Fasting Plasma TSH, mU/L | 2.4 ± 0.8 |
| Fasting Plasma T4, pmol/L | 15.0 ± 2.5 |
| Fasting Plasma T3, pmol/L | 4.9 ± 0.7 |
| Insulin sensitivity (M-value) (μmol/kg/min) | 46.3 ± 27.6 |
| Glucose Uptake Rates | Correlation Adjusted for BMI | Correlation Adjusted for Age | ||
|---|---|---|---|---|
| rho | p-Value | rho | p-Value | |
| Whole Brain | 0.72 | 0.029 | 0.71 | 0.03 |
| Frontal Lobe | 0.70 | 0.034 | 0.70 | 0.04 |
| Sublobar Region | 0.54 | 0.13 | 0.54 | 0.13 |
| Temporal Lobe | 0.63 | 0.07 | 0.62 | 0.07 |
| Parietal Lobe | 0.78 | 0.012 | 0.77 | 0.016 |
| Limbic Lobe | 0.73 | 0.026 | 0.72 | 0.029 |
| Occipital Lobe | 0.73 | 0.026 | 0.73 | 0.026 |
| Hypothalamus | 0.68 | 0.046 | 0.67 | 0.048 |
| Midbrain | 0.73 | 0.025 | 0.76 | 0.018 |
| Pons | 0.41 | 0.27 | 0.44 | 0.23 |
| Medulla | 0.71 | 0.03 | 0.72 | 0.03 |
| Cerebellum | 0.53 | 0.14 | 0.55 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
U-Din, M.; Rebelos, E.; Saari, T.; Niemi, T.; Kuellmer, K.; Eskola, O.; Fromme, T.; Rajander, J.; Taittonen, M.; Klingenspor, M.; et al. Thermogenic Capacity of Human Supraclavicular Brown Fat and Cold-Stimulated Brain Glucose Metabolism. Metabolites 2023, 13, 387. https://doi.org/10.3390/metabo13030387
U-Din M, Rebelos E, Saari T, Niemi T, Kuellmer K, Eskola O, Fromme T, Rajander J, Taittonen M, Klingenspor M, et al. Thermogenic Capacity of Human Supraclavicular Brown Fat and Cold-Stimulated Brain Glucose Metabolism. Metabolites. 2023; 13(3):387. https://doi.org/10.3390/metabo13030387
Chicago/Turabian StyleU-Din, Mueez, Eleni Rebelos, Teemu Saari, Tarja Niemi, Katharina Kuellmer, Olli Eskola, Tobias Fromme, Johan Rajander, Markku Taittonen, Martin Klingenspor, and et al. 2023. "Thermogenic Capacity of Human Supraclavicular Brown Fat and Cold-Stimulated Brain Glucose Metabolism" Metabolites 13, no. 3: 387. https://doi.org/10.3390/metabo13030387
APA StyleU-Din, M., Rebelos, E., Saari, T., Niemi, T., Kuellmer, K., Eskola, O., Fromme, T., Rajander, J., Taittonen, M., Klingenspor, M., Nuutila, P., Nummenmaa, L., & Virtanen, K. A. (2023). Thermogenic Capacity of Human Supraclavicular Brown Fat and Cold-Stimulated Brain Glucose Metabolism. Metabolites, 13(3), 387. https://doi.org/10.3390/metabo13030387







