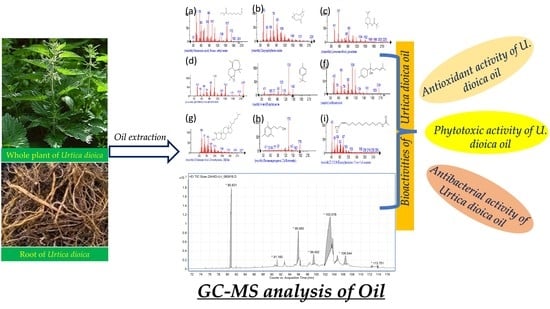
An Experimental and Computational Analysis of Plant Compounds from Whole Urtica dioica L. Plant’s Essential Oil for Antioxidant and Antibacterial Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extract Preparation
2.3. Gas Chromatography–Mass (GC–MS) Spectrum Analysis
2.4. Antioxidant Activity
2.5. Phytotoxic Activity
2.6. Antibacterial Activity
2.7. Computational Analysis
2.7.1. Selection of Protein Targets and Chemical Compounds
2.7.2. Molecular Docking (MD) and Interaction Analysis
2.7.3. Pharmacokinetic/ADMET Profile Estimation
3. Results and Discussion
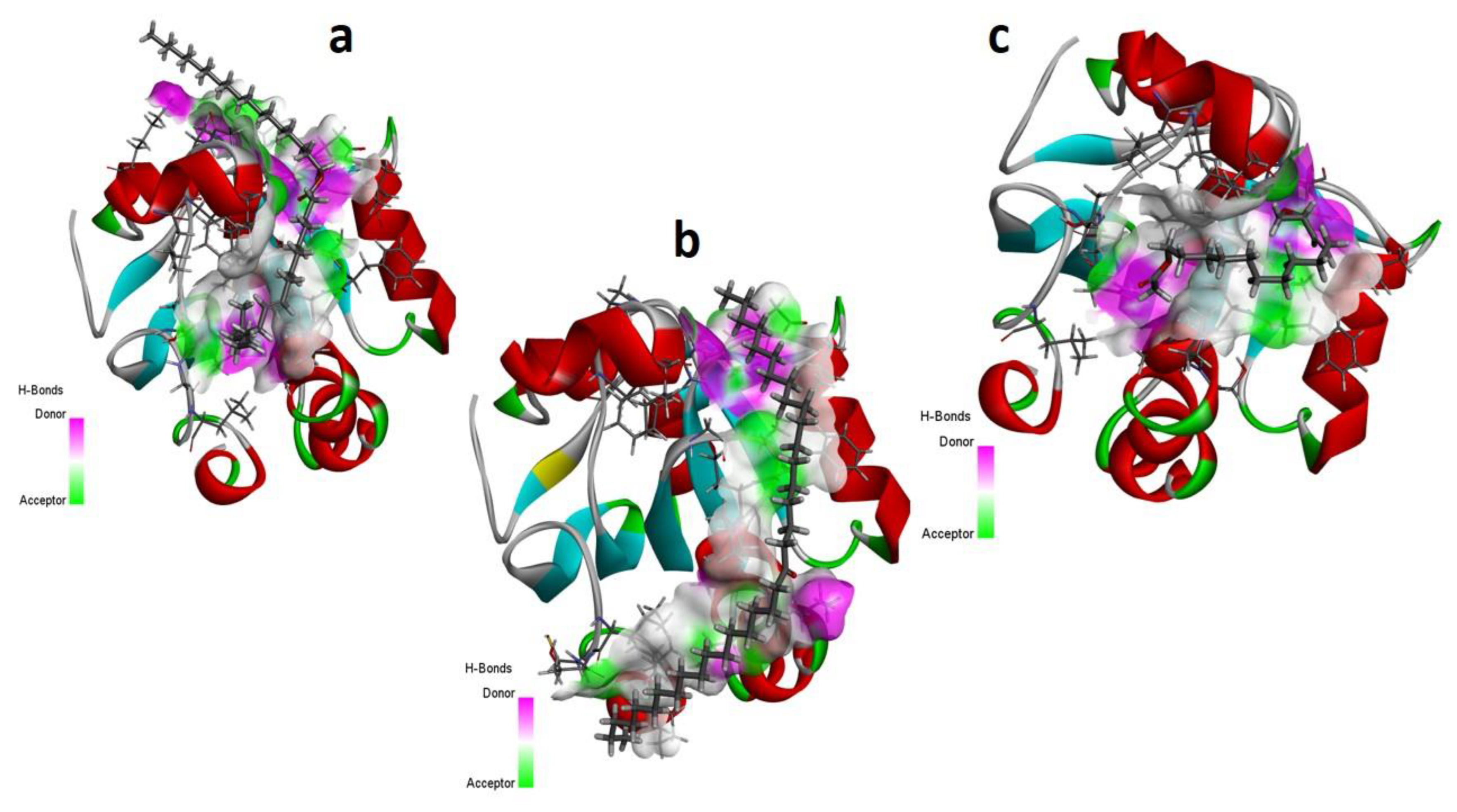
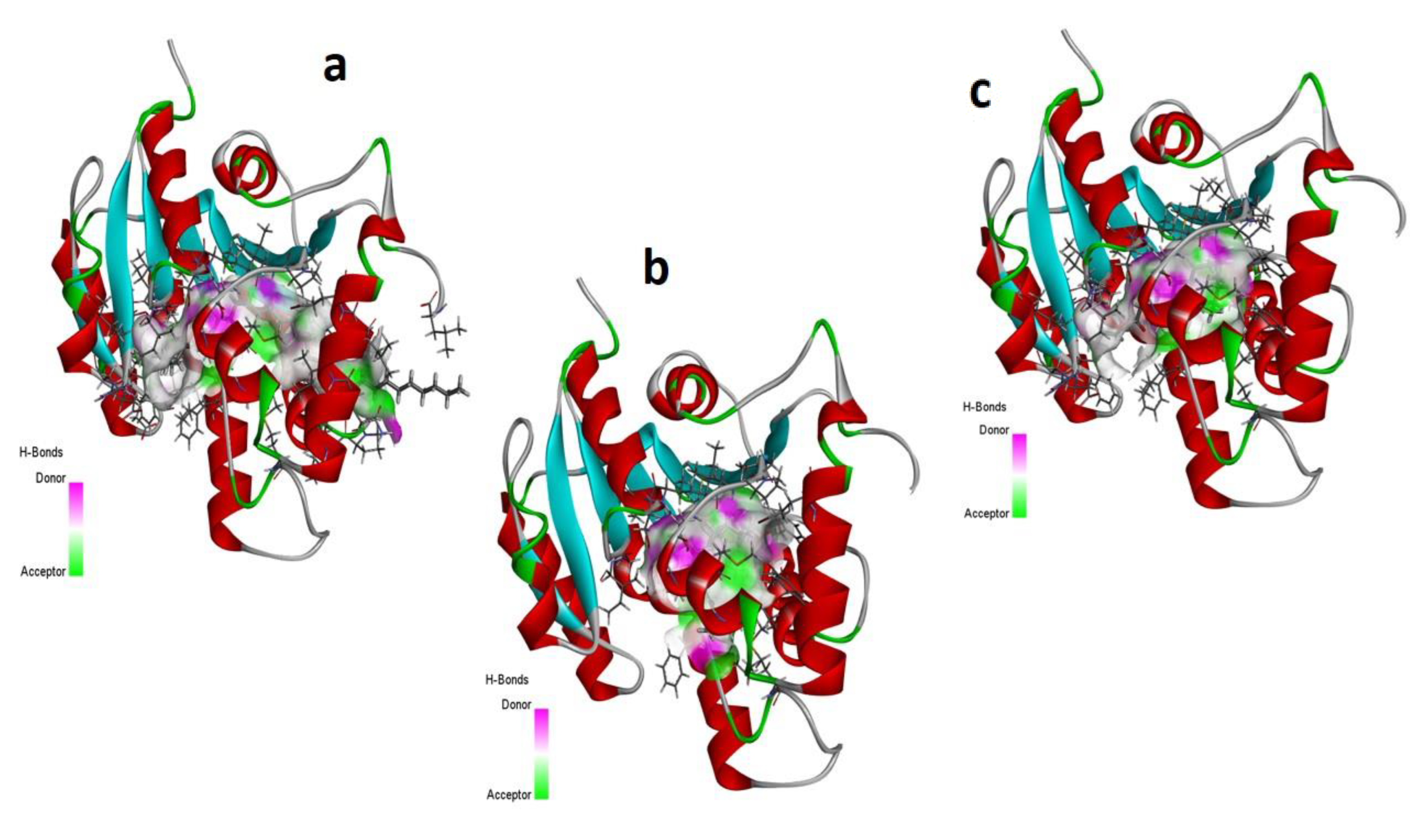
Computational Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motamedi, H.; Seyyednejad, S.M.; Bakhtiari, A.; Vafaei, M. Introducing Urtica dioica, a native plant of Khuzestan, as an antibacterial medicinal plant. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e15904. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary plants with extraordinary properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-F.; Monro, A.K.; Wen, F.; Xin, Z.-B.; Wei, Y.-G.; Zhang, Z.-X. The rediscovery and delimitation of Elatostemasetulosum WT Wang (Urticaceae). PhytoKeys 2019, 126, 79. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Mohammadian, M.; Dianat, M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran. J. Med. Sci. 2012, 37, 181. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Mittman, P. Randomized, double-blind study of freeze-dried Urtica dioica in the treatment of allergic rhinitis. Planta Med. 1990, 56, 44–47. [Google Scholar] [CrossRef]
- Durak, I.; Biri, H.; Devrim, E.; Sözen, S.; Avcı, A. Aqueous extract of Urtica dioica makes significant inhibition on adenosine deaminase activity in prostate tissue from patients with prostate cancer. Cancer Biol. Ther. 2004, 3, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Farzami, B.; Ahmadvand, D.; Vardasbi, S.; Majin, F.J.; Khaghani, S. Induction of Insulin Secretion by a Component of Urtica dioica Leaves Extract in Perfused Islets of Langehans and its Vivo Effects in normal and Streptozotocin Diabetic Rats. J. Ethnopharmacol. 2003, 89, 47–53. [Google Scholar] [CrossRef]
- Asghar, N.; Naqvi, S.A.R.; Hussain, Z.; Rasool, N.; Khan, Z.A.; Shahzad, S.A.; Sherazi, T.A.; Janjua, M.R.S.A.; Nagra, S.A.; Zia-Ul-Haq, M.; et al. Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chem. Cent. J. 2016, 10, 5. [Google Scholar] [CrossRef]
- Riehemann, K.; Behnke, B.; Schulze-Osthoff, K. Plant extracts from stinging nettle (Urtica dioica), an antirheumatic remedy, inhibit the proinflammatory transcription factor NF-κB. FEBS Lett. 1999, 442, 89–94. [Google Scholar] [CrossRef]
- Obertreis, B.; Giller, K.; Teucher, T.; Behnke, B.; Schmitz, H. Anti-inflammatory effect of Urtica dioica folia extract in comparison to caffeic malic acid. Arzneimittelforschung 1996, 46, 52–56. [Google Scholar] [PubMed]
- Bnouham, M.; Merhfour, F.-Z.; Ziyyat, A.; Mekhfi, H.; Aziz, M.; Legssyer, A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia 2003, 74, 677–681. [Google Scholar] [CrossRef]
- Tahri, A.; Yamani, S.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M.; Ziyyat, A. Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica in the rat. J. Ethnopharmacol. 2000, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, I.; Peivastegan, B.; Kolahi, M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak. J. Biol. Sci. 2009, 12, 58–63. [Google Scholar] [CrossRef]
- Gül, S.; Demirci, B.; Başer, K.H.C.; Akpulat, H.A.; Aksu, P. Chemical composition and in vitro cytotoxic, genotoxic effects of essential essential oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef]
- Fahad, K.; Hani, S.; Walid, M.; Fouad, A.S. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential essential oil s composition of Origanum majorana Annals of Agricultural cultivation. Sciences 2021, 66, 1–7. [Google Scholar]
- Huda, J.A.; Mohammed, Y.H.; Imad, H.H. Phytochemical analysis of Urtica dioica leaves by fourier-transform infrared spectroscopy and gas chromatography-mass spectrometry. J. Pharm. Phytoth. 2015, 7, 238–252. [Google Scholar]
- Gülçin, I.; Küfrevioǧlu, Ö.I.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Mzid, M.; Ben Khedir, S.; Ben Salem, M.; Regaieg, W.; Rebai, T. Antioxidant and antimicrobial activities of ethanol and aqueous extracts from Urtica urens. Pharm. Biol. 2017, 55, 775–781. [Google Scholar] [CrossRef]
- Körpe, D.A.; İşerİ, Ö.D.; Sahin, F.I.; Cabi, E.; Haberal, M. High-antibacterial activity of Urtica spp. seed extracts on food and plant pathogenic bacteria. Int. J. Food Sci. Nutr. 2013, 64, 355–362. [Google Scholar] [CrossRef]
- Ranjbar, R.; Shamami, K.S. Evaluation of antimicrobial activity of Eucalyptus essential essential oil and Urtica alcoholic extract on Salmonella enteritidis and Shigella dysenteriae in vitro conditions. Bull. Environ. Pharmacol. Life Sci. 2015, 4, 56–59. [Google Scholar]
- Salehzadeh, A.; Asadpour, L.; Naeemi, A.S.; Houshmand, E. Antimicrobial activity of methanolic extracts of Sambucus ebulus and Urtica dioica against clinical isolates of methicillin resistant Staphylococcus aureus. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Hasan, M.M.; Wang, Y.-B.; Papadakos, S.P.; Yu, H. Cordycepin as a Promising Inhibitor of SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp). Curr. Med. Chem. 2022, 29, 152–162. [Google Scholar] [CrossRef]
- Khan, M.S.; Mehmood, B.; Yousafi, Q.; Bibi, S.; Fazal, S.; Saleem, S.; Sajid, M.W.; Ihsan, A.; Azhar, M.; Kamal, M.A. Molecular docking studies reveal rhein from rhubarb (Rheum rhabarba-rum) as a putative inhibitor of atp-binding cassette super family g member 2. Med. Chem. 2021, 17, 273–288. [Google Scholar] [CrossRef]
- Saleem, U.; Shehzad, A.; Shah, S.; Raza, Z.; Shah, M.A.; Bibi, S.; Chauhdary, Z.; Ahmad, B. Antiparkinsonian activity of Cucurbita pepo seeds along with possible underlying mechanism. Metab. Brain Dis. 2021, 36, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, L.D. ChemDraw 8 ultra, windows and macintosh versions. J. Chem. Inf. Comput. Sci. 2004, 44, 2225–2226. [Google Scholar] [CrossRef]
- Declercq, J.-P.; Evrard, C.; Clippe, A.; Vander Stricht, D.; Bernard, A.; Knoops, B. Crystal structure of human peroxiredoxin 5, a novel type of mammalian peroxiredoxin at 1.5 Åresolution. J. Mol. Biol. 2001, 311, 751–759. [Google Scholar] [CrossRef]
- He, X.; Alian, A.; Stroud, R.; de Montellano, P.R. Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. J. Med. Chem. 2006, 49, 6308–6323. [Google Scholar] [CrossRef]
- Bibi, S.; Sakata, K. Current Status of Computer-Aided Drug Design for Type 2 Diabetes. Curr. Comput. Aided-Drug Des. 2016, 12, 167–177. [Google Scholar] [CrossRef]
- Bibi, S.; Sakata, K. An Integrated Computational Approach for Plant-Based Protein Tyrosine Phosphatase Non-Receptor Type 1 Inhibitors. Curr. Comput. Aided Drug Des. 2017, 13, 319–335. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Bibi, S.; Shah, M.A.; Ahmad, B.; Saleem, A.; Chauhdary, Z.; Anwar, F.; Javaid, N.; Hira, S.; Akhtar, M.F.; et al. Anti-Parkinson’s evaluation of Brassica juncea leaf extract and underlying mechanism of its phytochemicals. Front. Biosci.-Landmark 2021, 26, 1031–1051. [Google Scholar]
- Yousafi, Q.; Batool, J.; Khan, M.S.; Perveen, T.; Sajid, M.W.; Hussain, A.; Mehmood, A.; Saleem, S. In Silico Evaluation of Food Derived Bioactive Peptides as Inhibitors of Angiotensin Converting Enzyme (ACE). Int. J. Pept. Res. Ther. 2021, 27, 341–349. [Google Scholar] [CrossRef]
- Khan, M.; Patujo, J.; Mushtaq, I.; Ishtiaq, A.; Tahir, M.N.; Bibi, S.; Khan, M.S.; Mustafa, G.; Mirza, B.; Badshah, A.; et al. Anti-diabetic potential, crystal structure, molecular docking, DFT, and optical-electrochemical Studies of new Dimethyl and Diethyl Carbamoyl-N, N’-disubstituted based thioureas. J. Mol. Struct. 2021, 1253, 132207. [Google Scholar] [CrossRef]
- Zeb, M.A.; Rahman, T.U.; Sajid, M.; Xiao, W.; Musharraf, S.G.; Bibi, S.; Akitsu, T.; Liaqat, W. GC-MS Analysis and In Silico Approaches of Indigofera heterantha Root Essential oil Chemical Constituents. Compounds 2021, 1, 116–124. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Sander, T.; Freyss, J.; Von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Kukrić, Z.Z.; Topalić-Trivunović, L.N.; Kukavica, B.M.; Matoš, S.B.; Pavičić, S.S.; Boroja, M.M.; Savić, A. V Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Chaqroune, A.; Taleb, M. Effects of Extraction Technique and Solvent on Phytochemicals, Antioxidant, and Antimicrobial Activities of Cultivated and Wild Rosemary (Rosmarinus officinalis L.) from Taounate Region. Biointerface Res. Appl. Chem. 2022, 12, 8441–8452. [Google Scholar]
- Gul, H.; Naseer, R.D.; Abbas, I.; Khan, E.A.; Rehman, H.U.; Nawaz, A.; Abdel-Daim, M.M. The Therapeutic Application of Tamarix aphylla Extract Loaded Nanoemulsion Cream for Acid-Burn Wound Healing and Skin Regeneration. Medicina 2022, 59, 34. [Google Scholar] [CrossRef]
- Zenão, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Zeroual, A.; Sakar, E.H.; Eloutassi, N.; Mahjoubi, F.; Chaouch, M.; Chaqroune, A. Wild chamomile [Cladanthus mixtus (L.) chevall.] collected from central-northern Morocco: Phytochemical profiling, antioxidant, and antimicrobial activities. Biointerface Res. Appl. Chem. 2021, 11, 11440–11457. [Google Scholar]
- Khan, A.; Hussain, S.; Ahmad, S.; Suleman, M.; Bukhari, I.; Khan, T.; Wei, D.Q. Computational modelling of potentially emerging SARS-CoV-2 spike protein RBDs mutations with higher binding affinity towards ACE2: A structural modelling study. Comput. Biol. Med. 2022, 141, 105163. [Google Scholar] [CrossRef] [PubMed]
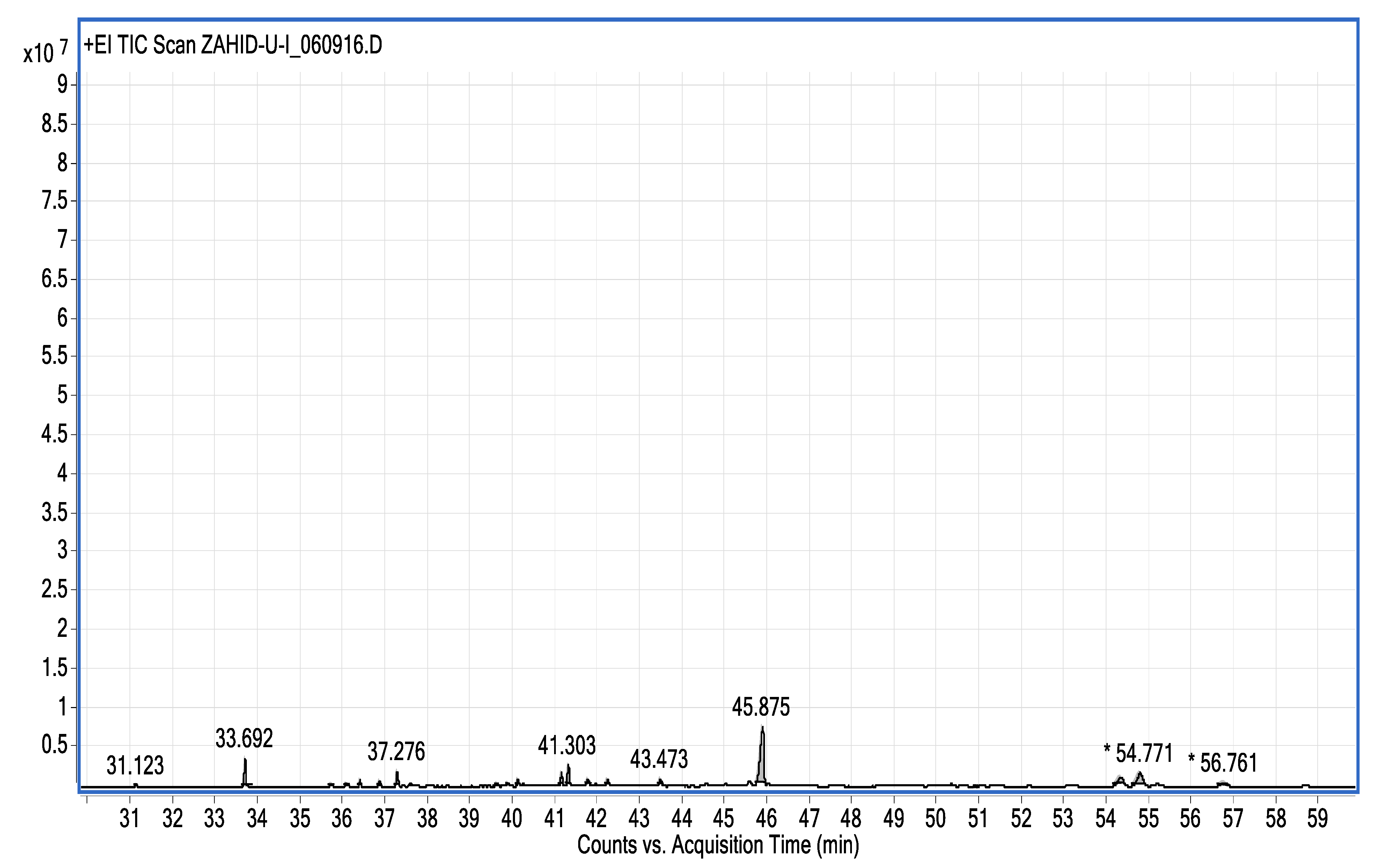
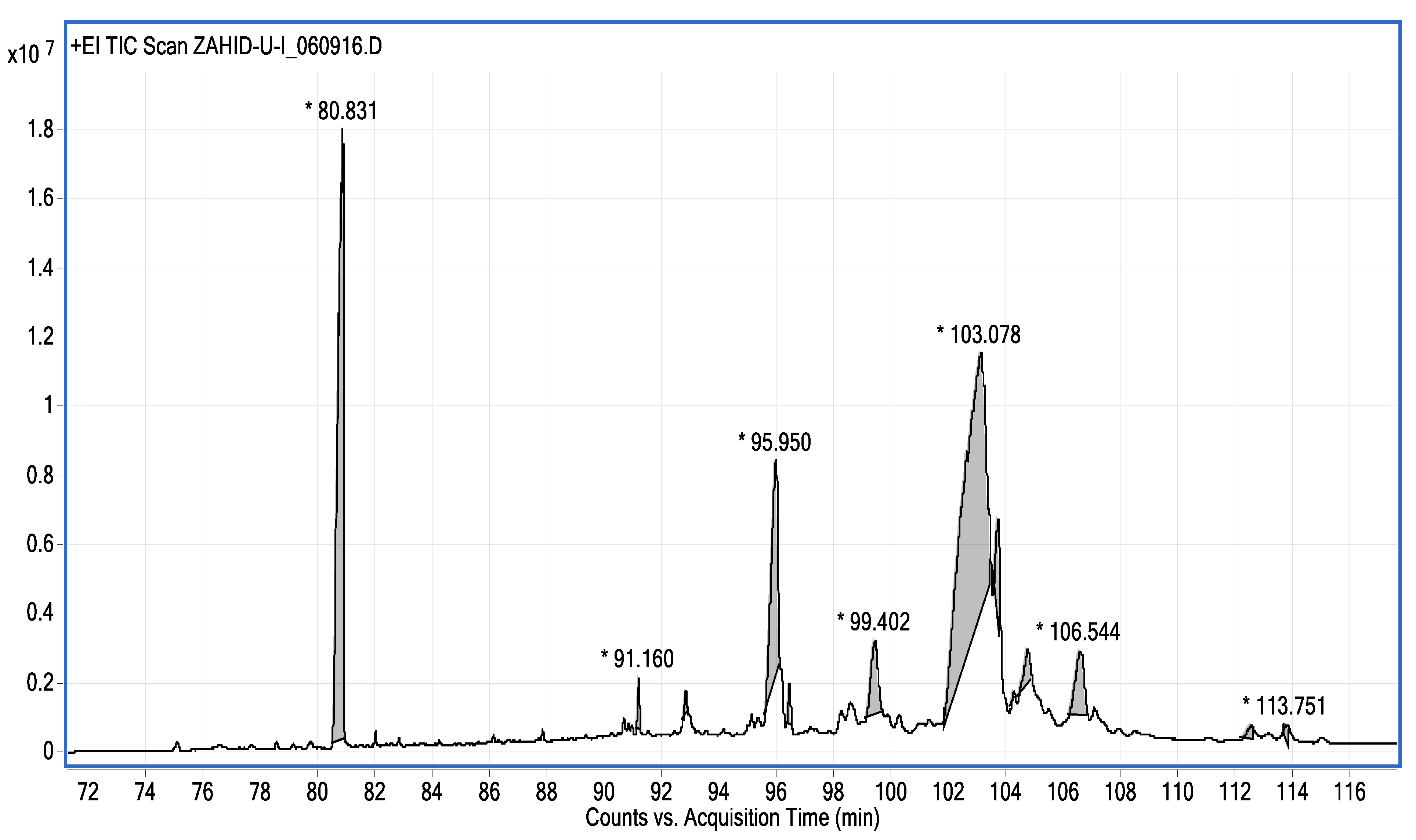
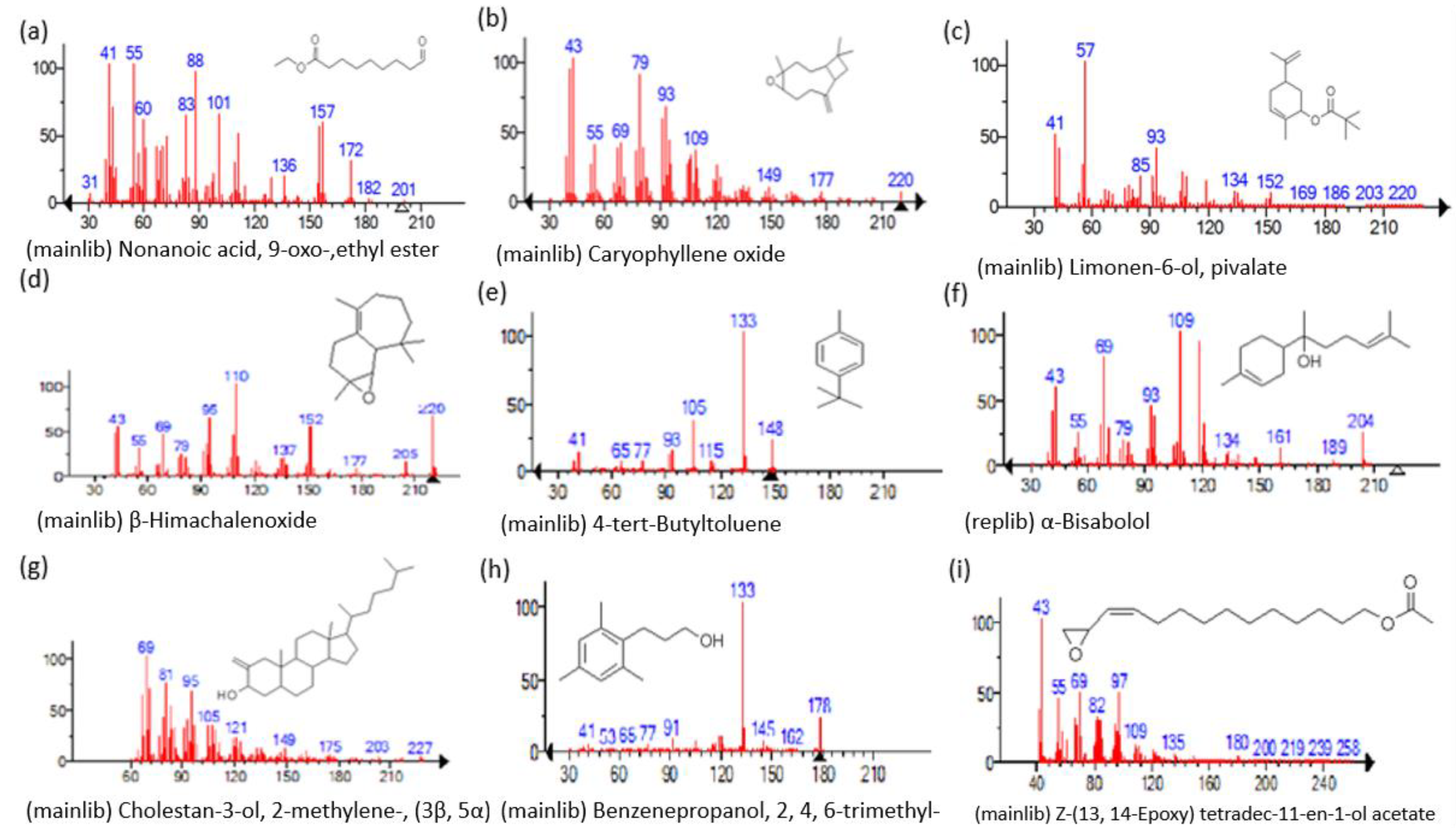
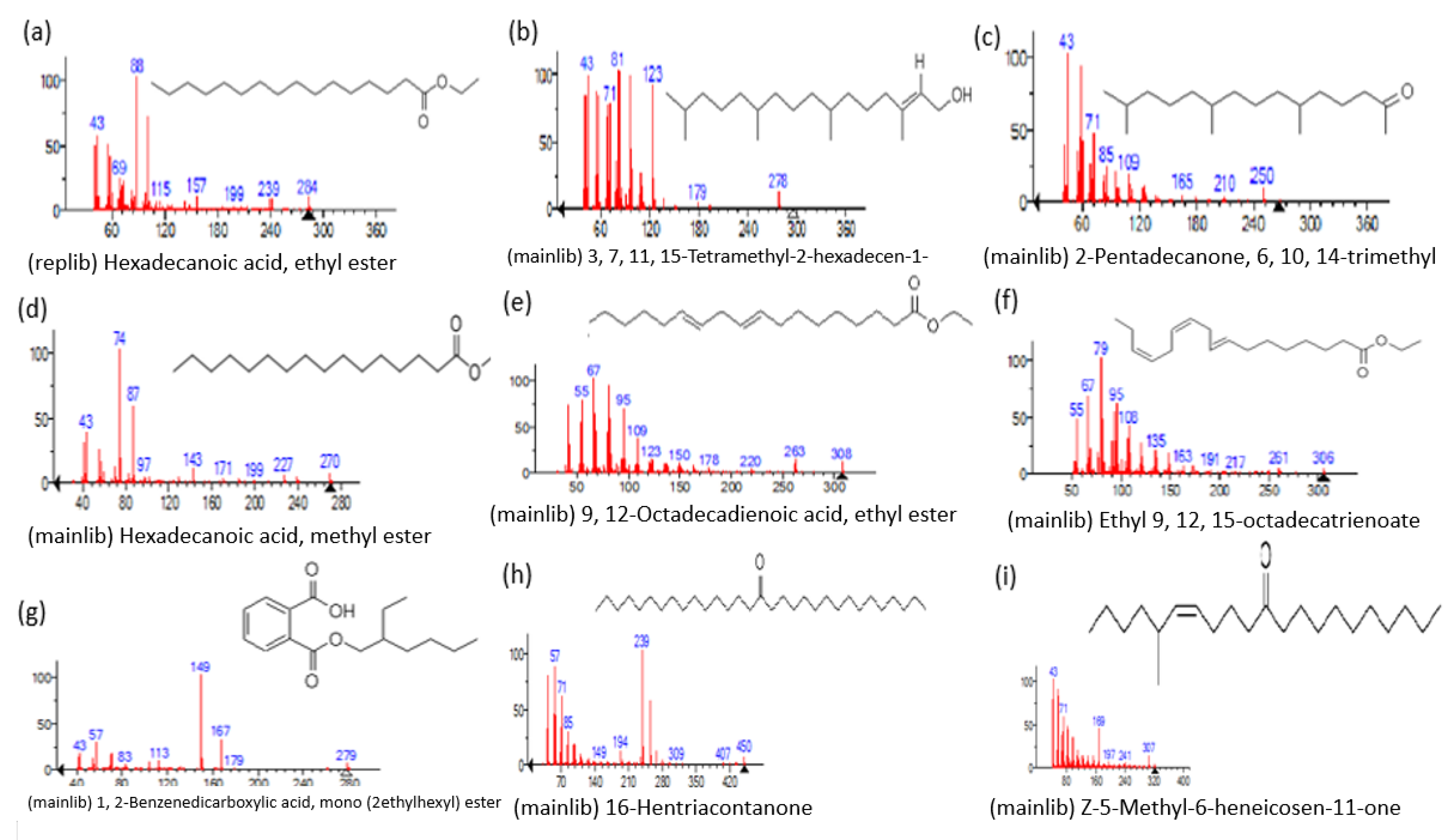
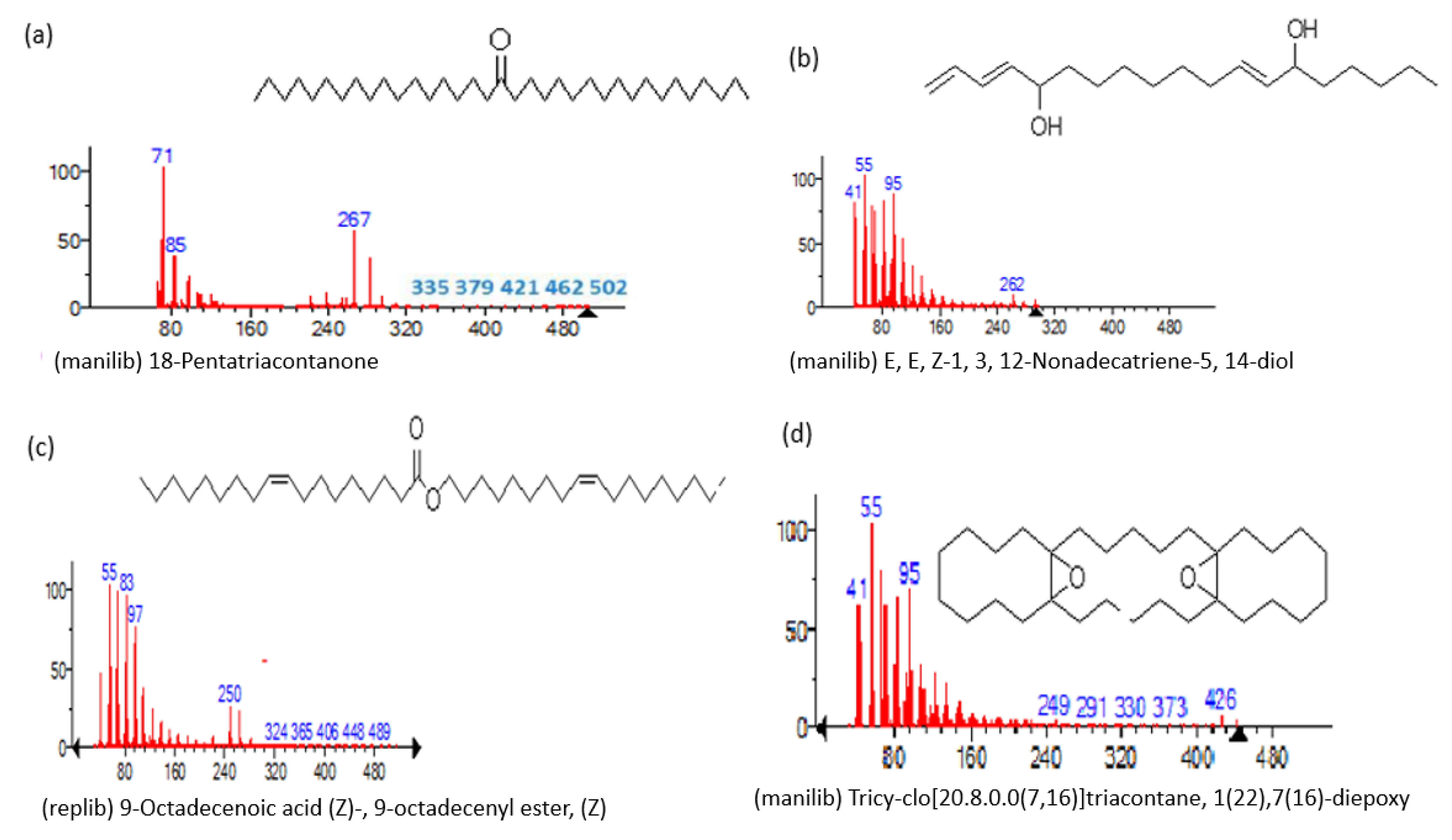
| Name of Compound | Mass/RT (min) | % Area | Molecular Formula | Fragments Ions | Structure |
|---|---|---|---|---|---|
| Nonanoic acid, 9-oxo-, ethyl ester | 200/33.69 | 2.32 | C11H20O3 | 41,55,88,29,43,101,83,60,157,155 | 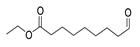 |
| Carophyllen oxide | 220/35.70 | 0.28 | C15H24O | 43,41,79,93,91,95,69,55,67,81 | 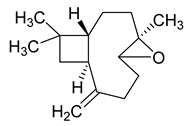 |
| Limonen-6-ol, pivalate | 236/36.06 | 0.23 | C15H24O2 | 57,41,43,93,55,107,109,91,85,119 | 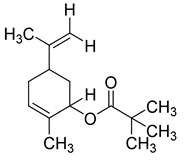 |
| β-Himachalenoxide | 220/36.39 | 0.45 | C15H24O | 110,220,95,192,43,41,69,109,151,93 |  |
| 4-tert-Butyltoluene | 148/36.85 | 0.4 | C11H16 | 133,105,148,93,41,91,134,39,115,77 | 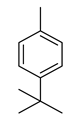 |
| α-Bisabolol | 222/37.27 | 1.25 | C15H26O | 109,119,69,43,93,41,95,121,67,71 | 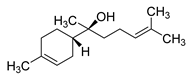 |
| Cholestan-3-ol, 2-methylene-, (3β, 5α)- | 400/36.32 | 0.18 | C28H48O | 69,81,71,95,67,83,79,93,97,105 |  |
| Benzenepropanol, 2, 4, 6-trimethyl- | 178/39.84 | 0.17 | C12H18O | 133,178,134,120,119,91,145,105,117,115 | 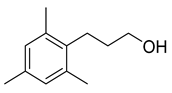 |
| Z-(13, 14-Epoxy) tetradec-11-en-1-ol acetate | 268/36.61 | 1.01 | C16H28O3 | 43,97,69,55,41,82,67,81,84,83 | 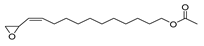 |
| Hexadecanoic acid, ethyl ester, | 284/40.11 | 0.47 | C18H36O2 | 88,101,43,55,41,57,69,73,71,70 | 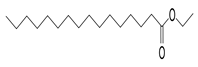 |
| 3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol | 296/40.14 | 1.01 | C20H40O | 81,82,43,95,123,55,41,57,71,68 |  |
| 2-Pentadecanone, 6, 10, 14-trimethyl | 268/41.30 | 1.76 | C18H36O | 43,58,71,57,59,41,55,69,85,95 |  |
| Hexadecanoic acid, methyl ester | 270/43.47 | 0.66 | C17H34O2 | 74,87,43,41,55,75,29,57, 143 | 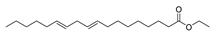 |
| 9, 12-Octadecadienoic acid, ethyl ester | 308/54.29 | 1.92 | C20H36O2 | 67,81,55,95,68,54,96,69 |  |
| Ethyl 9, 12, 15-octadecatrienoate | 306/54.76 | 3.33 | C20H34O2 | 79,67,95,93,81,55,8o,107,91 |  |
| 1, 2-Benzenedicarboxylic acid, mono (2-ethylhexyl) ester | 278/80.83 | 5.12 | C16H22O4 | 149,167,57,71,43,70 |  |
| 16-Hentriacontanone | 450/91.16 | 1.52 | C31H62O | 239,57,43,71,255,55,58,245,69,41 |  |
| Z-5-Methyl-6-heneicosen-11-one | 322/92.79 | 0.94 | C22H42O | 43,55,57,41,83,169,85,81,69 | 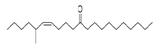 |
| 18-Pentatriacontanone | 506/96.41 | 1.161 | C35H70O | 71,267,69,85,83,283,97,82 | 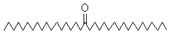 |
| E, E, Z-1, 3, 12-Nonadecatriene-5, 14-diol | 294/99.40 | 8.39 | C15H26O2 | 55,95,81,41,67,69,96,83,43,57 | 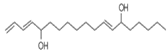 |
| 9-Octadecenoic acid (Z)-, 9-octadecenyl ester, (Z) | 532/103.82 | 1.00 | C36H68O2 | 55,69,83,97,82,96,57,95,67 |  |
| Tricyclo[20.8.0.0(7,16)]triacontane, 1(22),7(16)-diepoxy | 444/104.24 | 0.42 | C30H52O2 | 55,67,95,81,69,41,43,83,79,109 | 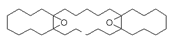 |
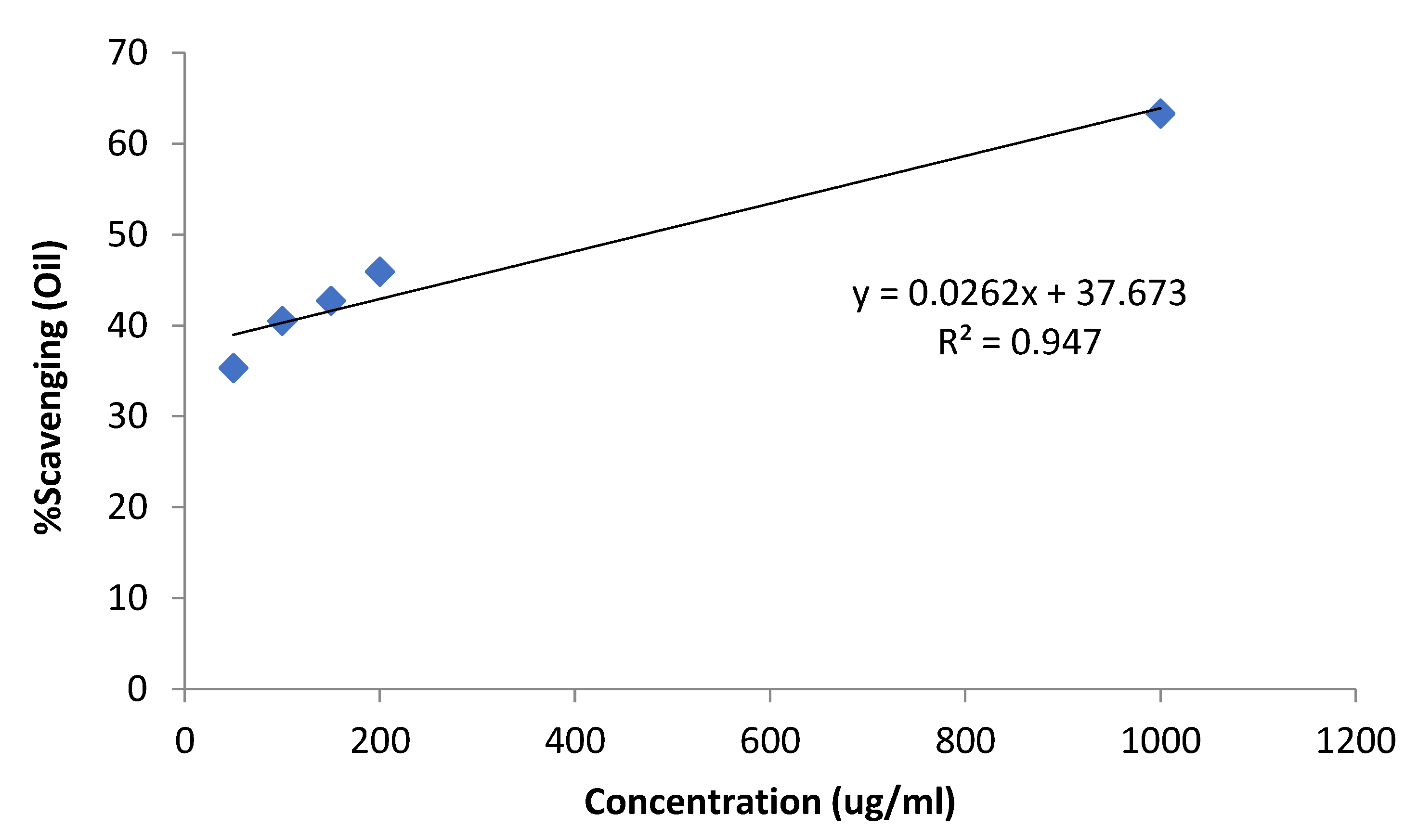
| Conc. (µg/mL) | %Scavenging (Essential Oil) | (Essential Oil) | %Scavenging (Ascorbic Acid) |
|---|---|---|---|
| 50 | 35.3 ± 1.8 | 70.5 ± 2.3 | |
| 100 | 40.5 ± 1.9 | 71.7 ± 3.1 | |
| 150 | 42.7 ± 2.1 | 470.4 | 72.9 ± 2.0 |
| 200 | 45.9 ± 1.7 | 73.4 ± 2.4 | |
| 1000 | 63.3 ± 1.8 | 87.4 ± 3.0 |
| Conc. (µg/mL) | Essential Oil | Control | %Growth Inhibition | Std. Drug Conc. (µg/mL) |
|---|---|---|---|---|
| 10 | 24 | 24 | 0 | 0.015 |
| 100 | 24 | 24 | 0 | |
| 250 | 24 | 24 | 0 | |
| 500 | 17 | 24 | 26 | |
| 1000 | 09 | 24 | 62.5 |
| Bacteria | U. dioica Essential Oil | Control (Ofloxacin) 0.25 µg/mL | ||
|---|---|---|---|---|
| 250 μg/mL | 500 μg/mL | 1000 μg/mL | ||
| Inhibition Zone Diameter (mm) | ||||
| E. coli | 0 | 10 ± 0.25 | 32 ± 1.4 | 92.47 ± 2.3 |
| B. subtilis | 0 | 12 ± 0.32 | 35 ± 1.3 | 91 ± 3.1 |
| S. aureus | 0 | 0 | 25 ± 1.2 | 94 ± 2.4 |
| P. aeruginosa | 0 | 0 | 26 ± 1.1 | 94 ± 3.1 |
| S. typhi | 0 | 0 | 20 ± 1.5 | 95 ± 2.3 |
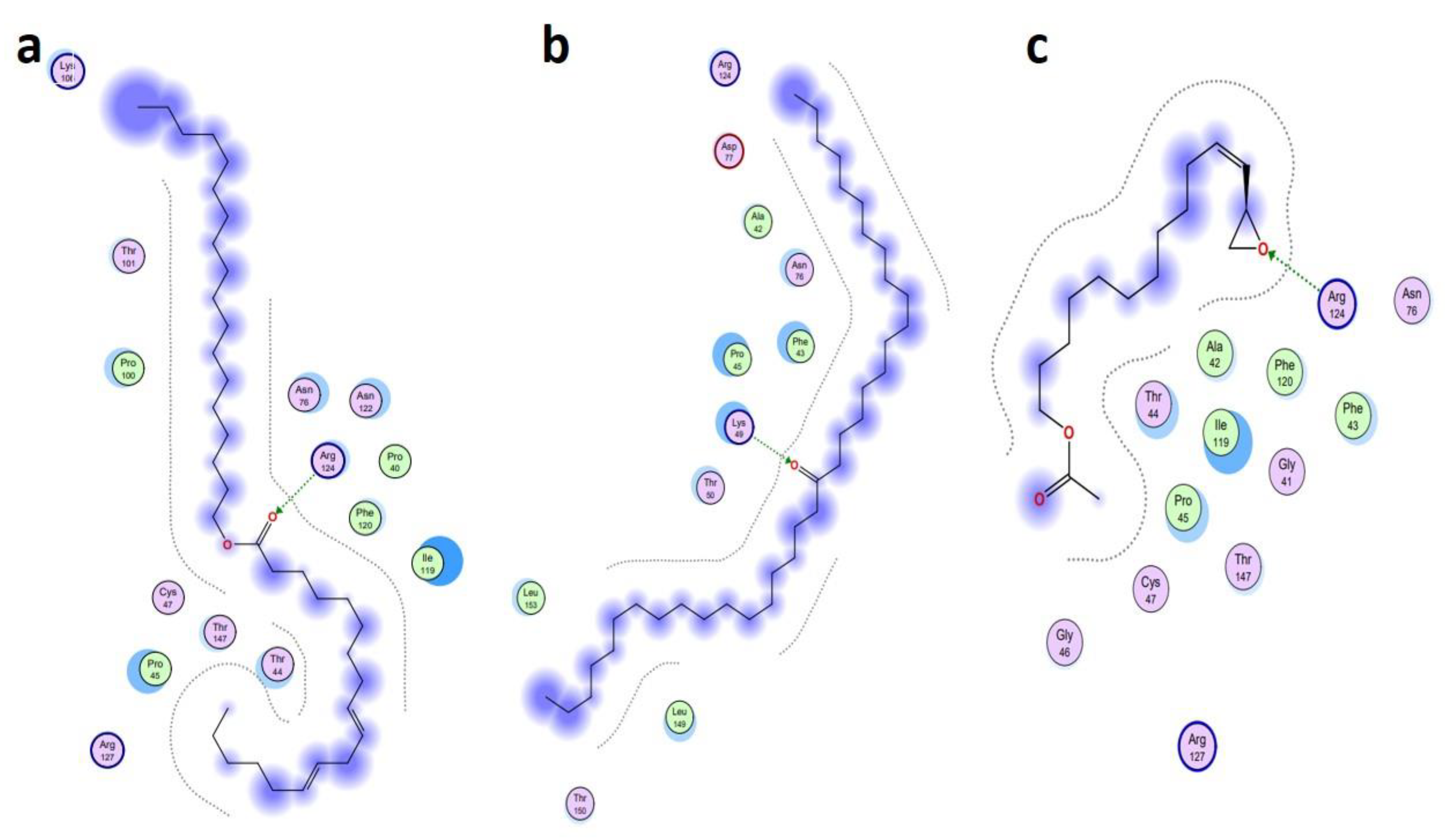
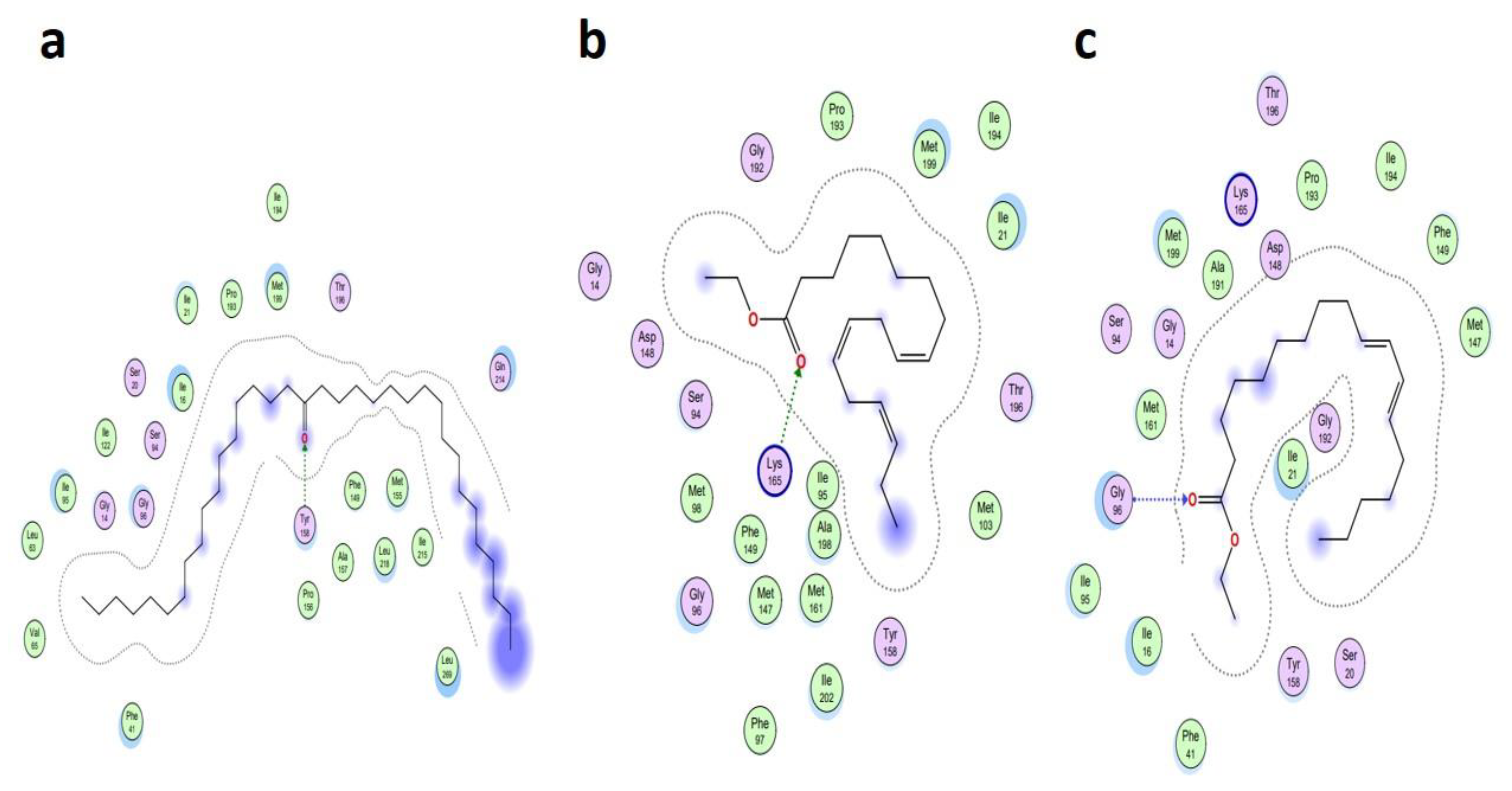
| Ligand Name | Binding Energy (Kcal/mol) | Binding Interaction | |||
|---|---|---|---|---|---|
| Interacting Residues | Interaction Type | Bond Distance | Bond Energy (Kcal/mol) | ||
| Antioxidant protein [PDB ID: 1HD2] | |||||
| 9-Octadecenoic acid (Z)-, 9-octadecenyl ester, (Z) [CID_22287839] | −6.1991 | O2–NH2 ARG 124 (A) | H-acceptor | 3.15 | −2.0 |
| 18-Pentatriacontanone [CID_10440] | −5.7512 | O1–NZ LYS 49 (A) | H-acceptor | 2.92 | −6.4 |
| Z-(13, 14-Epoxy) tetradec-11-en-1-ol acetate [CID_5363633] | −5.2222 | O1–NH2 ARG 124 (A) | H-acceptor | 3.05 | −0.5 |
| Antibacterial protein [PDB ID: 4TZK] | |||||
| 18-Pentatriacontanone [CID_10440] | −8.2366 | O1–OH TYR 158 (A) | H-acceptor | 2.93 | −2.3 |
| Ethyl 9, 12, 15-octadecatrienoate [CID_5367460] | −7.8228 | O2–NZ LYS 165 (A) | H-acceptor | 3.16 | −1.1 |
| 9, 12-Octadecadienoic acid, ethyl ester [CID_22371644] | −7.7674 | O2–N GLY 96 (A) | H-acceptor | 3.33 | −1.8 |
| Chemical Parameters | 9-Octadecenoic Acid (Z)-, 9-Octadecenyl Ester, (Z) | 18-Pentatriacontanone | Z-(13, 14-Epoxy) tetradec-11-en-1-ol Acetate | Ethyl 9, 12, 15-Octadecatrienoate | 9, 12-Octadecadienoic Acid, Ethyl Ester |
|---|---|---|---|---|---|
| Physicochemical Properties | |||||
| Molecular weight (MW) (g/mol) | 532.92 | 506 | 268.39 | 306.48 | 308.50 |
| Rotatable bonds | 32 | 32 | 13 | 15 | 16 |
| Hydrogen bond acceptors (HBA) | 2 | 1 | 3 | 2 | 2 |
| Hydrogen bond donors (HBD) | 0 | 0 | 0 | 0 | 0 |
| Molar Refractivity (MR) | 175.50 | 170.56 | 78.81 | 98.12 | 98.59 |
| Total polar surface area (TPSA) (Å) | 26.30 | 17.07 | 38.83 | 26.30 | 26.30 |
| Bioavailability Score | 0.17 | 0.17 | 0.55 | 0.55 | 0.55 |
| Lipophilicity | |||||
| Log Po/w (iLOGP) | 8.73 | 8.68 | 3.44 | 4.82 | 5.01 |
| Water Solubility | |||||
| Class | Insoluble | Insoluble | Soluble | Moderatley soluble | Moderatley soluble |
| Pharmacokinetics | |||||
| GI absorption | Low | Low | High | High | High |
| BBB permeant | No | No | Yes | No | No |
| P-gp substrate | Yes | Yes | No | No | No |
| CYP1A2 inhibitor | No | No | Yes | Yes | Yes |
| CYP2C19 Inhibitor | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | Yes | Yes | Yes |
| CYP2D6 inhibitor | No | No | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No |
| Log Kp (skin permeation) (cm/s) | 1.61 | 2.51 | −4.74 | −3.44 | −2.79 |
| Toxicity estimation | |||||
| Mutagenic | No | No | Yes | No | No |
| Tumorigenic | No | No | Yes | No | No |
| Reproductive effects | No | No | No | No | No |
| Irritant effects | No | No | Yes | No | No |
| Medicinal chemistry-related properties | |||||
| PAINS | No | No | No | No | No |
| Brenk | 1 t: isolated_alkene | No | 3: Three-membered_heterocycle, isolated_alkene | 1: isolated_alkene | 1: polyene |
| Synthetic accessibility | 5.29 | 4.57 | 3.60 | 3.26 | 3.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.Z.; Azad, A.K.; Jan, S.; Safdar, M.; Bibi, S.; Majid, A.M.S.A.; Albadrani, G.M.; Nouh, N.A.T.; Abdulhakim, J.A.; Abdel-Daim, M.M. An Experimental and Computational Analysis of Plant Compounds from Whole Urtica dioica L. Plant’s Essential Oil for Antioxidant and Antibacterial Activities. Metabolites 2023, 13, 502. https://doi.org/10.3390/metabo13040502
Khan MZ, Azad AK, Jan S, Safdar M, Bibi S, Majid AMSA, Albadrani GM, Nouh NAT, Abdulhakim JA, Abdel-Daim MM. An Experimental and Computational Analysis of Plant Compounds from Whole Urtica dioica L. Plant’s Essential Oil for Antioxidant and Antibacterial Activities. Metabolites. 2023; 13(4):502. https://doi.org/10.3390/metabo13040502
Chicago/Turabian StyleKhan, Muhammad Zahid, Abul Kalam Azad, Saleem Jan, Muhammad Safdar, Shabana Bibi, Amin Malik Shah Abdul Majid, Ghadeer M. Albadrani, Nehal Ahmed Talaat Nouh, Jawaher A. Abdulhakim, and Mohamed M. Abdel-Daim. 2023. "An Experimental and Computational Analysis of Plant Compounds from Whole Urtica dioica L. Plant’s Essential Oil for Antioxidant and Antibacterial Activities" Metabolites 13, no. 4: 502. https://doi.org/10.3390/metabo13040502
APA StyleKhan, M. Z., Azad, A. K., Jan, S., Safdar, M., Bibi, S., Majid, A. M. S. A., Albadrani, G. M., Nouh, N. A. T., Abdulhakim, J. A., & Abdel-Daim, M. M. (2023). An Experimental and Computational Analysis of Plant Compounds from Whole Urtica dioica L. Plant’s Essential Oil for Antioxidant and Antibacterial Activities. Metabolites, 13(4), 502. https://doi.org/10.3390/metabo13040502