Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Mushroom Material
2.3. UAE Methodology
2.4. Experimental Design
2.5. Evaluation of TPC
2.6. In Vitro Antioxidant Capacity
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of the Extraction Solvent
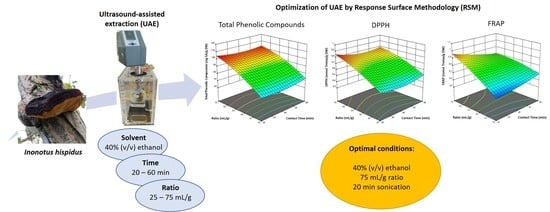
3.2. Optimization of the Extraction Conditions
3.3. Correlation between the Evaluated Responses of I. hispidus Extracts
3.4. HPLC Analysis of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Rai, S.N.; Dubey, S.K.; Pandey, A.T.; Tabassum, N.; Chaturvedi, V.K.; Singh, N.B. Biomolecules of mushroom: A recipe of human wellness. Crit. Rev. Biotechnol. 2022, 42, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Sánchez, C. Bioactives from Mushroom and Their Application. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 23–57. [Google Scholar]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.; Afonso, A.; Fernandes, C.; Nunes, F.M.; Marques, G.; Saavedra, M.J. Comparative antioxidant and antimicrobial properties of Lentinula edodes Donko and Koshin varieties against priority multidrug-resistant pathogens. S. Afr. J. Chem. Eng. 2021, 35, 98–106. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Castro, F.; Aires, A.; Marques, G.; Saavedra, M.J. Antimicrobial, Antibiofilm, and Antioxidant Properties of Boletus edulis and Neoboletus luridiformis Against Multidrug-Resistant ESKAPE Pathogens. Front. Nutr. 2022, 8, 1093. [Google Scholar] [CrossRef]
- Ryvarden, L.; Gilbertson, R.L. European Polypores, Part 1; Fungiflora: Oslo, Norway, 1993; Volume 6. [Google Scholar]
- Wang, Z.-X.; Feng, X.-L.; Liu, C.; Gao, J.-M.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics 2022, 11, 1097. [Google Scholar] [CrossRef]
- Yang, H.; Li, S.; Qu, Y.; Li, L.; Li, Y.; Wang, D. Anti-Colorectal Cancer Effects of Inonotus hispidus (Bull.: Fr.) P. Karst. Spore Powder through Regulation of Gut Microbiota-Mediated JAK/STAT Signaling. Nutrients 2022, 14, 3299. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, J.; Liu, Z.; Li, Z.; Teng, L.; Wang, D. Inonotus hispidus Protects against Hyperlipidemia by Inhibiting Oxidative Stress and Inflammation through Nrf2/NF-κB Signaling in High Fat Diet Fed Mice. Nutrients 2022, 14, 3477. [Google Scholar] [CrossRef]
- Awadh Ali, N.A.; Mothana, R.A.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Bao, H.; Wang, H.; Li, Q. Anti-tumour Effect and Pharmacokinetics of an Active Ingredient Isolated from Inonotus hispidus. Biol. Pharm. Bull. 2019, 42, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hou, R.; Xu, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019, 123, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Shomali, N.; Onar, O.; Alkan, T.; Demirtaş, N.; Akata, I.; Yildirim, Ö. Investigation of the Polyphenol Composition, Biological Activities, and Detoxification Properties of Some Medicinal Mushrooms from Turkey. Turk. J. Pharm. Sci. 2019, 16, 155–160. [Google Scholar] [CrossRef]
- Zan, L.F.; Qin, J.C.; Zhang, Y.M.; Yao, Y.H.; Bao, H.Y.; Li, X. Antioxidant hispidin derivatives from medicinal mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011, 59, 770–772. [Google Scholar] [CrossRef] [Green Version]
- Benarous, K.; Bombarda, I.; Iriepa, I.; Moraleda, I.; Gaetan, H.; Linani, A.; Tahri, D.; Sebaa, M.; Yousfi, M. Harmaline and hispidin from Peganum harmala and Inonotus hispidus with binding affinity to Candida rugosa lipase: In silico and in vitro studies. Bioorganic Chem. 2015, 62, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Yan, J.; Xu, K.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019, 129, 41–49. [Google Scholar] [CrossRef]
- Fogarasi, M.; Socaciu, M.-I.; Sălăgean, C.-D.; Ranga, F.; Fărcaș, A.C.; Socaci, S.A.; Socaciu, C.; Țibulcă, D.; Fogarasi, S.; Semeniuc, C.A. Comparison of Different Extraction Solvents for Characterization of Antioxidant Potential and Polyphenolic Composition in Boletus edulis and Cantharellus cibarius Mushrooms from Romania. Molecules 2021, 26, 7508. [Google Scholar] [CrossRef]
- Hu, Y.-N.; Sung, T.-J.; Chou, C.-H.; Liu, K.-L.; Hsieh, L.-P.; Hsieh, C.-W. Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Akbar, N.; Shah, S.M.A.; Ali, S.; Abbas, A. Green approaches for the extraction of bioactive from natural sources for pharmaceutical applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula, R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 269–291. [Google Scholar]
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Barbero, G.F.; Carrera, C. Ultrasound-Assisted Extraction of Total Phenolic Compounds and Antioxidant Activity in Mushrooms. Agronomy 2022, 12, 1812. [Google Scholar] [CrossRef]
- Martín-García, B.; Pasini, F.; Verardo, V.; Díaz-de-Cerio, E.; Tylewicz, U.; Gómez-Caravaca, A.M.; Caboni, M.F. Optimization of Sonotrode Ultrasonic-Assisted Extraction of Proanthocyanidins from Brewers’ Spent Grains. Antioxidants 2019, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Extraction Techniques for the Determination of Phenolic Compounds in Food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 159–180. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Selection of the Solvent and Extraction Conditions for Maximum Recovery of Antioxidant Phenolic Compounds from Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Kasina, M.; Joseph, K.; John, M. Application of Central Composite Design to Optimize Spawns Propagation. Open J. Optim. 2020, 9, 47–70. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Dominguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crop. Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. Biomed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonsong, S.; Klaypradit, W.; Wilaipun, P. Antioxidant activities of extracts from five edible mushrooms using different extractants. Agric. Nat. Resour. 2016, 50, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Ianni, F.; Blasi, F.; Angelini, P.; Simone, S.C.D.; Angeles Flores, G.; Cossignani, L.; Venanzoni, R. Extraction Optimization by Experimental Design of Bioactives from Pleurotus ostreatus and Evaluation of Antioxidant and Antimicrobial Activities. Processes 2021, 9, 743. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Wong, B.Y.; Tan, C.P.; Ho, C.W. Effect of solid-to-solvent ratio on phenolic content and antioxidant capacities of “Dukung Anak” (Phyllanthus niruri). Int. Food Res. J. 2013, 20, 325–330. [Google Scholar]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Martins, S.; Aguilar, C.N.; de la Garza-Rodriguez, I.; Mussatto, S.I.; Teixeira, J.A. Kinetic study of nordihydroguaiaretic acid recovery from Larrea tridentata by microwave-assisted extraction. J. Chem. Technol. Biotechnol. 2010, 85, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Belmont, CA, USA, 1996; pp. xxii, 600. [Google Scholar]
- Bristy, A.T.; Islam, T.; Ahmed, R.; Hossain, J.; Reza, H.M.; Jain, P. Evaluation of Total Phenolic Content, HPLC Analysis, and Antioxidant Potential of Three Local Varieties of Mushroom: A Comparative Study. Int. J. Food Sci. 2022, 2022, 3834936. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Maouni, A.; Pinto da Silva, L.; Saidi, R.; Legssyer, M.; Lamrani, Z.; Esteves da Silva, J.C.G. Antioxidant Properties, Bioactive Compounds Contents, and Chemical Characterization of Two Wild Edible Mushroom Species from Morocco: Paralepista flaccida (Sowerby) Vizzini and Lepista nuda (Bull.) Cooke. Molecules 2023, 28, 1123. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Xu, B. Phenolic profiles, antioxidant capacities and metal chelating ability of edible mushrooms commonly consumed in China. LWT Food Sci. Technol. 2016, 72, 423–431. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Palkina, K.A.; Ipatova, D.A.; Shakhova, E.S.; Balakireva, A.V.; Markina, N.M. Therapeutic Potential of Hispidin-Fungal and Plant Polyketide. J. Fungi 2021, 7, 323. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, H.; Lu, T.; Zhao, Y.; Zheng, W. Ultraviolet radiation promotes the production of hispidin polyphenols by medicinal mushroom Inonotus obliquus. Fungal Biol. 2022, 126, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, R.; Zhang, J.; Bu, Q.; Wang, W.; Liu, Y.; Li, Q.; Guo, Y.; Zhang, L.; Yang, Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019, 9, 16172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gründemann, C.; Arnhold, M.; Meier, S.; Bäcker, C.; Garcia-Käufer, M.; Grunewald, F.; Steinborn, C.; Klemd, A.M.; Wille, R.; Huber, R.; et al. Effects of Inonotus hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med. 2016, 82, 1359–1367. [Google Scholar] [CrossRef] [PubMed]






| Run Order | Contact Time (min) | Solvent-to-Solid Ratio (mL/g) | Response TPC (mg GA/g DW) | Response DPPH (mmol Trolox/g DW) | Response FRAP (mmol Trolox/g DW) |
|---|---|---|---|---|---|
| 1 | 60 | 25 | R1 | R1 | R1 |
| 2 | 60 | 75 | R2 | R2 | R2 |
| 3 | 40 | 50 | R3 | R3 | R3 |
| 4 | 20 | 25 | R4 | R4 | R4 |
| 5 | 68.28 | 50 | R5 | R5 | R5 |
| 6 | 40 | 50 | R6 | R6 | R6 |
| 7 | 40 | 14.64 | R7 | R7 | R7 |
| 8 | 40 | 50 | R8 | R8 | R8 |
| 9 | 40 | 50 | R9 | R9 | R9 |
| 10 | 11.72 | 50 | R10 | R10 | R10 |
| 11 | 20 | 75 | R11 | R11 | R11 |
| 12 | 40 | 50 | R12 | R12 | R12 |
| 13 | 40 | 85.36 | R13 | R13 | R13 |
| Independent Variables | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −α (−2) | −1 | 0 | +1 | +α (+2) | ||
| Contact Time (min) | X1 | 11.72 | 20 | 40 | 60 | 68.28 |
| Solvent-to-Solid Ratio (mL/g) | X2 | 14.64 | 25 | 50 | 75 | 85.36 |
| Contact Time | Organic Solvent | TPC (mg GA/g DW) | DPPH (mmol Trolox/g DW) | FRAP (mmol Trolox/g DW) |
|---|---|---|---|---|
| 40 min | Ethanol 40% (v/v) | 100.70 ± 5.08 **** | 1.09 ± 0.08 **** | 0.78 ± 0.03 **** |
| Methanol 80% (v/v) | 85.94 ± 2.41 | 0.88 ± 0.05 | 0.69 ± 0.03 |
| Assay | Coded Level (Real Values) | TPC (mg GA/g DW) | DPPH (mmol Trolox/g DW) | FRAP (mmol Trolox/g DW) | ||||
|---|---|---|---|---|---|---|---|---|
| Contact Time (min) | Solvent-to-Solid Ratio (mL/g) | Observed | Predicted | Observed | Predicted | Observed | Predicted | |
| 1 | +1 (60) | −1 (25) | 72.00 | 73.47 | 0.51 | 0.52 | 0.61 | 0.69 |
| 2 | +1 (60) | +1 (75) | 102.89 | 102.87 | 0.77 | 0.79 | 0.81 | 0.80 |
| 3 Z | 0 (40) | 0 (50) | 96.60 | 89.22 | 0.57 | 0.65 | 0.79 | 0.77 |
| 4 | −1 (20) | −1 (25) | 68.83 | 70.08 | 0.52 | 0.50 | 0.55 | 0.59 |
| 5 | +2 (68.28) | 0 (50) | 95.96 | 95.19 | 0.76 | 0.74 | 0.82 | 0.78 |
| 6 Z | 0 (40) | 0 (50) | 82.94 | 89.22 | 0.58 | 0.65 | 0.77 | 0.77 |
| 7 | 0 (40) | −2 (14.64) | 60.00 | 58.33 | 0.35 | 0.36 | 0.62 | 0.54 |
| 8 Z | 0 (40) | 0 (50) | 88.71 | 89.22 | 0.60 | 0.65 | 0.73 | 0.77 |
| 9 Z | 0 (40) | 0 (50) | 90.66 | 89.22 | 0.77 | 0.65 | 0.81 | 0.77 |
| 10 | −2 (11.72) | 0 (50) | 94.38 | 93.91 | 0.75 | 0.77 | 0.81 | 0.82 |
| 11 | −1 (20) | +1 (75) | 104.68 | 104.45 | 0.86 | 0.85 | 1.01 | 0.96 |
| 12 Z | 0 (40) | 0 (50) | 87.19 | 89.22 | 0.72 | 0.65 | 0.77 | 0.77 |
| 13 | 0 (40) | +2 (85.36) | 103.00 | 103.43 | 0.80 | 0.79 | 0.81 | 0.89 |
| Responses | Statistics | X1 | X2 | X1,2 | X12 | X22 | Model |
|---|---|---|---|---|---|---|---|
| TPC | p value | 0.7661 (n.s.) | <0.0001 (****) | 0.5477 (n.s.) | 0.1164 (n.s.) | 0.0265 (*) | 0.0002 (***) |
| F value | 0.1060 | 131.94 | 0.3990 | 3.21 | 7.84 | 29.00 | |
| DPPH | p value | 0.6801 (n.s.) | 0.0004 (***) | 0.5886 (n.s.) | 0.0857 (n.s) | 0.2148 (n.s.) | 0.0057 (**) |
| F value | 0.1850 | 38.35 | 0.3212 | 4.00 | 1.86 | 9.11 | |
| FRAP | p value | 0.4650 (n.s.) | 0.0006 (***) | 0.0586 (n.s.) | 0.5524 (n.s.) | 0.2276 (n.s.) | 0.0064 (**) |
| F value | 0.5970 | 35.54 | 5.10 | 0.3894 | 1.75 | 8.73 | |
| TPC = 89.22 + 0.45X1 + 15.9439X2 − 1.24X1X2 + 2.66625X12 − 4.16875X22; R2 = 0.95 | |||||||
| DPPH = 0.648 − 0.0107322X1 + 0.15455X2 − 0.02X1X2 + 0.0535X12 − 0.0365X22; R2 = 0.87 | |||||||
| FRAP = 0.774 − 0.0157322X1 + 0.121391X2 − 0.065X1X2 + 0.013625X12 − 0.028875X22; R2 = 0.86 | |||||||
| Experimental Assays | Independent Variables | Responses | |||
|---|---|---|---|---|---|
| Contact Time (min) | Solvent-to-Solid Ratio (mL/g) | TPC (mg GA/g DW) | DPPH (mmol Trolox/g DW) | FRAP (mmol Trolox/g DW) | |
| 1 | 20 | 75 | 121.27 ± 2.11 | 1.01 ± 0.14 | 1.11 ± 0.02 |
| 2 | 20 | 75 | 95.72 ± 1.14 | 0.77 ± 0.02 | 0.98 ± 0.01 |
| 3 | 20 | 75 | 96.84 ± 1.62 | 0.80 ± 0.19 | 0.93 ± 0.01 |
| Average | 104.61 ± 13.82 | 0.86 ± 0.15 | 1.01 ± 0.08 | ||
| Results predicted by the statistical analysis | 104.45 | 0.85 | 0.96 | ||
| Compound | Retention Time (min) | Concentration (µg/g DW) |
|---|---|---|
| Glycitin | 5.62 | 2.26 ± 0.006 |
| Diosmetin | 8.03 | 18.39 ± 0.050 |
| Hydroxybenzoic acid | 12.32 | 3.48 ± 0.032 |
| Caffeic acid | 19.42 | 1.24 ± 0.012 |
| Luteolin-7-O-glucoside | 20.64 | 1.22 ± 0.033 |
| Myricetin | 20.91 | 13.45 ± 0.130 |
| Luteolin-4′-O-glucoside | 22.27 | 3.75 ± 0.087 |
| Quercetin | 22.37 | 1.91 ± 0.030 |
| Hispidin | 23.61 | 122.80 ± 1.456 |
| Hispidin-like compound | 24.98 | 4.08 ± 0.115 |
| Hispidin-like compound | 26.37 | 3.86 ± 0.095 |
| Hispidin-like compound | 27.31 | 5.70 ± 0.057 |
| Hispidin-like compound | 29.11 | 19.50 ± 0.101 |
| Hispidin-like compound | 30.11 | 3.62 ± 0.101 |
| Isorhamnetin | 33.90 | 13.75 ± 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. https://doi.org/10.3390/metabo13040524
Machado-Carvalho L, Martins T, Aires A, Marques G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites. 2023; 13(4):524. https://doi.org/10.3390/metabo13040524
Chicago/Turabian StyleMachado-Carvalho, Liliana, Tânia Martins, Alfredo Aires, and Guilhermina Marques. 2023. "Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology" Metabolites 13, no. 4: 524. https://doi.org/10.3390/metabo13040524
APA StyleMachado-Carvalho, L., Martins, T., Aires, A., & Marques, G. (2023). Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites, 13(4), 524. https://doi.org/10.3390/metabo13040524