Lipidomic Profiling Reveals Concerted Temporal Patterns of Functionally Related Lipids in Aedes aegypti Females Following Blood Feeding
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Preparation
2.2. Female Mosquito Measurement
2.3. Lipid Extraction
2.4. Mass Spectrometry Analyses
2.5. Lipid Identification and Quantification
2.6. Data Analysis
3. Results
3.1. Female Body Weight and Wing Length Measurement
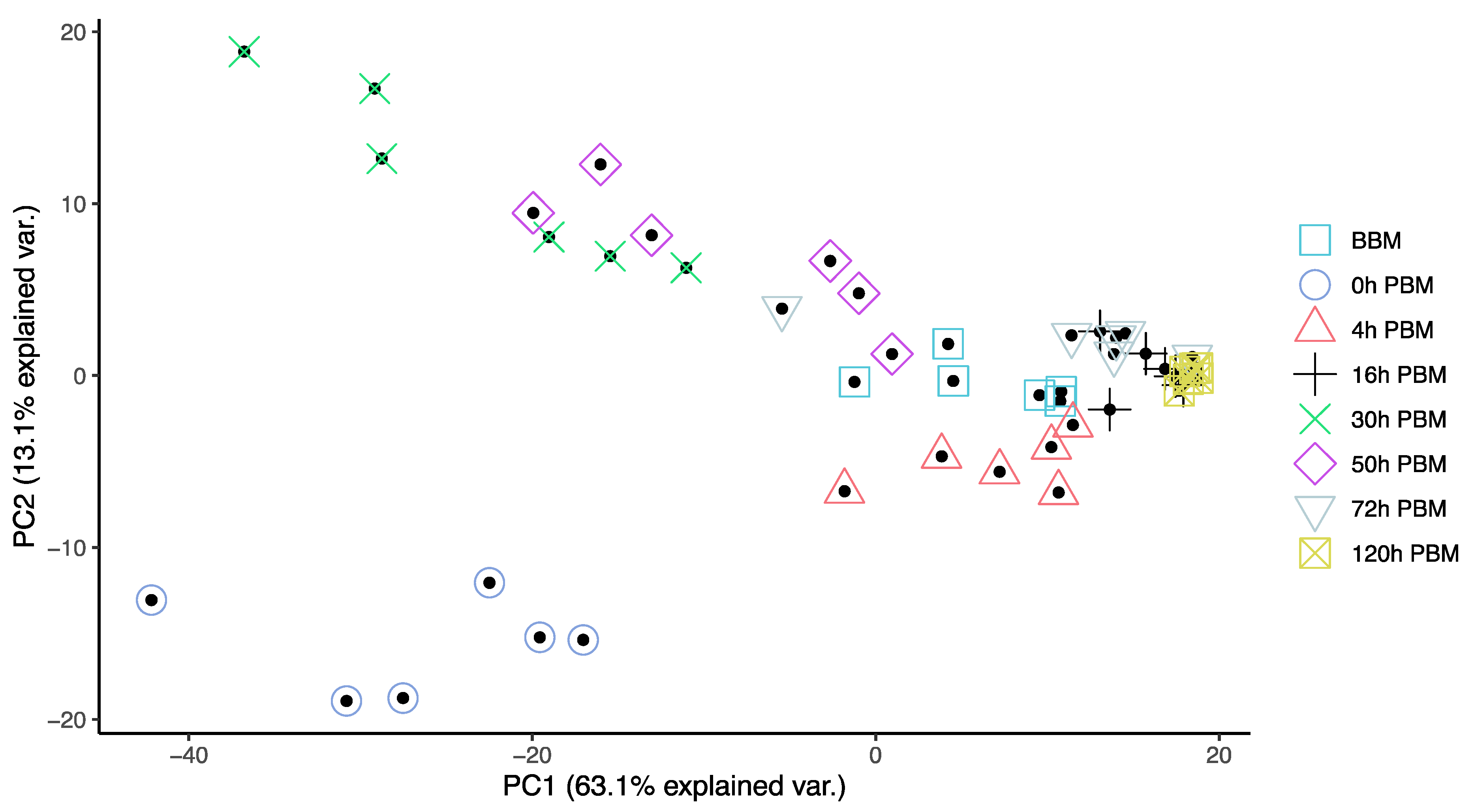
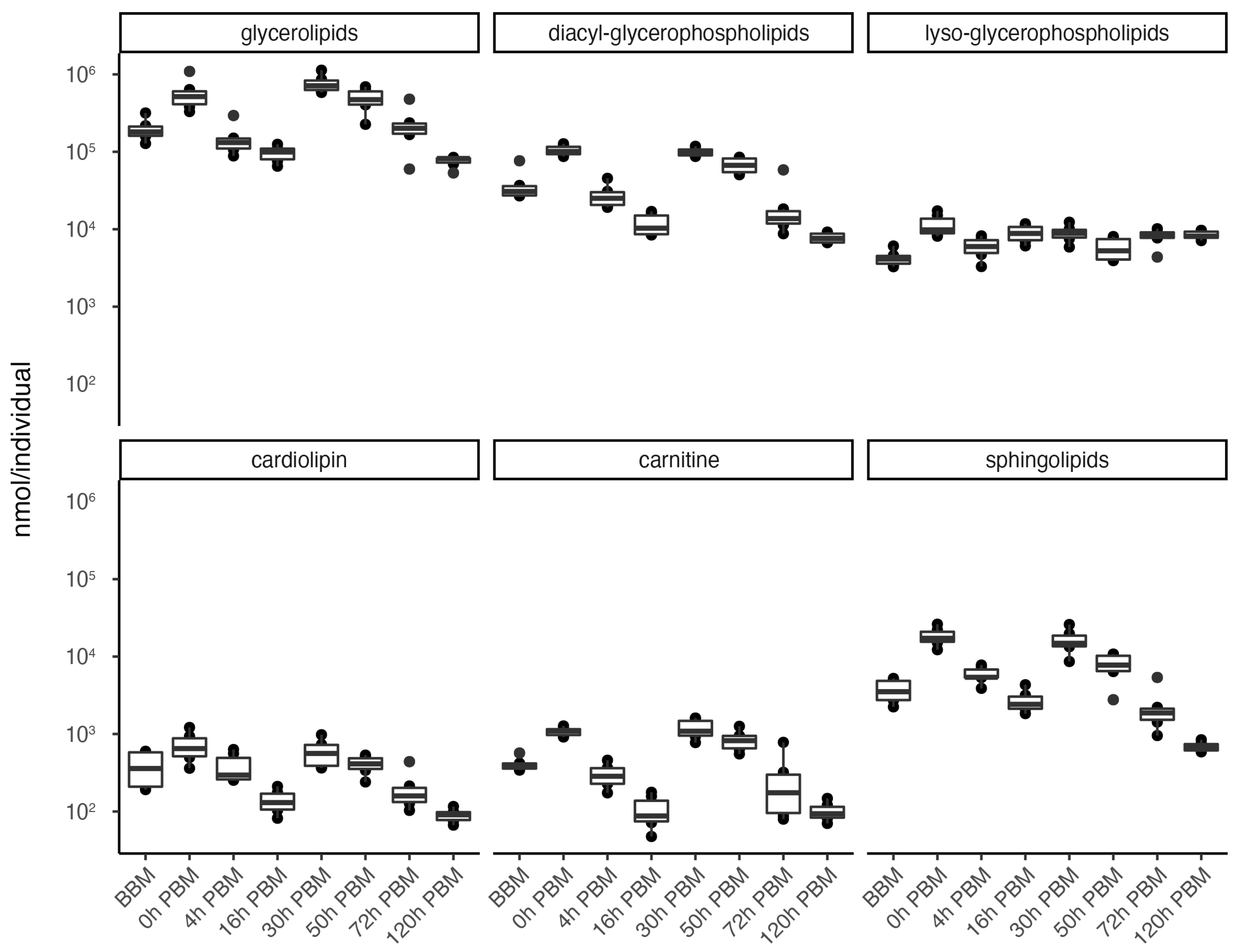
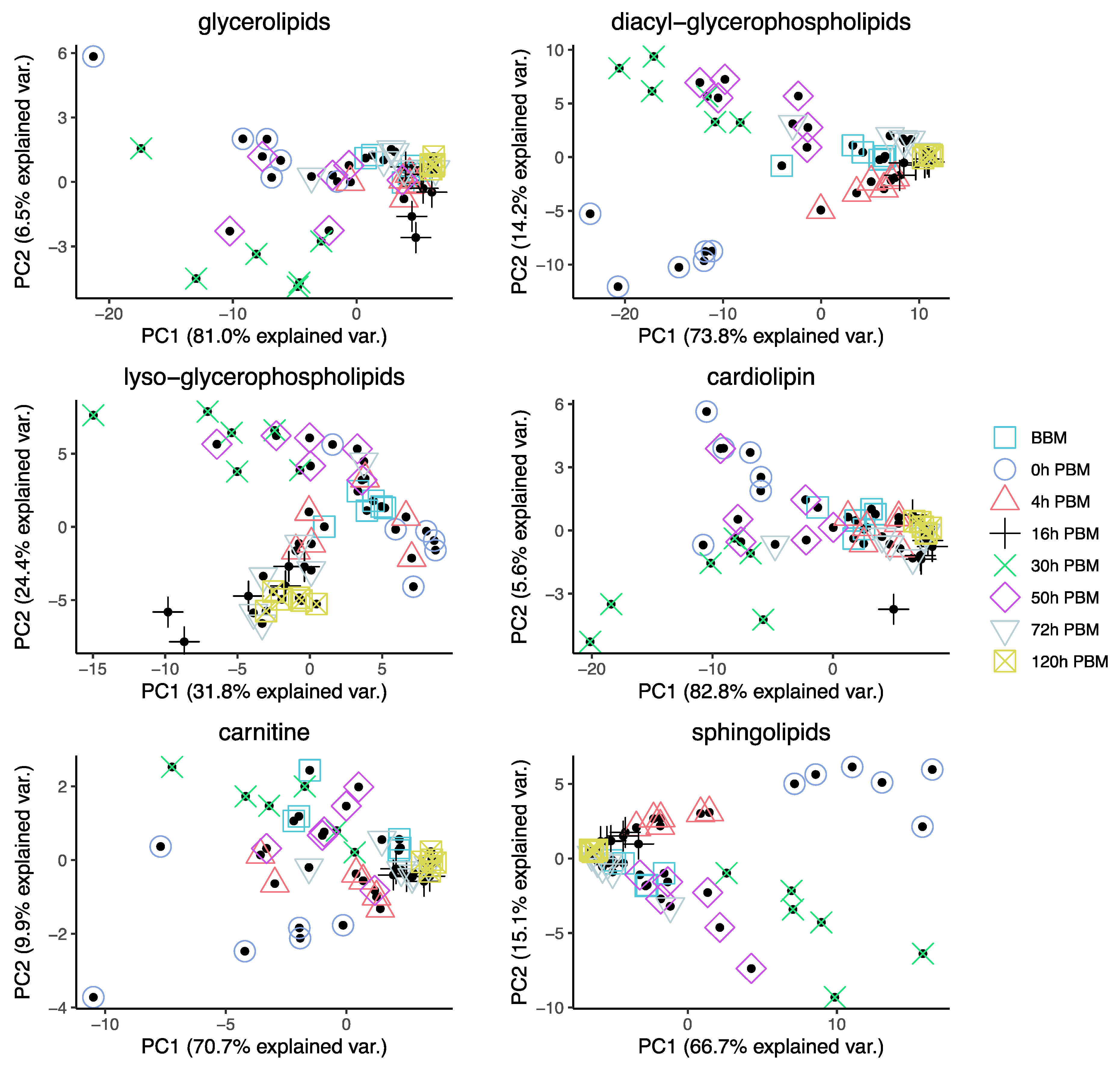
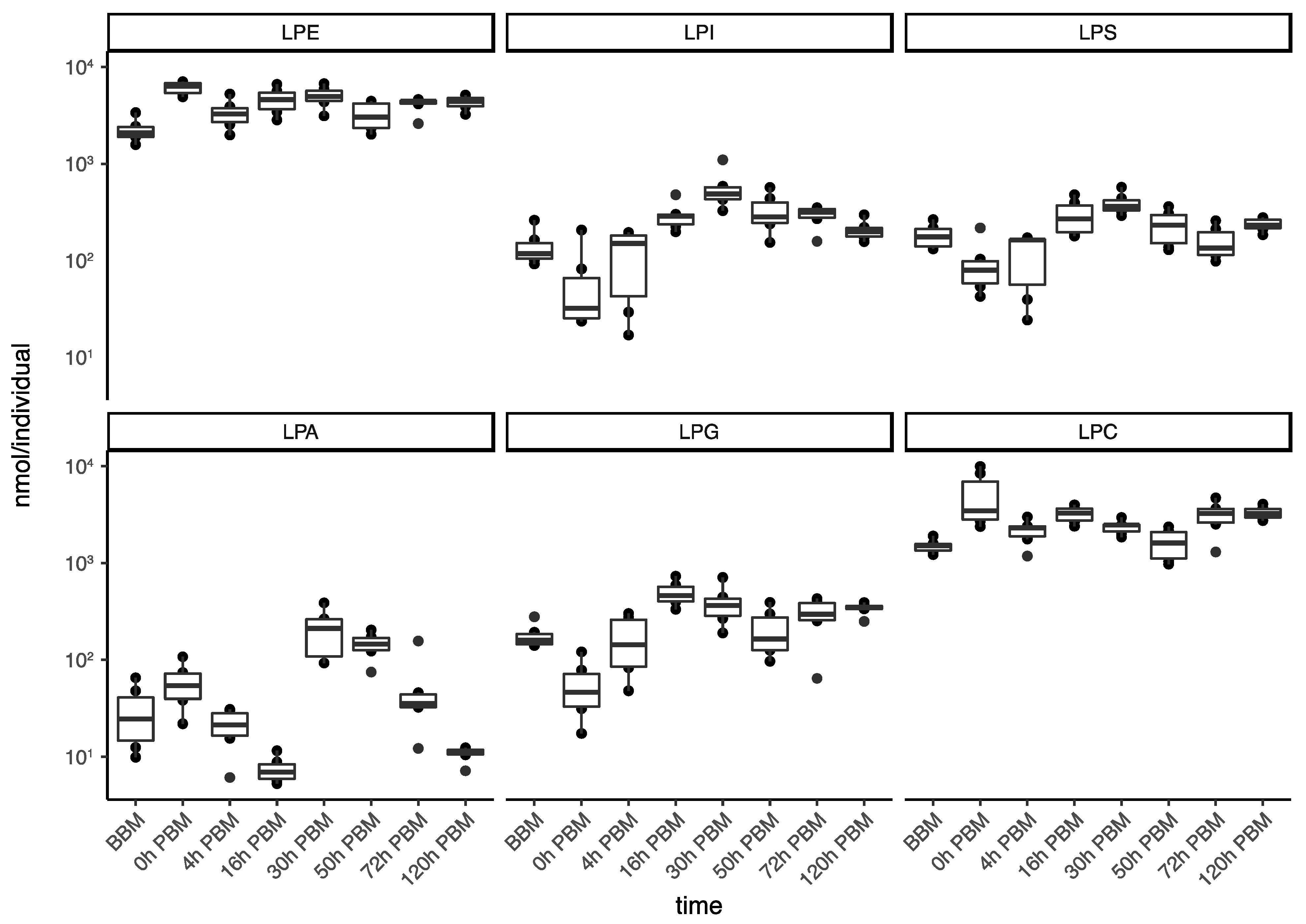
3.2. Lipids Identification and Their Changes during Female Reproductive Process
3.3. Identification of Change Patterns for Specific Lipids
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution 2014, 68, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Gulia-Nuss, M.; Elliot, A.; Brown, M.R.; Strand, M.R. Multiple factors contribute to anautogenous reproduction by the mosquito Aedes aegypti. J. Insect Physiol. 2015, 82, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, T.W.; Naksathit, A.; Day, J.F.; Kittayapong, P.; Edman, J.D. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am. J. Trop. Med. Hyg. 1997, 57, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, R.; Ibrahim, M.M. Formation of lipid reserves in fat body and eggs of the yellow fever mosquito, Aedes aegypti. J. Insect Physiol. 2001, 47, 623–627. [Google Scholar] [CrossRef]
- Zhou, G.; Pennington, J.E.; Wells, M.A. Utilization of pre-existing energy stores of female Aedes aegypti mosquitoes during the first gonotrophic cycle. Insect Biochem Mol. Biol. 2004, 34, 919–925. [Google Scholar] [CrossRef]
- Zhou, G.; Flowers, M.; Friedrich, K.; Horton, J.; Pennington, J.; Wells, M.A. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J. Insect Physiol. 2004, 50, 337–349. [Google Scholar] [CrossRef]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef] [Green Version]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, H.H.; O’Connor, J.D.; Fuchs, M.S.; Sage, B.; Schlaeger, D.A.; Bohm, M.K. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Natl. Acad. Sci. USA 1975, 72, 3255–3259. [Google Scholar] [CrossRef] [Green Version]
- Belles, X.; Piulachs, M.D. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta 2015, 1849, 181–186. [Google Scholar] [CrossRef]
- Klowden, M.J. Endocrine aspects of mosquito reproduction. Physiol. Insect Biochem. Physiol. 1997, 35, 491–512. [Google Scholar] [CrossRef]
- Briegel, H.; Gut, T.; Lea, A.O. Sequential deposition of yolk components during oogenesis in an insect, Aedes aegypti (Diptera: Culicidae). J. Insect Physiol. 2003, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Casas-Martínez, M.; Tamayo-Domínguez, R.; Bond-Compeán, J.G.; Rojas, J.C.; Weber, M.; Ulloa-García, A. Oogenic development and gonotrophic cycle of Aedes aegypti and Aedes albopictus in laboratory. Salud Publica Mex. 2020, 62, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.W.; Knapek, K.J.; Kendall, L.V. Rearing Aedes aegypti mosquitoes in a laboratory setting. Lab. Animal Sci. Prof. 2020, 55, 42–45. [Google Scholar]
- Hansen, I.A.; Attardo, G.M.; Park, J.H.; Peng, Q.; Raikhel, A.S. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc. Natl. Acad. Sci. USA 2004, 101, 10626–10631. [Google Scholar] [CrossRef] [Green Version]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attardo, G.M.; Hansen, I.A.; Raikhel, A.S. Nutritional regulation of vitellogenesis in mosquitoes: Implications for anautogeny. Insect Biochem. Mol. Biol. 2005, 35, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Phasomkusolsil, S.; Tawong, J.; Monkanna, N.; Pantuwatana, K.; Damdangdee, N.; Khongtak, W.; Kertmanee, Y.; Evans, B.P.; Schuster, A.L. Maintenance of mosquito vectors: Effects of blood source on feeding, survival, fecundity, and egg hatching rates. J. Vector Ecol. 2013, 38, 38–45. [Google Scholar] [CrossRef]
- Ross, P.A.; Lau, M.-J.; Hoffmann, A.A. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS ONE 2019, 14, e0224268. [Google Scholar] [CrossRef] [Green Version]
- Dutra, H.L.C.; Rodrigues, S.L.; Mansur, S.B.; de Oliveira, S.P.; Caragata, E.P.; Moreira, L.A. Development and physiological effects of an artificial diet for Wolbachia-infected Aedes aegypti. Sci. Rep. 2017, 7, 15687. [Google Scholar] [CrossRef] [Green Version]
- Talyuli, O.A.; Bottino-Rojas, V.; Taracena, M.L.; Soares, A.L.; Oliveira, J.H.; Oliveira, P.L. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. J. Insect Physiol. 2015, 83, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Caragata, E.P.; Rancès, E.; Hedges, L.M.; Gofton, A.W.; Johnson, K.N.; O’Neill, S.L.; McGraw, E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013, 9, e1003459. [Google Scholar] [CrossRef] [Green Version]
- Caragata, E.P.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H.; Hefti, M.; DiMarco, E. Lipid metabolism during sequential gonotrophic cycles in large and small female Aedes aegypti. J. Insect Physiol. 2002, 48, 547–554. [Google Scholar] [CrossRef]
- Troy, S.; Anderson, W.A.; Spielman, A. Lipid content of maturing ovaries of Aedes aegypti mosquitoes. Comp. Biochem. Physiol. B 1975, 50, 457–461. [Google Scholar] [CrossRef]
- Vrablik, T.L.; Watts, J.L. Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Mol. Reprod. Dev. 2013, 80, 244–259. [Google Scholar] [CrossRef] [Green Version]
- Toprak, U.; Hegedus, D.; Doğan, C.; Güney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990, 36, 165–172. [Google Scholar] [CrossRef]
- Lydic, T.A.; Townsend, S.; Adda, C.G.; Collins, C.; Mathivanan, S.; Reid, G.E. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods 2015, 87, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. A lipidome atlas in MS-DIAL 4. Nat. Biotechnol. 2020, 38, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid. Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Oshaghi, M.A.; Chavshin, A.R.; Vatandoost, H.; Yaaghoobi, F.; Mohtarami, F.; Noorjah, N. Effects of post-ingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Exp. Parasitol. 2006, 112, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Mukabana, W.R.; Takken, W.; Seda, P.; Killeen, G.F.; Hawley, W.A.; Knols, B.G. Extent of digestion affects the success of amplifying human DNA from blood meals of Anopheles gambiae (Diptera: Culicidae). Bull. EntoMol. Res. 2002, 92, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, L.C.; Edman, J.D.; Scott, T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. EntoMol. 2001, 38, 411–422. [Google Scholar] [CrossRef]
- Roy, D. On the role of blood in ovulation in Aedes aegypti, Linn. Bull. EntoMol. Res. 1936, 27, 423–429. [Google Scholar] [CrossRef]
- Arifin, S.A.; Falasca, M. Lysophosphatidylinositol signalling and metabolic diseases. Metabolites 2016, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro, R.; Falasca, M. Lysophosphatidylinositol signalling: New wine from an old bottle. Biochim. Biophys. Acta 2012, 1821, 694–705. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Kim, Y.J. Overview of innate immunity in Drosophila. J. Biochem. Mol. Biol. 2005, 38, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Sohlenkamp, C.; Galindo-Lagunas, K.A.; Guan, Z.; Vinuesa, P.; Robinson, S.; Thomas-Oates, J.; Raetz, C.R.; Geiger, O. The lipid lysyl-phosphatidylglycerol is present in membranes of Rhizobium tropici CIAT899 and confers increased resistance to polymyxin B under acidic growth conditions. Mol. Plant Microbe Interact. 2007, 20, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinch, M.; Mitra, S.; Rodriguez, S.D.; Li, Y.; Kandel, Y.; Dungan, B.; Holguin, F.O.; Attardo, G.M.; Hansen, I.A. Fat and happy: Profiling mosquito fat body lipid storage and composition post-blood meal. Front. Insect Sci. 2021, 1, 693168. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.L.; Saha, T.T.; Roy, S.; Zhao, B.; Raikhel, A.S.; Zou, Z. Temporal coordination of carbohydrate metabolism during mosquito reproduction. PLoS Genet. 2015, 11, e1005309. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, H.H.; Fallon, A.M.; Laufer, H. Vitellogenin synthesis by the fat body of the mosquito Aedes aegypti: Evidence of transcriptional control. Dev. Biol. 1973, 31, 285–294. [Google Scholar] [CrossRef]
- Sun, J.; Hiraoka, T.; Dittmer, N.T.; Cho, K.H.; Raikhel, A.S. Lipophorin as a yolk protein precursor in the mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2000, 30, 1161–1171. [Google Scholar] [CrossRef]
- Kawooya, J.K.; Law, J.H. Role of lipophorin in lipid transport to the insect egg. J. Biol. Chem. 1988, 263, 8748–8753. [Google Scholar] [CrossRef]
- Ford, P.S.; Van Heusden, M.C. Triglyceride-rich lipophorin in Aedes aegypti (Diptera: Culicidae). J. Med. EntoMol. 1994, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.E.; Nussenzveig, R.H.; Van Heusden, M.C. Lipid transfer from insect fat body to lipophorin: Comparison between a mosquito triacylglycerol-rich lipophorin and a sphinx moth diacylglycerol-rich lipophorin. J. Lipid. Res. 1996, 37, 1144–1152. [Google Scholar] [CrossRef]
- Arrese, E.L.; Canavoso, L.E.; Jouni, Z.E.; Pennington, J.E.; Tsuchida, K.; Wells, M.A. Lipid storage and mobilization in insects: Current status and future directions. Insect Biochem. Mol. Biol. 2001, 31, 7–17. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Wells, M.A. Metabolic pathways for diacylglycerol biosynthesis and release in the midgut of larval Manduca sexta. Insect Biochem. Mol. Biol. 2000, 30, 1173–1180. [Google Scholar] [CrossRef]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Black, P.N.; DiRusso, C.C. Transmembrane movement of exogenous long-chain fatty acids: Proteins, enzymes, and vectorial esterification. MicroBiol. Mol. Biol. Rev. 2003, 67, 454–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietschel, E.T.; Kirikae, T.; Schade, F.U.; Mamat, U.; Schmidt, G.; Loppnow, H.; Ulmer, A.J.; Zähringer, U.; Seydel, U.; Di Padova, F.; et al. Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB J. 1994, 8, 217–225. [Google Scholar] [CrossRef]
- Ishii, I.; Fukushima, N.; Ye, X.; Chun, J. Lysophospholipid receptors: Signaling and biology. Annu. Rev. Biochem. 2004, 73, 321–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid. Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid. Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and biophysical homeostasis of mammalian membranes counteracts dietary lipid perturbations to maintain cellular fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef] [Green Version]
- Minard, G.; Mavingui, P.; Moro, C.V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 2013, 6, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muturi, E.J.; Njoroge, T.M.; Dunlap, C.; Cáceres, C.E. Blood meal source and mixed blood-feeding influence gut bacterial community composition in Aedes aegypti. Parasit. Vectors 2021, 14, 83. [Google Scholar] [CrossRef]
- Gaio Ade, O.; Gusmão, D.S.; Santos, A.V.; Berbert-Molina, M.A.; Pimenta, P.F.; Lemos, F.J. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: Culicidae) (L.). Parasit. Vectors 2011, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Luquain, C.; Sciorra, V.A.; Morris, A.J. Lysophosphatidic acid signaling: How a small lipid does big things. Trends. Biochem. Sci. 2003, 28, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Toscano, S.; Trivedi, D.; Jones, D.R.; Mathre, S.; Clarke, J.H.; Divecha, N.; Raghu, P. Phosphatidylinositol 5-phosphate 4-kinase (PIP4K) regulates TOR signaling and cell growth during Drosophila development. Proc. Natl. Acad. Sci. USA 2013, 110, 5963–5968. [Google Scholar] [CrossRef] [Green Version]
- Milligan, S.C.; Alb, J.G., Jr.; Elagina, R.B.; Bankaitis, V.A.; Hyde, D.R. The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J. Cell Biol. 1997, 139, 351–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Murillas, I.; Pettitt, T.; Macdonald, E.; Okkenhaug, H.; Georgiev, P.; Trivedi, D.; Hassan, B.; Wakelam, M.; Raghu, P. lazaro encodes a lipid phosphate phosphohydrolase that regulates phosphatidylinositol turnover during Drosophila phototransduction. Neuron 2006, 49, 533–546. [Google Scholar] [CrossRef] [Green Version]
- Schlame, M.; Ren, M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim. Biophys. Acta 2009, 1788, 2080–2083. [Google Scholar] [CrossRef] [Green Version]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Bell, R.M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science 1989, 243, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.; Acharya, J.K. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol. Life Sci. 2005, 62, 128–142. [Google Scholar] [CrossRef]

| Name (Abbreviation) | Standard | Adduct Type | Number of Lipids Identified |
|---|---|---|---|
| Diacylglycerol (DG) | 15:0_18:1(d7) DAG | [M + NH4]+ | 14 |
| Triglyceride (TG) | 15:0_18:1(d7)_15:0 TAG | [M + NH4]+ | 41 |
| Phosphatidylethanolamine (PE) | 15:0_18:1(d7) PE | [M − H]− | 43 |
| Ether-linked PE (EtherPE) | 15:0_18:1(d7) PE | [M − H]− | 18 |
| Oxidized PE (OxPE) | 15:0_18:1(d7) PE | [M − H]− | 8 |
| Phosphatidylinositol (PI) | 15:0_18:1(d7) PI | [M − H]− | 33 |
| Ether-linked PI (EtherPI) | 15:0_18:1(d7) PI | [M − H]− | 3 |
| Phosphatidylserine (PS) | 15:0_18:1(d7) PS | [M − H]− | 10 |
| Phosphatidic acid (PA) | 15:0_18:1(d7) PA | [M − H]− | 5 |
| Phosphatidylglycerol (PG) | 15:0_18:1(d7) PG | [M − H]− | 24 |
| Phosphatidylcholine (PC) | 15:0_18:1(d7) PC | [M + H]+ | 52 |
| Ether-linked PC (EtherPC) | 15:0_18:1(d7) PC | [M + H]+ | 7 |
| Lyso-PE (LPE) | 18:1(d7) Lyso PE | [M − H]− | 13 |
| Ether-linked lyso-PE (EtherLPE) | 18:1(d7) Lyso PE | [M − H]− | 2 |
| Lyso-PI (LPI) | 15:0_18:1(d7) PI | [M − H]− | 7 |
| Lyso-PS (LPS) | 15:0_18:1(d7) PS | [M − H]− | 6 |
| Lyso-PA (LPA) | 15:0_18:1(d7) PA | [M − H]− | 4 |
| Lyso-PG (LPG) | 15:0_18:1(d7) PG | [M − H]− | 7 |
| Lyso-PC (LPC) | 18:1(d7) Lyso PC | [M + H]+ | 9 |
| Cardiolipin (CL) | 15:0_18:1(d7) PG | [M − H]− | 66 |
| Carnitine (CAR) | 15:0_18:1(d7) PC | [M + H]+ | 15 |
| Sphingomyelin (SM) | d18:1_18:1(d9) SM | [M + H]+ | 26 |
| Ceramide (Cer) | d18:1_18:1(d9) SM | [M − H]− | 23 |
| Hexosylceramides (HexCer) | d18:1_18:1(d9) SM | [M − H]− | 4 |
| Sulfatides (SHexCer) | d18:1_18:1(d9) SM | [M − H]− | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, M.-J.; Nie, S.; Yang, Q.; Harshman, L.G.; Mao, C.; Williamson, N.A.; Hoffmann, A.A. Lipidomic Profiling Reveals Concerted Temporal Patterns of Functionally Related Lipids in Aedes aegypti Females Following Blood Feeding. Metabolites 2023, 13, 421. https://doi.org/10.3390/metabo13030421
Lau M-J, Nie S, Yang Q, Harshman LG, Mao C, Williamson NA, Hoffmann AA. Lipidomic Profiling Reveals Concerted Temporal Patterns of Functionally Related Lipids in Aedes aegypti Females Following Blood Feeding. Metabolites. 2023; 13(3):421. https://doi.org/10.3390/metabo13030421
Chicago/Turabian StyleLau, Meng-Jia, Shuai Nie, Qiong Yang, Lawrence G. Harshman, Cungui Mao, Nicholas A. Williamson, and Ary A. Hoffmann. 2023. "Lipidomic Profiling Reveals Concerted Temporal Patterns of Functionally Related Lipids in Aedes aegypti Females Following Blood Feeding" Metabolites 13, no. 3: 421. https://doi.org/10.3390/metabo13030421
APA StyleLau, M.-J., Nie, S., Yang, Q., Harshman, L. G., Mao, C., Williamson, N. A., & Hoffmann, A. A. (2023). Lipidomic Profiling Reveals Concerted Temporal Patterns of Functionally Related Lipids in Aedes aegypti Females Following Blood Feeding. Metabolites, 13(3), 421. https://doi.org/10.3390/metabo13030421










