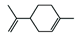
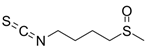
Abstract
The overuse of antibiotics in the healthcare, veterinary, and agricultural industries has led to the development of antimicrobial resistance (AMR), resulting in significant economic losses worldwide and a growing healthcare problem that urgently needs to be solved. Plants produce a variety of secondary metabolites, making them an area of interest in the search for new phytochemicals to cope with AMR. A great part of agri-food waste is of plant origin, constituting a promising source of valuable compounds with different bioactivities, including those against antimicrobial resistance. Many types of phytochemicals, such as carotenoids, tocopherols, glucosinolates, and phenolic compounds, are widely present in plant by-products, such as citrus peels, tomato waste, and wine pomace. Unveiling these and other bioactive compounds is therefore very relevant and could be an important and sustainable form of agri-food waste valorisation, adding profit for local economies and mitigating the negative impact of these wastes’ decomposition on the environment. This review will focus on the potential of agri-food waste from a plant origin as a source of phytochemicals with antibacterial activity for global health benefits against AMR.
1. Introduction
Food waste is an inevitable outcome of food production processes, and substantial quantities of by-products are generated along the food chain. According to the United Nations Food and Agriculture Organization (FAO), approximately one-third of all food produced for human consumption globally is wasted at various stages of the food supply chain and during production, post-harvest handling and storage, processing, distribution, and consumption [1].
Such waste, estimated at 1.3 billion tonnes of food per year [1], constitutes a burden for the industry, which often has to pay to discard it. Moreover, there are obvious economic and environmental impacts, particularly when the wastes are deposited in landfills with no or minimal processing, contributing to greenhouse gas emissions and groundwater contamination [1]. This scenario will gradually worsen as the human population continues to grow exponentially, and, despite the huge amounts of wasted food, more food will be produced.
In general terms, agri-food waste refers to any organic material that is discarded during food production or processing activities. This can include everything from animal and plant by-products, such as bones and fat, to leftover crops or spoiled produce that cannot be sold for human consumption. Most of this food wastage, particularly that of a plant origin, contains considerable amounts of phytochemicals with interesting bioactivities for animal and human health management. This includes valuable compounds with the potential to generate enough revenues for the valorisation of agri-food residues. This is an important goal that can greatly benefit the environment and improve the food chain’s security and sustainability [2]. This strategy requires a deeper understanding of the composition of agri-food waste and the properties of its main components to unveil potential uses and routes for waste processing, envisaging a zero-waste policy promoted, for instance, by EU authorities [3]. As an example of such a strategy, it is worthwhile to refer to the work of Šeregelj et al. [4]. The authors encapsulated red pepper waste bioactive compounds, which were used to develop a functional yoghurt without losing its original sensorial properties. D-phytochemicals were found in red pepper wastes, namely carotenoids (β-carotene, lutein, zeaxanthin, β-cryptoxanthin), hydroxybenzoic acids (gallic, vanillic, protocatechuic acid), hydroxycinnamic acids (sinapic, caffeic, rosmarinic, chlorogenic acid), flavan-3-ols (epicatechin), and flavonols (rutin, quercetin, and myricetin). The fortification of the yoghourt had a positive influence on maintaining the initial number of lactic acid bacteria during storage, which retained carotenoids and increased polyphenol retention.
Antimicrobial resistance (AMR) involving the transfer of bacteria and genes between humans, animals, and the environment constitutes a global challenge [5], and researchers are actively trying to unveil new drugs able to mitigate this health problem. As referred to above, plants produce a variety of secondary metabolites, and a substantial part of agri-food waste is from a plant origin. Therefore, there is great potential in delving into agri-food waste composition to unveil potential antibacterial phytochemicals. In this context, this review will briefly refer to the impact of bacterial activity in the food chain and human health and the challenges posed by AMR, discussing then the use of extraction and chromatographic technologies to identify and quantify antibacterial phytochemicals in agri-food wastes and how these phytochemicals can be used as alternative antibiotics and food additives. To obtain a focused discussion of this topic, only applications reported in the literature since 2018 were considered.
2. The Impact of Bacterial Activity
2.1. Most Relevant Bacteria Affecting Human and Animal Health, Food Preservation, and Environment
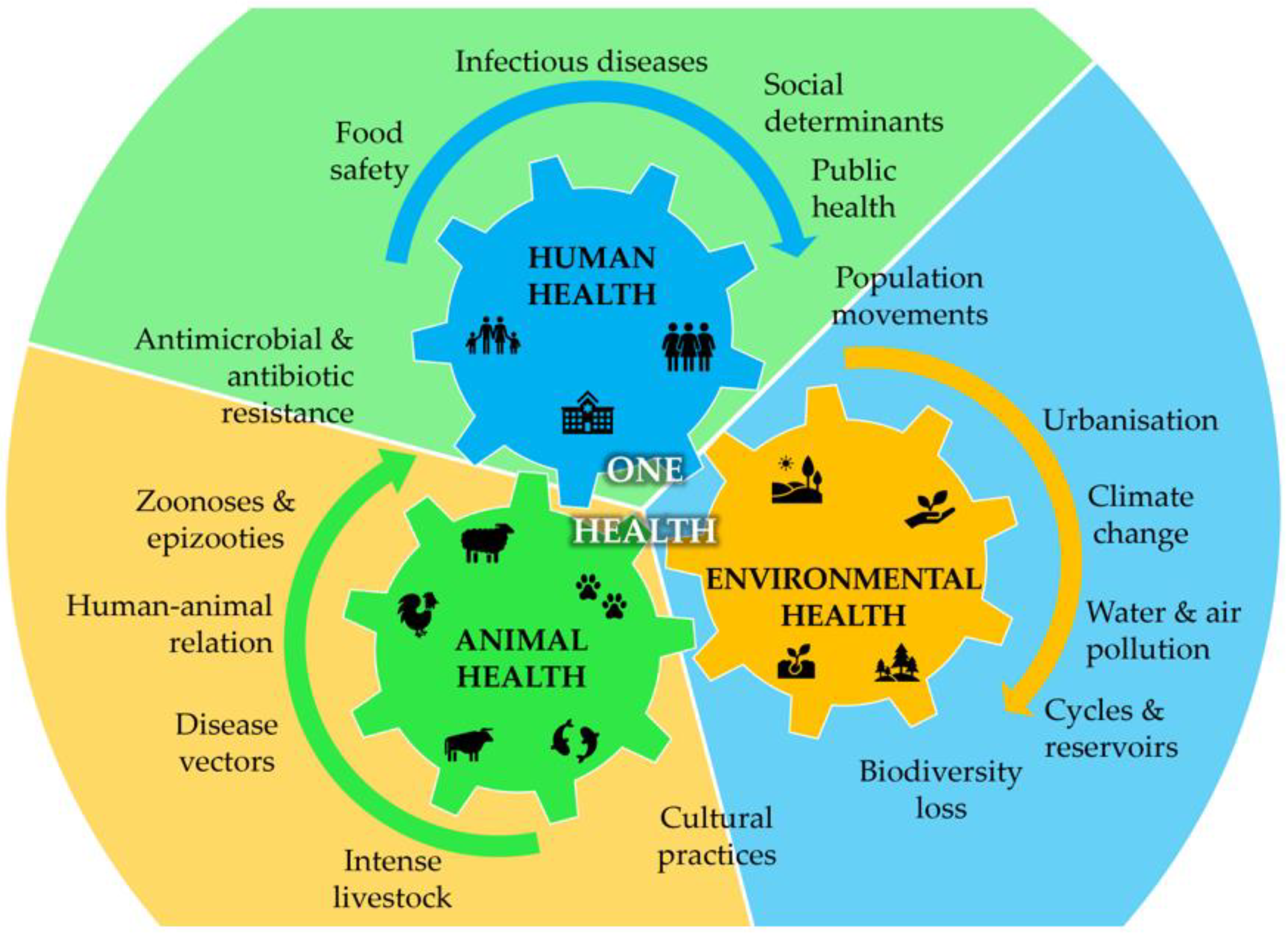
Bacteria are present everywhere, including in humans, where there are roughly as many bacteria as host cells [6]. Most of these bacteria are essential to host metabolism, and a delicate balance is established between host and foreign cells to maintain homeostasis. However, human activity in the environment continually puts pressure on the human-animal–ecosystems interface, leading to the disruption and extinction of natural ecosystems and species that have evolved over millions of years. Recently, this has been acknowledged through the One Health initiative, which aims to oversee the challenges for human health integrated holistically with animal and environmental health [7,8,9]. Zoonotic diseases caused by bacterial outbreaks, for instance, are easily transmitted through contact with animals, food, water, and contaminated environments, emerging as serious challenges to public health. These zoonoses can easily affect aquaculture, agriculture, and other food systems [10], disrupting the food chain supply to millions of people on the planet (Figure 1). Several bacteria are more prone to elicit the referred problems, mostly by causing food poisoning and environmental contamination or directly infecting the human host. Table 1 describes the most important bacteria causing food poisoning and environmental contamination or acting directly as human pathogens. Food spoilage from bacteria is often accompanied by a decay in the sensory attributes that makes consumers reject ingestion. However, cross-contamination and toxin production by pathogenic microorganisms present in food is not so easily observed and poses risks to consumers. Raw or poorly processed foods, such as milk and dairy products, meat, and poultry, can be contaminated with different bacteria, such as Bacillus cereus [11], Brucella spp. [12], Campylobacter spp. [13], Clostridium difficile (C. difficile) [14], Escherichia coli (E. coli) [15], Staphylococcus aureus (S. aureus) [16], or Yersinia spp. [17,18]. In turn, eggs, poultry, and meat are more often contaminated with Salmonella spp. [19,20] and seafood with C. difficile [14], Listeria monocytogenes [21,22], or Vibrio spp. [23,24,25]. These infections may cause different alterations in the host depending on the susceptibility and severity of the pathogen contamination, ranging from asymptomatic contaminations to transitory digestive alterations (gastroenteritis, diarrhoea, vomiting, nausea, and mild fevers), or the infections can even affect other systems (hepatobiliary, genitourinary, musculoskeletal, cardiovascular, and integumentary systems), representing a serious threat to humans. These harsh effects are often associated with toxigenic strains such as C. difficile, E. coli, S. aureus, or V. cholerae (Table 1), whose toxins can be very harmful to the infected host. There are, however, other bacteria that form spores to withstand unfavourable conditions, such as B. cereus [11]. In this context, the combination of these two characteristics, which occurs with C. difficile, can make this bacterial infection particularly dangerous. C. difficile can survive in harsh conditions (e.g., antibiotic therapy) and later, under favourable conditions, produce toxins soon after the germination of the spores, causing serious illness or even death, especially in vulnerable populations, such as elderly people and those with weakened immune systems. The effect of food poisoning caused by bacteria on human health is, therefore, broad, and it is even more dangerous when the infection is caused by bacteria that evolved as human pathogens. As shown in Table 1, several bacteria can cause severe disruptions in human health. Some of them, such as Acinetobacter baumannii, Klebsiella pneumoniae, or Pseudomonas aeruginosa (P. aeruginosa), are opportunistic bacteria populating clinical environments and infecting immunocompromised patients [26,27,28]. Others, such as the Neisseria strains Neisseria meningitidis and Neisseria gonorrhoeae, can cause serious infections in humans, such as meningitis or gonorrhoea, respectively. Finally, Mycobacterium tuberculosis is responsible for tuberculosis (TB), a primary respiratory and incapacitating infection. TB is the leading cause of death caused by a single infectious agent [29].
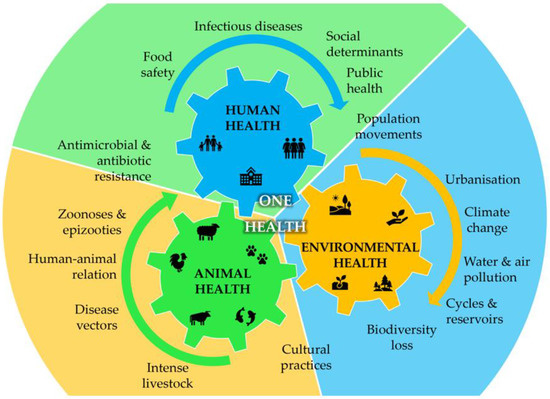
Figure 1.
Integrated overview of the One Health concept showing the interdependence between human, animal, and environmental health and the interplay of the main factors that drive each one of the three categories (adapted from [7,8,9]).

Table 1.
Most important bacteria causing food poisoning, environmental hazards, or human health complications.
2.2. The Challenge of Antimicrobial Resistance (AMR)
AMR is a significant problem in the field of medicine and healthcare. It refers to microorganisms, mainly bacteria, viruses, and fungi, that become resistant to multiple drugs and treatments that were primarily designed to target them. This is often the result of the indiscriminate use of antibiotics in the healthcare, veterinary, and agricultural industries [68,69]. Inadequate antibiotic and dosing choices and unnecessarily extended treatment have also contributed to the problem. Overall, these strategies have boosted antibiotic resistance in different environments, such as hospitals, nursing homes, and communities [70]. Consequently, AMR has become a major public health challenge, described by the World Health Organization as one of the top 10 public health challenges worldwide. In 2019 alone, it was estimated that 4.95 million deaths were associated with bacterial antimicrobial resistance, with 1.27 million being a direct cause of bacterial antimicrobial resistance [71]. These figures are concerning because the treatment of infectious diseases involving AMR will become progressively harder with a limited portfolio of effective drugs. As a result, prohibitive healthcare costs, morbidity, and mortality rates will likely increase soon [72]. Antibiotic resistance is not only a serious threat to humans but also to the environment. The use of antibiotics in food-producing animals is already a major public health problem that needs to be addressed. Different antibiotics have been used not only to prevent and treat infectious diseases but also to promote faster growth and higher productivity, further inducing and spreading antibiotic resistance between animals and from animals to humans [69]. Ultimately, this can result in environmental antibiotic resistance that can affect human health because the consumption of food contaminated with both pathogenic and nonpathogenic bacteria facilitate the transfer of antibiotic resistance among bacterial strains [69,73]. Bacteria can adapt to antibiotic resistance through two major genetic strategies, mutational resistance and horizontal gene transfer [5,74]. In mutational resistance, a subset of bacterial cells derived from a susceptible population develop mutations in gene(s) associated with the mechanism of action of the compound or drug [74]. Consequently, the resistant mutant will show preserved cell survival in the presence of the antimicrobial molecule, resulting in antimicrobial resistance. Acquired mutational changes are diverse and vary in complexity and include modification of the antimicrobial target, leading to a decreased affinity for the drug [74] and, thus, a decreased drug uptake: activation of efflux mechanisms to expel the antimicrobial molecule [75], loss of porin proteins preventing the accumulation of antimicrobial drugs [76], and important changes in metabolic pathways via the modulation of regulatory networks [5,77,78,79]. The acquisition of foreign DNA material for resistance determinants through horizontal gene transfer is also responsible for antimicrobial resistance. Horizontal gene transfer can occur through different strategies, namely transformation, transduction, or conjugation. Integrons are another important mechanism for accumulating antimicrobial resistance genes, representing one of the main drivers of bacterial evolution [5,79,80]. Moreover, as some of these bacteria are essential to our metabolism, there are growing concerns that human microbiota can also be severely affected by resistant bacteria, further increasing the burden of AMR in the health systems [81]. Currently, the most common multidrug-resistant bacteria include methicillin-resistant Staphylococcus aureus (MRSA) [82], vancomycin-resistant Enterococcus (VRE) [83], extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL) [84], carbapenem-resistant Enterobacteriaceae (CRE) [85], P. aeruginosa [86], Acinetobacter baumannii [87], Klebsiella pneumoniae [88], Mycobacterium tuberculosis [55], Salmonella enterica [89], and Neisseria gonorrhoeae [62]. These bacteria develop different strategies to overcome the host’s defences, which can occur mainly in three types: efflux-mediated multidrug resistance, target modification, or enzymatic inactivation [72,90]. The efflux-mediated multidrug resistance involves the expulsion of drugs from the cell by pumps that are normally responsible for removing toxic molecules and waste products. Another type of AMR is target modification [72]. In this type of resistance, the target of the drug is modified so that it can no longer recognize or bind to the drug, rendering it ineffective. This type of resistance is commonly observed in bacteria and viruses where they alter the structure or expression of the target molecule to evade drug action. Finally, enzymatic inactivation occurs when microorganisms produce enzymes that can break down or modify the drugs before they can reach their intended target. This mechanism is particularly common in bacteria, which may produce enzymes such as β-lactamases that can degrade antibiotics like penicillin and cephalosporins [72]. Overall, multidrug resistance is a complex phenomenon that poses significant challenges to the development of effective treatments for infectious diseases. Efforts are underway to tackle this problem through the development of new drugs and alternative treatment strategies, such as combination therapy and immunotherapy [91,92,93].
3. Antibacterial Phytochemicals Identified in Food Wastes
Phytochemicals are natural chemical compounds found in plant foods, such as fruits, vegetables, legumes, whole grains, nuts, seeds, and herbs. These compounds act as a natural defence system for plants, protecting them from infections and microbial invasions and giving them colour, aroma, and flavour [94]. Phytochemicals have emerged as safe alternatives to conventional antibiotics to treat antibiotic-resistant pathogen-originated infections, as well as an alternative to chemical additives to foodborne bacteria [95]. Many phytochemicals have demonstrated their potential as bactericidal agents and have proved to inhibit the vital events for the sustenance and resistance of the pathogen, including efflux pumps, replication machinery, and cell permeability, among others [96]. Phytochemicals are grouped according to their structural characteristics into four large groups: nitrogen alkaloids, phenolic compounds, terpenoids, and organosulfur compounds [94].
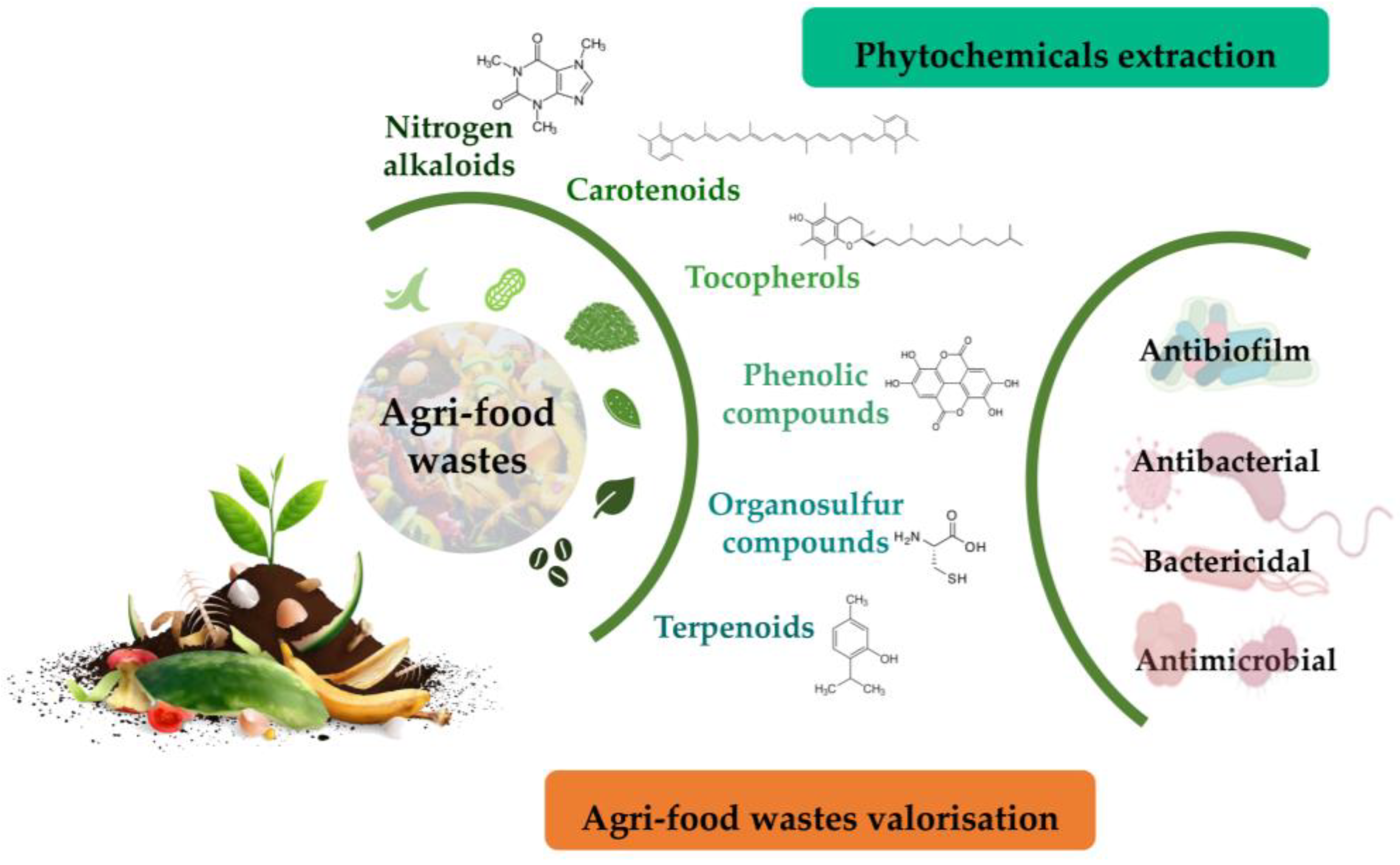
Agri-food wastes comprise peels, seeds, shells, pomace, and leaves. These residues are important substrates for phytochemicals, including polyphenols, carotenoids, essential oils, tocopherols, and terpenes. In addition to their antibiotic activities, phytochemicals found in agri-food wastes can be easily managed via their valorisation to produce value-added products, food additives, therapeutics, or other environmental applications due to their antioxidant, therapeutic, and nutritional properties [97,98,99,100] (Figure 2).
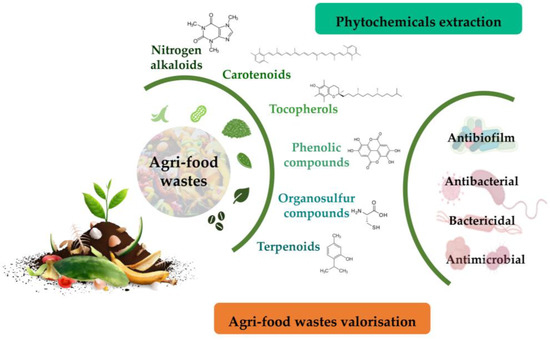
Figure 2.
Overall strategy to unveil phytochemicals with antimicrobial activity from food wastes as a strategy for their valorisation.
Several studies have shown the antibacterial potential of phytochemicals found in agri-food wastes (Table 2). For instance, Carmo and collaborators [101] isolated coumarins (bergapten, xanthotoxin, dimethyl allyl xanthyletin) and an imidazole alkaloid from the crude extract of leaves and bark of Pilocarpus pennatifolius Lemaire. The extracts and pure compounds were tested against different strains of bacteria and fungi, which showed promising antimicrobial and antifungal activities. The alkaloid identified showed a minimal inhibitory concentration of 1.56 μg·mL−1 against Enterococcus fecalis, and 1.56 μg·mL−1 and 6.25 μg·mL−1 against Salmonella enteritidis and Pseudomonas aeruginosa, respectively. The extracts of the studied species proved to be an alternative source in the search for new antimicrobial agents for the treatment of diseases caused by bacteria.

Table 2.
Antibacterial potential of phytochemicals found in agri-food wastes.
Overall, phytochemicals found in agri-food wastes have a significant potential to be used as alternative antibiotics and food additives. Additionally, their valorisation can lead to the production of value-added products with beneficial properties.
3.1. Nitrogen Alkaloids
Alkaloids are a type of organic nitrogen heterocyclic compound that have a wide range of chemical structures based on the rings in the molecule [94]. Nicotine, morphine, caffeine, and mescaline are some of the well-known alkaloids. Plants produce alkaloids as a defence mechanism against insects and herbivores. These compounds also have antibacterial properties against a range of microorganisms, such as Mycobacterium fortuitum, Mycobacterium tuberculosis, Mycobacterium smegmatis, E. coli, S. aureus, Salmonella typhimurium, Klebsiella pneumonia, and P. aeruginosa [94,96].
The coffee industry generates a significant amount of by-products that can be used as a source of bioactive compounds [102,103]. Researchers have evaluated the antibacterial activity of arabica coffee leaves and found that the extracts contain the alkaloids trigonelline and caffeine [102]. These extracts were found to be effective against E. coli.
3.2. Phenolic Compounds
Plant polyphenols, also known as phenolic compounds, are organic compounds that contain at least one phenol group and have an aromatic ring with one or more hydroxyl groups in their molecular structure [95], and they are classified into flavonoids and non-flavonoids based on their structural characteristics [94,96] secondary metabolites that play a crucial role in plant physiology, including defence against herbivores and pathogens and mechanical support for the plant. [94] have shown antimicrobial properties against a wide range of microorganisms, and they can sensitize multidrug-resistant strains to bacteriostatic or bactericidal antibiotics, making them promising natural antimicrobial agents [96]. Additionally, polyphenols have been established as chemopreventive and therapeutic agents due to their potential health-benefiting properties, including antioxidant, antiallergic, anti-inflammatory, anticancer, antihypertensive, and antimicrobial features [94,95,96]. Sharma et al. [108] investigated the biological activities of polyphenols in skinned fresh and ageing onions. The authors found that the antibiofilm activity against E. coli, P. aeruginosa, S. aureus, and Bacillus cereus increased with ageing onions as the levels of quercetin and total phenolic content also increased upon aging in the studied varieties.
3.2.1. Flavonoids
Plant flavonoids, which have a 2-phenyl-benzo-γ-pyrane nucleus with two benzene rings, have demonstrated promising antimicrobial activities and antioxidant properties [94,96]. Many classes of flavonoids, including flavonols, flavanols, flavanones, isoflavonoids, chalcones, and dihydrochalcones, have been identified as allelochemicals that inhibit microbial growth. Flavonoids are also known to inhibit quorum sensing and biofilm formation, as well as act as resistant-reversal agents [96]. Catechins and proanthocyanidins possess antioxidant properties and have been proposed to neutralize bacterial toxic factors originating from V. cholerae, V. vulnificus, S. aureus, Bacillus anthracis, and C. botulinum. Additionally, citrus flavonoids, such as apigenin, kaempferol, quercetin, and naringenin, are effective antagonists of cell–cell signalling [95,120]. Chrysin and kaempferol restrict the DNA gyrase activity, which is an essential enzyme in DNA replication in E. coli, while aglycone flavonoids, such as myricetin, hesperetin, and phloretin, inhibit biofilm formation in Staphylococcus strains [96].
3.2.2. Non-Flavonoids
Phenolic acids, including benzoic, phenylacetic, and phenylpropionic acids, have been discovered to have inhibitory effects on both pathogenic and non-pathogenic bacteria and fungi. These include E. coli, Lactobacillus spp., S. aureus, P. aeruginosa, and Candida albicans [95,96].
Hydroxycinnamic acids, such as caffeic, coumaric, ferulic, and sinapic acids, have also been found to inhibit the growth of Bacillus cereus, S. aureus, and Pseudomonas fluorescens [95]. Ferulic acid and gallic acid have also demonstrated antibacterial properties against various bacterial isolates. Both acids damage the cell walls of E. coli, P. aeruginosa, and S. aureus, leading to local damage and cellular material leakage [96]; gallic acid has been shown to exhibit strong antibacterial potential against Enterococcus faecalis, Streptococcus pneumonia, P. aeruginosa, Moraxella catarrhalis, S. aureus, Enterococcus faecalis, E. coli, and Streptococcus agalactiae strains [96].
3.3. Terpenoids
Terpenoids are a diverse group of organic compounds that are similar to terpenes. They consist of mono- and sesquiterpenoids [94], which are the main components of essential oils. Essential oils are volatile plant products [96] that can be extracted from various plant parts, such as flowers and fruits. They contain a mixture of low-mass plant natural products or phytochemicals, including myrcene, o-cimene, citral, geraniol, eugenol, carvacrol, linalool, citronellal, carvone, limonene, terpinenes, menthol, and menthone [94,96].
Essential oils have strong antimicrobial properties and are commonly used in traditional medicine. They are considered safe for consumption and vital host tissues. However, their stability is crucial for their quality and pharmacological potency [96]. Essential oils are known for their remarkable antibacterial activities against both Gram-positive and negative pathogens, including bactericidal and re-potentiating or re-sensitizing of antibiotics potentials against pathogenic microbes. They have also demonstrated their potential in targeting and disturbing the most prevalent drug-resistance-determining mechanisms of microbes, namely the cell wall, cell membrane and permeability, drug efflux pumps, mobile genetic elements, quorum sensing, and biofilm [96].
Citrus fruits are the main source of essential oils [94,113,114,116]. For example, Djenane [113] evaluated the chemical composition of citrus peel (orange, lemon, and bergamot) essential oils. The essential oils analysed were mainly composed of limonene (77.4%) for orange essential oil; linalyl acetate (37.3%) and linalool (23.4%) for bergamot essential oil; and limonene (51.4%), β-pinene (17.0%), and γ-terpinene (13.5%) for lemon essential oil. The in vitro antimicrobial activity of the essential oils was evaluated against S. aureus, which revealed that lemon essential oil had more antibacterial effects than the other essential oils.
3.4. Organosulfur Compounds
Organosulfur compounds, also known as thiols, are present in various plants and vegetables. These compounds include glucosinolates and allyl sulphides, which contain sulfur in their structure. Glucosinolates are found in cruciferous vegetables of the Brassicales order while allyl sulphides are abundant in garlic [94].
Glucosinolates play a vital role in plant defence against microbial pathogens and insect herbivores. They act as signalling molecules that initiate pathways such as stomatal closure, apoptosis, and callose accumulation [121]. A study by Blažević et al. [118] investigated the glucosinolate profile and antibacterial activity of Lepidium latifolium L. against food spoilage bacteria. The results showed that allyl isothiocyanate, a compound found in the plant, was highly effective against E. coli.
4. Potential Applications, Limitations, and Challenges for Antibacterial Phytochemicals from Agri-Food Wastes
The examples that are given in Table 2 point to the potential use of several antibacterial phytochemicals as an alternative to conventional antibiotics to treat antibiotic-resistant pathogen-originated infections, as well as an alternative to chemical additives to foodborne bacteria. These phytochemicals have demonstrated potential as bactericidal agents and have proved to inhibit the vital events for the sustenance and resistance of the pathogen, including efflux pumps, replication machinery, and cell permeability, among others. Additionally, their valorisation can lead to the production of other value-added products with beneficial properties spanning other fields of applications, such as cosmetics. Nevertheless, despite all the potential shown in the extraction of compounds from agri-food wastes to unveil new antibacterial compounds, there are several limitations and challenges to overcome. Agri-food wastes are complex mixtures containing compounds in a wide range of concentrations, and often, the bioactive compounds are present in very low amounts. Therefore, efficient extraction, purification, and characterization methods are required to obtain the active compounds from the waste materials. Additionally, the variability of the composition of agri-food wastes affects the quality and quantity of the extracted compounds. Furthermore, in many cases, the bioactive effect is not elicited by a single phytochemical but instead results from the synergistic effect of several compounds present in a single extract. This poses important constraints to the definition of sustainable and scalable production methods to ensure the availability of the extracted compounds for global health applications. For the new molecules identified with antibacterial activity, potential toxicity effects must be considered, which may limit their use in human and animal health applications. This requires further research and assays to determine the safety and efficacy of the extracted compounds. Furthermore, the lack of regulatory frameworks for the use of the new antibacterial compounds in human and animal health applications will delay their use for several years or decades. Finally, we have to consider the potential for the development of resistance to the extracted compounds, which may limit their long-term effectiveness against bacterial infections and result in the development of more aggressive forms of AMR.
5. Conclusions
Agri-food wastes uncover a plethora of naturally occurring phytochemicals that could hold significant bioactive potential for many uses in animal and human applications. Bacteria and other microbes are very relevant to human activity, including our metabolism. For this reason, growing AMR against antibiotics constitutes a severe health problem. To unveil new phytochemicals and methods able to mitigate this challenge, many researchers around the world have turned their attention towards delving into the composition of agri-food wastes. The exploration of this field can pave the way for novel and effective drugs against resistant bacteria and help to alleviate the AMR pressure in healthcare systems worldwide. This strategy is continuously driving the isolation and characterization in agri-food wastes of many promising antibacterial compounds from different chemical families, mainly nitrogen alkaloids, phenolic and organosulfur compounds, and terpenoids. Hopefully, some of these molecules will be effective against AMR.
Author Contributions
Funding
This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through the CQM Base Fund, UIDB/00674/2020, the Programmatic Fund, UIDP/00674/2020, Madeira 14–20 Program, project PROEQUIPRAM, Reforço do Investimento em Equipamentos e Infraestruturas Científicas RAM (M1420-01-0145-FEDER-000008), Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação (ARDITI) through the project M1420-01-0145-FEDER-000005-Centro de Química da Madeira-CQM + (Madeira 14–20 Program) and Project M1420-09-5369-FSE-000001 for the post-doctoral fellowship granted to J.A.M.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. FAO Statistical Yearbook 2021—World Food and Agriculture; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Berenguer, C.V.; Andrade, C.; Pereira, J.A.M.; Perestrelo, R.; Câmara, J.S. Current Challenges in the Sustainable Valorisation of Agri-Food Wastes: A Review. Processes 2022, 11, 20. [Google Scholar] [CrossRef]
- European Commission. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Seregelj, V.; Tumbas Saponjac, V.; Levic, S.; Kalusevic, A.; Cetkovic, G.; Canadanovic-Brunet, J.; Nedovic, V.; Stajcic, S.; Vulic, J.; Vidakovic, A. Application of encapsulated natural bioactive compounds from red pepper waste in yogurt. J. Microencapsul. 2019, 36, 704–714. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Overgaauw, P.A.M.; Vinke, C.M.; van Hagen, M.A.E.; Lipman, L.J.A. A One Health Perspective on the Human–Companion Animal Relationship with Emphasis on Zoonotic Aspects. Int. J. Environ. Res. Public Health 2020, 17, 3789. [Google Scholar] [CrossRef]
- Elnaiem, A.; Mohamed-Ahmed, O.; Zumla, A.; Mecaskey, J.; Charron, N.; Abakar, M.F.; Raji, T.; Bahalim, A.; Manikam, L.; Risk, O.; et al. Global and regional governance of One Health and implications for global health security. Lancet 2023, 401, 688–704. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, X.; Liu, J.; Visbeck, M.; Guo, H.; Murray, V.; McGillycuddy, C.; Ke, B.; Kalonji, G.; Zhai, P.; et al. From concept to action: A united, holistic and One Health approach to respond to the climate change crisis. Infect. Dis. Poverty 2022, 11, 17. [Google Scholar] [CrossRef]
- Li, A.M. Ecological determinants of health: Food and environment on human health. Environ. Sci. Pollut. Res. Int. 2017, 24, 9002–9015. [Google Scholar] [CrossRef]
- Glasset, B.; Sperry, M.; Dervyn, R.; Herbin, S.; Brisabois, A.; Ramarao, N. The cytotoxic potential of Bacillus cereus strains of various origins. Food Microbiol. 2021, 98, 103759. [Google Scholar] [CrossRef]
- Béjaoui, A.; Ben Abdallah, I.; Maaroufi, A. Brucella spp. Contamination in Artisanal Unpasteurized Dairy Products: An Emerging Foodborne Threat in Tunisia. Foods 2022, 11, 2269. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwata, T.; Uema, M.; Yonemitsu, K.; Igimi, S.; Asakura, H. Campylobacter spp. prevalence and fluoroquinolone resistance in chicken layer farms. J. Vet. Med. Sci. 2022, 84, 743–746. [Google Scholar] [CrossRef]
- Rodriguez, C.; Taminiau, B.; Van Broeck, J.; Delmee, M.; Daube, G. Clostridium difficile in Food and Animals: A Comprehensive Review. Adv. Exp. Med. Biol. 2016, 932, 65–92. [Google Scholar] [CrossRef]
- Hussein, H.S.; Sakuma, T. Prevalence of shiga toxin-producing Escherichia coli in dairy cattle and their products. J. Dairy Sci. 2005, 88, 450–465. [Google Scholar] [CrossRef]
- Ahmad-Mansour, N.; Loubet, P.; Pouget, C.; Dunyach-Remy, C.; Sotto, A.; Lavigne, J.-P.; Molle, V. Staphylococcus aureus Toxins: An Update on Their Pathogenic Properties and Potential Treatments. Toxins 2021, 13, 677. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Academic Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Platt-Samoraj, A.; Kończyk-Kmiecik, K.; Bakuła, T. Occurrence and Genetic Correlations of Yersinia spp. Isolated from Commensal Rodents in Northeastern Poland. Pathogens 2021, 10, 1247. [Google Scholar] [CrossRef]
- Cardoso, M.J.; Nicolau, A.I.; Borda, D.; Nielsen, L.; Maia, R.L.; Moretro, T.; Ferreira, V.; Knochel, S.; Langsrud, S.; Teixeira, P. Salmonella in eggs: From shopping to consumption-A review providing an evidence-based analysis of risk factors. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2716–2741. [Google Scholar] [CrossRef]
- Guerrero, T.; Bayas-Rea, R.; Erazo, E.; Zapata Mena, S. Nontyphoidal Salmonella in Food from Latin America: A Systematic Review. Foodborne Pathog. Dis. 2022, 19, 85–103. [Google Scholar] [CrossRef]
- Awofisayo-Okuyelu, A.; Verlander, N.Q.; Amar, C.; Elson, R.; Grant, K.; Harris, J. Factors influencing the time between onset of illness and specimen collection in the diagnosis of non-pregnancy associated listeriosis in England and Wales. BMC Infect. Dis. 2016, 16, 311. [Google Scholar] [CrossRef]
- Kiefer, S.; Kling, K.; Stephan, R.; Bratschi, M.W.; Jost, M.; Bless, P.J.; Schmutz, C.; Mausezahl, D.; Wyss, K.; Mausezahl-Feuz, M.; et al. How can patients and their physicians contribute to an outbreak investigation? Experiences from a nationwide listeriosis outbreak in Switzerland. Swiss Med. Wkly. 2016, 146, w14366. [Google Scholar] [CrossRef]
- Elmahdi, S.; DaSilva, L.V.; Parveen, S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016, 57, 128–134. [Google Scholar] [CrossRef]
- Elmahdi, S.; Parveen, S.; Ossai, S.; DaSilva, L.V.; Jahncke, M.; Bowers, J.; Jacobs, J. Vibrio parahaemolyticus and Vibrio vulnificus Recovered from Oysters during an Oyster Relay Study. Appl. Environ. Microbiol. 2018, 84, e01790-17. [Google Scholar] [CrossRef]
- Crisan, C.V.; Hammer, B.K. The Vibrio cholerae type VI secretion system: Toxins, regulators and consequences. Environ. Microbiol. 2020, 22, 4112–4122. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef]
- Koch, A.; Mizrahi, V. Mycobacterium tuberculosis. Trends Microbiol. 2018, 26, 555–556. [Google Scholar] [CrossRef]
- Rahnama, H.; Azari, R.; Yousefi, M.H.; Berizi, E.; Mazloomi, S.M.; Hosseinzadeh, S.; Derakhshan, Z.; Ferrante, M.; Conti, G.O. A systematic review and meta-analysis of the prevalence of Bacillus cereus in foods. Food Control 2023, 143, 109250. [Google Scholar] [CrossRef]
- Meng, J.N.; Liu, Y.J.; Shen, X.; Wang, J.; Xu, Z.K.; Ding, Y.; Beier, R.C.; Luo, L.; Lei, H.T.; Xu, Z.L. Detection of emetic Bacillus cereus and the emetic toxin cereulide in food matrices: Progress and perspectives. Trends Food Sci. Technol. 2022, 123, 322–333. [Google Scholar] [CrossRef]
- Salih, S.B.; Alothman, A. Acute brucellosis presenting as gastroenteritis: Case report. Infect. Dis. Res. Treat. 2013, 6, 35–37. [Google Scholar] [CrossRef]
- Roman, K.; Castillo, R.; Gilman, R.H.; Calderon, M.; Vivar, A.; Cespedes, M.; Smits, H.L.; Melendez, P.; Gotuzzo, E.; Guerra, H.; et al. A foodborne outbreak of brucellosis at a police station cafeteria, Lima, Peru. Am. J. Trop. Med. Hyg. 2013, 88, 552–558. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Lis, L.; Lasagabaster, A.; Nafarrate, I.; Ferrocino, I.; Cocolin, L.; Rantsiou, K. Campylobacter spp. prevalence and mitigation strategies in the broiler production chain. Food Microbiol. 2022, 104, 103998. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Béjaoui, A.; Ben Hamda, C.; Alaya, N.; Hamrouni, S.; Bessoussa, G.; Ghram, A.; Maaroufi, A. Campylobacter spp. in Eggs and Laying Hens in the North-East of Tunisia: High Prevalence and Multidrug-Resistance Phenotypes. Vet. Sci. 2022, 9, 108. [Google Scholar] [CrossRef]
- Dilnessa, T.; Getaneh, A.; Hailu, W.; Moges, F.; Gelaw, B. Prevalence and antimicrobial resistance pattern of Clostridium difficile among hospitalized diarrheal patients: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0262597. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, Y.M.; Park, K.H.; Kim, Y.J.; Kang, K.C.; Lee, C.K.; Lee, M.S. Clostridium difficile infection after orthopedic surgery: Incidence, associated factors, and impact on outcome. Am. J. Infect. Control 2022, 50, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Portinha, I.M.; Douillard, F.P.; Korkeala, H.; Lindström, M. Sporulation Strategies and Potential Role of the Exosporium in Survival and Persistence of Clostridium botulinum. Int. J. Mol. Sci. 2022, 23, 754. [Google Scholar] [CrossRef]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT-Food Sci. Technol. 2022, 163, 113603. [Google Scholar] [CrossRef]
- Juneja, V.K.; Sidhu, G.; Xu, X.R.; Osoria, M.; Glass, K.A.; Schill, K.M.; Golden, M.C.; Schaffner, D.W.; Kumar, G.D.; Shrestha, S.; et al. Predictive model for growth of Clostridium botulinum from spores at temperatures applicable to cooling of cooked ground pork. Innov. Food Sci. Emerg. Technol. 2022, 77, 102960. [Google Scholar] [CrossRef]
- Ramos, S.; Silva, V.; Dapkevicius, M.D.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Zając, M.; Sztromwasser, P.; Bortolaia, V.; Leekitcharoenphon, P.; Cavaco, L.M.; Ziȩtek-Barszcz, A.; Hendriksen, R.S.; Wasyl, D. Occurrence and Characterization of mcr-1-Positive Escherichia coli Isolated from Food-Producing Animals in Poland, 2011–2016. Front. Microbiol. 2019, 10, 1753. [Google Scholar] [CrossRef]
- Fagerlund, A.; Wagner, E.; Moretro, T.; Heir, E.; Moen, B.; Rychli, K.; Langsrud, S. Pervasive Listeria monocytogenes Is Common in the Norwegian Food System and Is Associated with Increased Prevalence of Stress Survival and Resistance Determinants. Appl. Environ. Microbiol. 2022, 88, e0086122. [Google Scholar] [CrossRef]
- Morasi, R.M.; Rall, V.L.M.; Dantas, S.T.A.; Alonso, V.P.P.; Silva, N.C.C. Salmonella spp. in low water activity food: Occurrence, survival mechanisms, and thermoresistance. J. Food Sci. 2022, 87, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Sadat, A.; Shata, R.R.; Farag, A.M.M.; Ramadan, H.; Alkhedaide, A.; Soliman, M.M.; Elbadawy, M.; Abugomaa, A.; Awad, A. Prevalence and Characterization of PVL-Positive Staphylococcus aureus Isolated from Raw Cow’s Milk. Toxins 2022, 14, 97. [Google Scholar] [CrossRef]
- Wang, D.; Flint, S.H.; Palmer, J.S.; Gagic, D.; Fletcher, G.C.; On, S.L.W. Global expansion of Vibrio parahaemolyticus threatens the seafood industry: Perspective on controlling its biofilm formation. LWT-Food Sci. Technol. 2022, 158, 113182. [Google Scholar] [CrossRef]
- Jugder, B.E.; Batista, J.H.; Gibson, J.A.; Cunningham, P.M.; Asara, J.M.; Watnick, P.I. Vibrio cholerae high cell density quorum sensing activates the host intestinal innate immune response. Cell Rep. 2022, 40, 111368. [Google Scholar] [CrossRef] [PubMed]
- Teschler, J.K.; Nadell, C.D.; Drescher, K.; Yildiz, F.H. Mechanisms Underlying Vibrio cholerae Biofilm Formation and Dispersion. Annu. Rev. Microbiol. 2022, 76, 503–532. [Google Scholar] [CrossRef]
- Durofil, A.; Maddela, N.R.; Naranjo, R.A.; Radice, M. Evidence on antimicrobial activity of essential oils and herbal extracts against Yersinia enterocolitica-A review. Food Biosci. 2022, 47, 101712. [Google Scholar] [CrossRef]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2022, 50, 83–92. [Google Scholar] [CrossRef]
- Upmanyu, K.; Haq, Q.M.R.; Singh, R. Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics. Curr. Res. Microb. Sci. 2022, 3, 100131. [Google Scholar] [CrossRef]
- Rocha, J.; Ferreira, C.; Mil-Homens, D.; Busquets, A.; Fialho, A.M.; Henriques, I.; Gomila, M.; Manaia, C.M. Third generation cephalosporin-resistant Klebsiella pneumoniae thriving in patients and in wastewater: What do they have in common? BMC Genom. 2022, 23, 72. [Google Scholar] [CrossRef]
- Wang, M.; Earley, M.; Chen, L.; Hanson, B.M.; Yu, Y.; Liu, Z.; Salcedo, S.; Cober, E.; Li, L.; Kanj, S.S.; et al. Clinical outcomes and bacterial characteristics of carbapenem-resistant Klebsiella pneumoniae complex among patients from different global regions (CRACKLE-2): A prospective, multicentre, cohort study. Lancet Infect. Dis. 2022, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gygli, S.M.; Borrell, S.; Trauner, A.; Gagneux, S. Antimicrobial resistance in Mycobacterium tuberculosis: Mechanistic and evolutionary perspectives. FEMS Microbiol. Rev. 2017, 41, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Acharya, A.; Gautam, S.; Ghimire, S.P.; Mishra, G.; Parajuli, N.; Sapkota, B. Advances in diagnosis of Tuberculosis: An update into molecular diagnosis of Mycobacterium tuberculosis. Mol. Biol. Rep. 2020, 47, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, N.G.; Stephens, D.S. Neisseria meningitidis: Biology, microbiology, and epidemiology. Methods Mol. Biol. 2012, 799, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Lucidarme, J.; Parikh, S.R.; Campbell, H.; Borrow, R.; Ramsay, M.E. Meningococcal disease and sexual transmission: Urogenital and anorectal infections and invasive disease due to Neisseria meningitidis. Lancet 2020, 395, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Booy, R.; Gentile, A.; Nissen, M.; Whelan, J.; Abitbol, V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum. Vaccines Immunother. 2019, 15, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef]
- Vallely, L.M.; Egli-Gany, D.; Wand, H.; Pomat, W.S.; Homer, C.S.E.; Guy, R.; Silver, B.; Rumbold, A.R.; Kaldor, J.M.; Vallely, A.J.; et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: Systematic review and meta-analysis. Sex. Transm. Infect. 2021, 97, 104–111. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 2011, 1230, E19–E28. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Lichtenberg, M.; Jakobsen, T.H.; Kuhl, M.; Kolpen, M.; Jensen, P.O.; Bjarnsholt, T. The structure-function relationship of Pseudomonas aeruginosa in infections and its influence on the microenvironment. FEMS Microbiol. Rev. 2022, 46, fuac018. [Google Scholar] [CrossRef]
- Adil, M.; Khan, R.; Rupasinghe, H.P.V. Application of Medicinal Plants as a Source for Therapeutic Agents against Streptococcus pyogenes Infections. Curr. Drug Metab. 2018, 19, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.R.; Keller, N.; Brouwer, S.; Jespersen, M.G.; Cork, A.J.; Hayes, A.J.; Pitt, M.E.; De Oliveira, D.M.P.; Harbison-Price, N.; Bertolla, O.M.; et al. Detection of Streptococcus pyogenes M1(UK) in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat. Commun. 2023, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Imran, S.; Frost, H.R.; Azzopardi, K.I.; Jalali, S.; Novakovic, B.; Osowicki, J.; Steer, A.C.; Licciardi, P.V.; Pellicci, D.G. Immune signature of acute pharyngitis in a Streptococcus pyogenes human challenge trial. Nat. Commun. 2022, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, W.Y.; Bisht, A.; Gopinath, A.; Cheema, A.H.; Chaludiya, K.; Khalid, M.; Nwosu, M.; Konka, S.; Khan, S. A Systematic Review of Antibiotic Resistance Trends and Treatment Options for Hospital-Acquired Multidrug-Resistant Infections. Cureus 2022, 14, e29956. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.A.; Frewer, L.J.; Jones, G.; Brereton, P.A.; Whittingham, M.J.; Stewart, G. The agri-food chain and antimicrobial resistance: A review. Trends Food Sci. Technol. 2017, 69, 131–147. [Google Scholar] [CrossRef]
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updat. 2020, 51, 100695. [Google Scholar] [CrossRef]
- Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Eltai, N.O.; Yassine, H.M.; El-Obeid, T.; Al-Hadidi, S.H.; Al Thani, A.A.; Alali, W.Q. Prevalence of Antibiotic-Resistant Escherichia coli Isolates from Local and Imported Retail Chicken Carcasses. J. Food Prot. 2020, 83, 2200–2208. [Google Scholar] [CrossRef]
- Duval, R.E.; Grare, M.; Demoré, B. Fight against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, R.; Zhang, Y.; Chen, L.; Huang, N.; Yu, K.; Zhou, C.; Cao, J.; Zhou, T. Characterization of resistance mechanisms of Enterobacter cloacae Complex co-resistant to carbapenem and colistin. BMC Microbiol. 2021, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Eger, E.; Schwabe, M.; Schulig, L.; Hübner, N.-O.; Bohnert, J.A.; Bornscheuer, U.T.; Heiden, S.E.; Müller, J.U.; Adnan, F.; Becker, K.; et al. Extensively Drug-Resistant Klebsiella pneumoniae Counteracts Fitness and Virulence Costs That Accompanied Ceftazidime-Avibactam Resistance Acquisition. Microbiol. Spectr. 2022, 10, e00148-22. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.T.; Chen, E.Z.; Yang, L.; Peng, C.; Wang, Q.; Xu, Z.; Chen, D.Q. Emerging resistance mechanisms for 4 types of common anti-MRSA antibiotics in Staphylococcus aureus: A comprehensive review. Microb. Pathog. 2021, 156, 104915. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4,, VMBF-0016-2015. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and Barriers to, Horizontal Gene Transfer between Bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef]
- Ahmad, N.; Joji, R.M.; Shahid, M. Evolution and implementation of One Health to control the dissemination of antibiotic-resistant bacteria and resistance genes: A review. Front. Cell Infect. Microbiol. 2022, 12, 1065796. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum beta-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef]
- Ballén, V.; Gabasa, Y.; Ratia, C.; Ortega, R.; Tejero, M.; Soto, S. Antibiotic Resistance and Virulence Profiles of Klebsiella pneumoniae Strains Isolated From Different Clinical Sources. Front. Cell. Infect. Microbiol. 2021, 11, 738223. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, S.; Kuang, D.; Hsu, C.H.; Yin, L.; Luo, Y.; Chen, Z.; Xu, X.; Strain, E.; McDermott, P.; et al. Geography shapes the genomics and antimicrobial resistance of Salmonella enterica Serovar Enteritidis isolated from humans. Sci. Rep. 2023, 13, 1331. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Rastogi, A.; Pandey, S.; Gupta, S.; Sohal, J.S. Multidrug-Resistant Bacteria: Their Mechanism of Action and Prophylaxis. BioMed Res. Int. 2022, 2022, 5419874. [Google Scholar] [CrossRef]
- Reig, S.; Le Gouellec, A.; Bleves, S. What Is New in the Anti-Pseudomonas aeruginosa Clinical Development Pipeline Since the 2017 WHO Alert? Front. Cell Infect. Microbiol. 2022, 12, 909731. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Lü, Y.; Yue, C. Development and Research Progress of Anti-Drug Resistant Bacteria Drugs. Infect. Drug Resist. 2021, 14, 5575–5593. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.B.; Yan, J.; Slarve, M.; Lu, P.; Li, R.; Ruiz, J.; Lee, B.; Burk, E.; Talyansky, Y.; Oelschlaeger, P.; et al. Monoclonal Antibody Therapy against Acinetobacter baumannii. Infect. Immun. 2021, 89, e0016221. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Delgado-Adámez, J. Agrifood by-Products as a Source of Phytochemical Compounds. In Descriptive Food Science; Díaz, A.V., García-Gimeno, R.M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Panda, L.; Duarte-Sierra, A. Recent Advancements in Enhancing Antimicrobial Activity of Plant-Derived Polyphenols by Biochemical Means. Horticulturae 2022, 8, 401. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Singh, A.; Singh, P.; Singh, A. Anaerobic Digestion of Agri-Food Wastes for Generating Biofuels. Indian J. Microbiol. 2021, 61, 427–440. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Mazzei, R.; Bazzarelli, F.; Piacentini, E.; Giannakopoulos, V.; Roberts, M.R.; Giorno, L. Agri-Food Industry Waste as Resource of Chemicals: The Role of Membrane Technology in Their Sustainable Recycling. Sustainability 2022, 14, 1483. [Google Scholar] [CrossRef]
- do Carmo, G.; Fernandes, T.S.; Pedroso, M.; Ferraz, A.; Neto, A.T.; Silva, U.F.; Mostardeiro, M.A.; Back, D.F.; Dalcol, I.I.; Morel, A.F. Phytochemical and antimicrobial study of Pilocarpus pennatifolius Lemaire. Fitoterapia 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Mesquita Junior, G.A.; da Costa, Y.F.G.; Mello, V.; Costa, F.F.; Rodarte, M.P.; Costa, J.C.D.; Alves, M.S.; Vilela, F.M.P. Chemical characterisation by UPLC-Q-ToF-MS/MS and antibacterial potential of Coffea arabica L. leaves: A coffee by-product. Phytochem. Anal. 2022, 33, 1036–1044. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food. Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Miedzianka, J.; Peksa, A.; Nems, A.; Drzymala, K.; Zambrowicz, A.; Kowalczewski, P. Trypsin inhibitor, antioxidant and antimicrobial activities as well as chemical composition of potato sprouts originating from yellow- and colored-fleshed varieties. J. Environ. Sci. Health B 2020, 55, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsul. 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Shan, Y.; Yang, Z.; Zhang, L.; Ling, W.; Liang, Y.; Ouyang, Z.; Zhong, B.; Zhang, J. Chemical composition, antioxidant, antibacterial, and tyrosinase inhibition activity of extracts from Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) peel. J. Sci. Food Agric. 2020, 100, 2664–2674. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D.; et al. Superior Antibacterial Activity of Integral Lemon Pectin Extracted via Hydrodynamic Cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Zhang, D.; Nie, S.; Xie, M.; Hu, J. Antioxidant and antibacterial capabilities of phenolic compounds and organic acids from Camellia oleifera cake. Food Sci. Biotechnol. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Gupta, N.; Poddar, K.; Sarkar, D.; Kumari, N.; Padhan, B.; Sarkar, A. Fruit waste management by pigment production and utilization of residual as bioadsorbent. J. Environ. Manag. 2019, 244, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of different drying methods on bitter orange (Citrus aurantium L.) peel waste: Changes in physical (density and color) and essential oil (yield, composition, antioxidant and antibacterial) properties of powders. J. Food Meas. Charact. 2019, 14, 862–875. [Google Scholar] [CrossRef]
- Pereira, M.G.; Maciel, G.M.; Haminiuk, C.W.I.; Bach, F.; Hamerski, F.; Scheer, A.D.; Corazza, M.L. Effect of Extraction Process on Composition, Antioxidant and Antibacterial Activity of Oil from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Seeds. Waste Biomass Valorization 2019, 10, 2611–2625. [Google Scholar] [CrossRef]
- Hou, H.S.; Bonku, E.M.; Zhai, R.; Zeng, R.; Hou, Y.L.; Yang, Z.H.; Quan, C. Extraction of essential oil from Citrus reticulate Blanco peel and its antibacterial activity against Cutibacterium acnes (formerly Propionibacterium acnes). Heliyon 2019, 5, e02947. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Akhtar, N.; Okla, M.K.; Shah, A.N.; Shah, A.A.; Abdel-Mawgoud, M.; AbdElgayed, G.; Al-Hashimi, A.; AbdElgawad, H. Seed Priming with Pomegranate Peel Extract Improves Growth, Glucosinolates Metabolism and Antimicrobial Potential of Brassica oleraceae Varieties. J. Plant Growth Regul. 2022, 42, 3043–3055. [Google Scholar] [CrossRef]
- Blazevic, I.; Dulovic, A.; Maravic, A.; Cikes Culic, V.; Montaut, S.; Rollin, P. Antimicrobial and Cytotoxic Activities of Lepidium latifolium L. Hydrodistillate, Extract and Its Major Sulfur Volatile Allyl Isothiocyanate. Chem. Biodivers. 2019, 16, e1800661. [Google Scholar] [CrossRef] [PubMed]
- Abukhabta, S.; Khalil Ghawi, S.; Karatzas, K.A.; Charalampopoulos, D.; McDougall, G.; Allwood, J.W.; Verrall, S.; Lavery, S.; Latimer, C.; Pourshahidi, L.K.; et al. Sulforaphane-enriched extracts from glucoraphanin-rich broccoli exert antimicrobial activity against gut pathogens in vitro and innovative cooking methods increase in vivo intestinal delivery of sulforaphane. Eur. J. Nutr. 2021, 60, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Chamkhi, I.; Balahbib, A.; Rebezov, M.; Shariati, M.A.; Wilairatana, P.; Mubarak, M.S.; Benali, T.; El Omari, N. Mechanisms, Anti-Quorum-Sensing Actions, and Clinical Trials of Medicinal Plant Bioactive Compounds against Bacteria: A Comprehensive Review. Molecules 2022, 27, 1484. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, D.; Lanzanova, C.; Pagnotta, E.; Bassolino, L.; Mastrangelo, A.M.; Marone, D.; Matteo, R.; Lo Scalzo, R.; Balconi, C. Sustainable Use of Bioactive Compounds from Solanum Tuberosum and Brassicaceae Wastes and by-Products for Crop Protection—A Review. Molecules 2021, 26, 2174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).