Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
Study Population
- A1. 1st day NICU LPs (n = 51) and healthy age-matched LPs (controls) (n = 23).
- A2. 3rd day NICU LPs (n = 31) and healthy age-matched LPs (n = 12; controls).
- B1. 1st day NICU LPs with RDS (n = 17) and 1st day NICU LPs without RDS (n = 14).
- B2. 3rd day NICU LPs with RDS (n = 9) and 3rd day NICU LPs without RDS (n = 9).
- C1. 1st day NICU LPs with RDS (n = 17) and healthy age-matched LPs (n = 21).
- C2. 3rd day NICU LPs with RDS (n = 9) and healthy age-matched LPs (n = 12).
- D1. 1st day NICU LPs without RDS (n = 14) and healthy age-matched LPs (n = 21).
- D2. 3rd day NICU LPs without RDS (n = 12) and healthy age-matched LPs (n = 9).
3. Sample Preparation and NMR Analysis Methodology
4. Statistical Analysis
5. Results
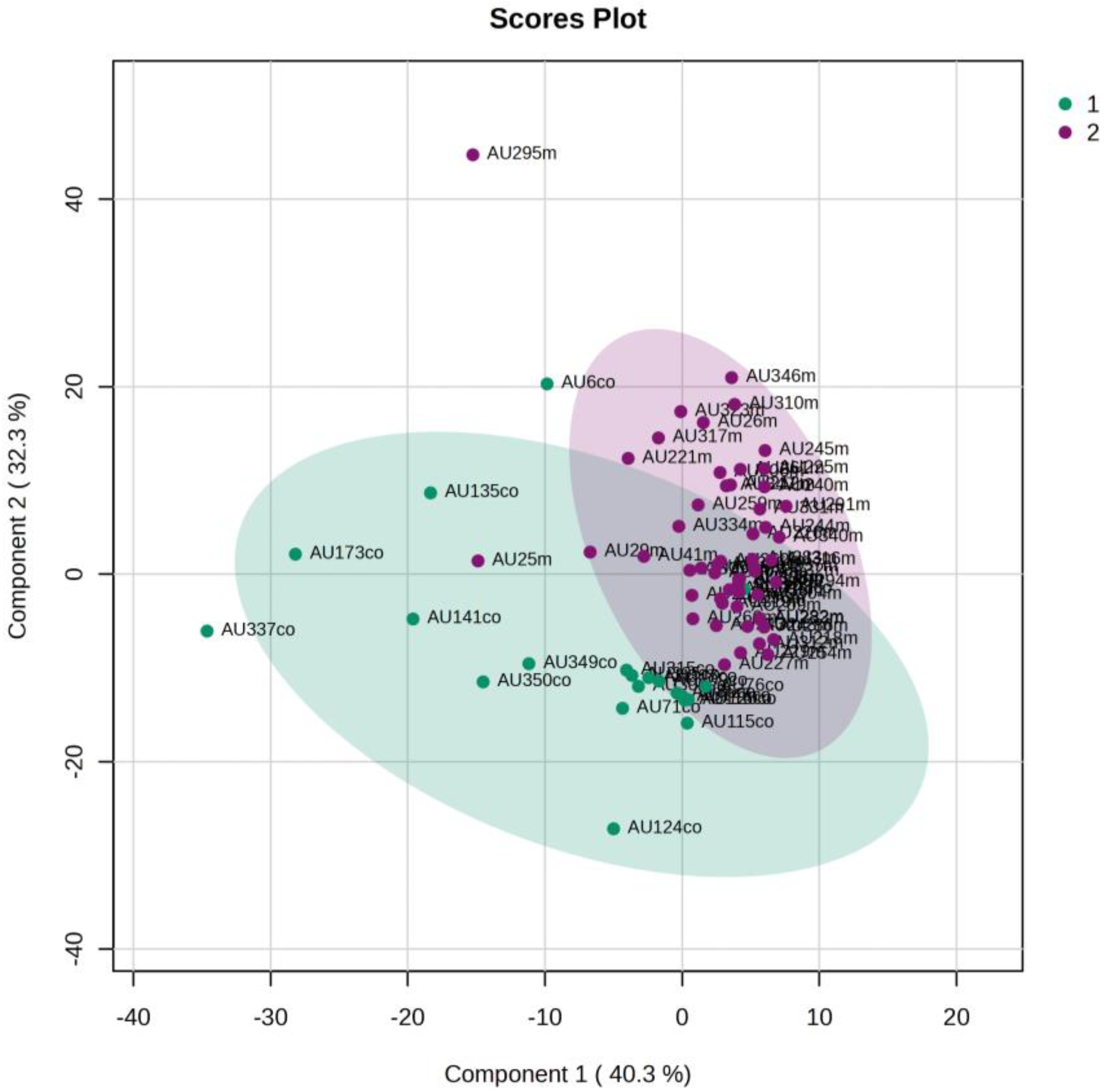
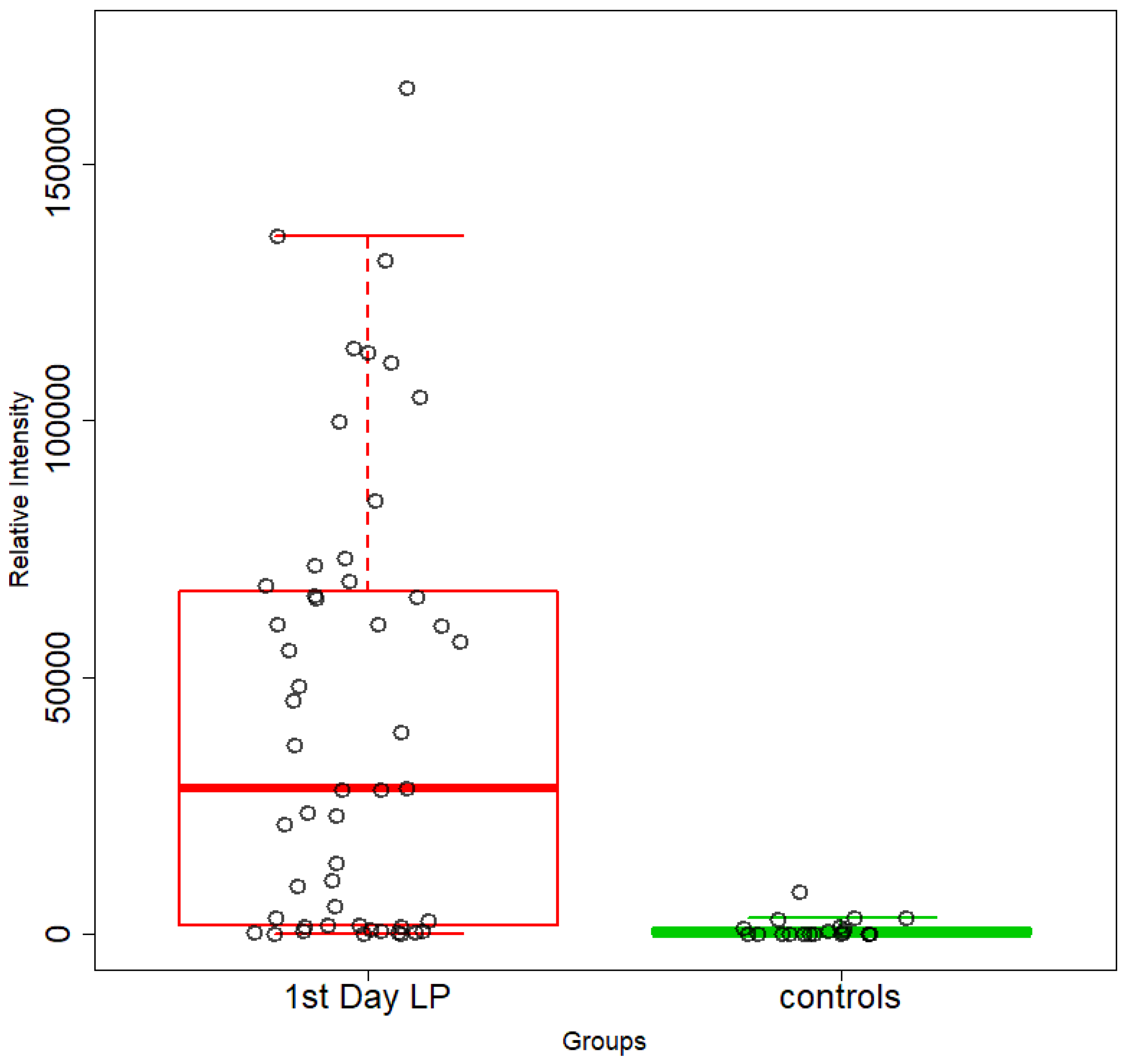
5.1. NICU LPs vs. Healthy (Age-Matched) LPs (1st and 3rd Days of Life)
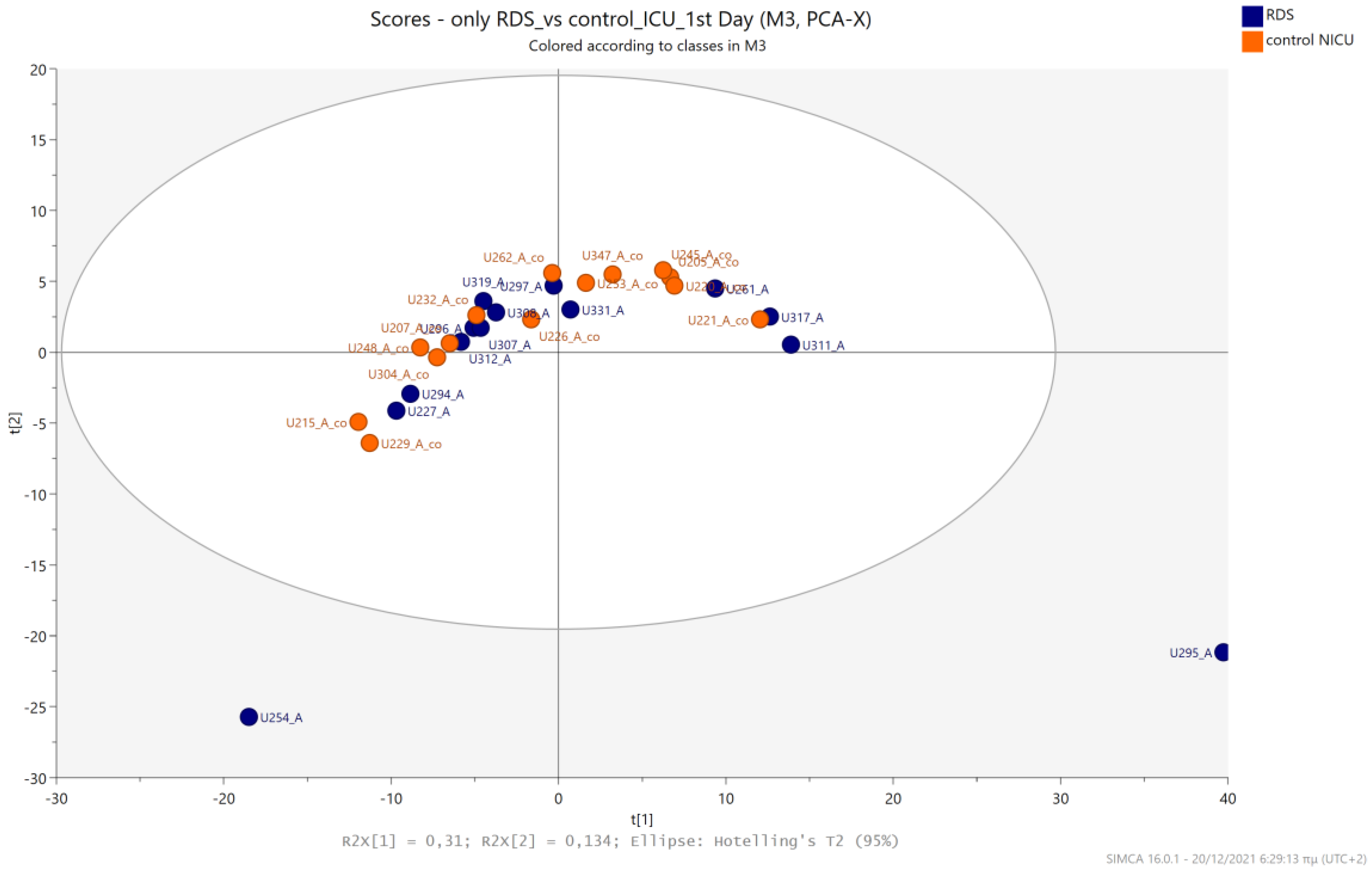
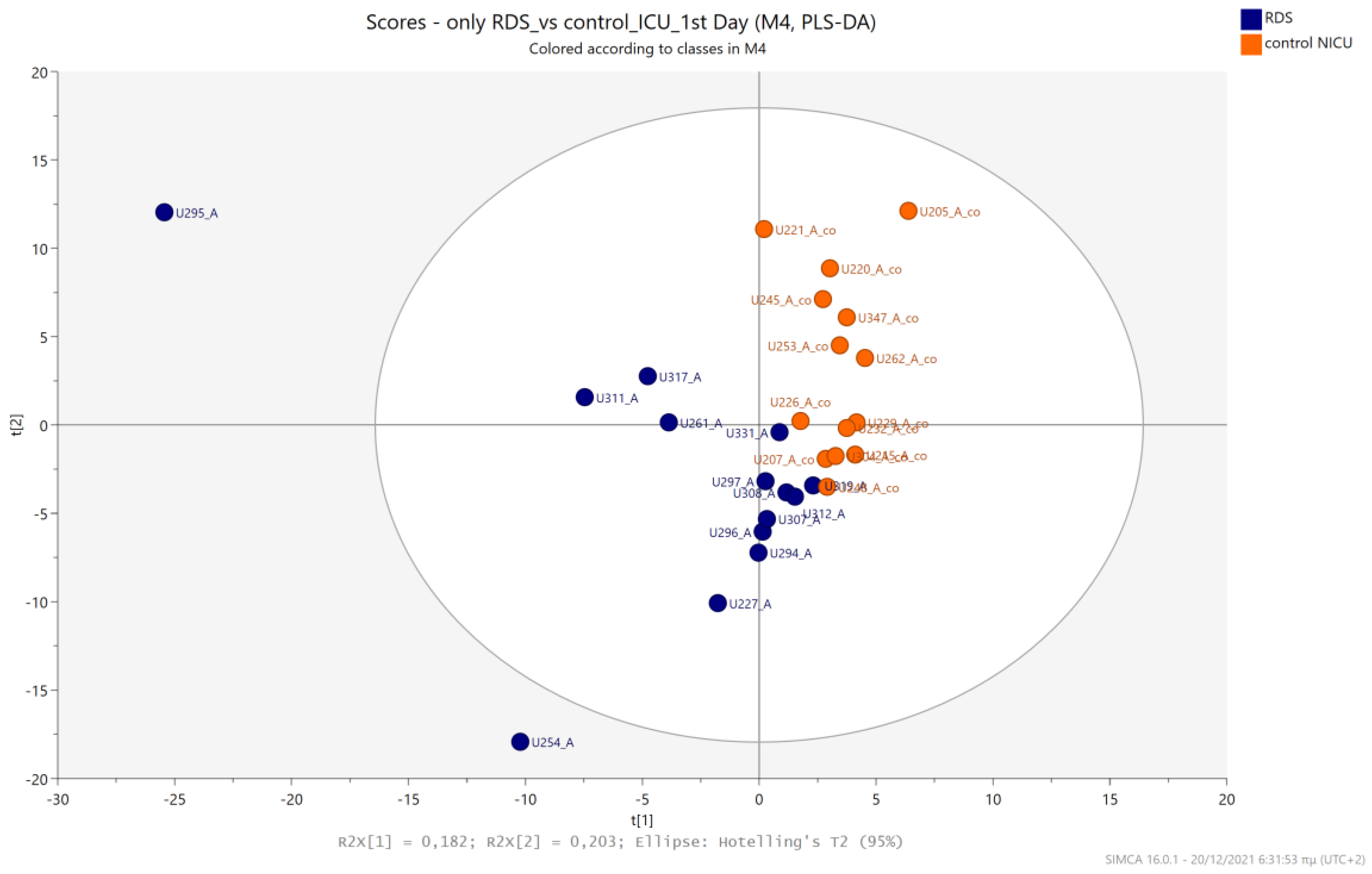
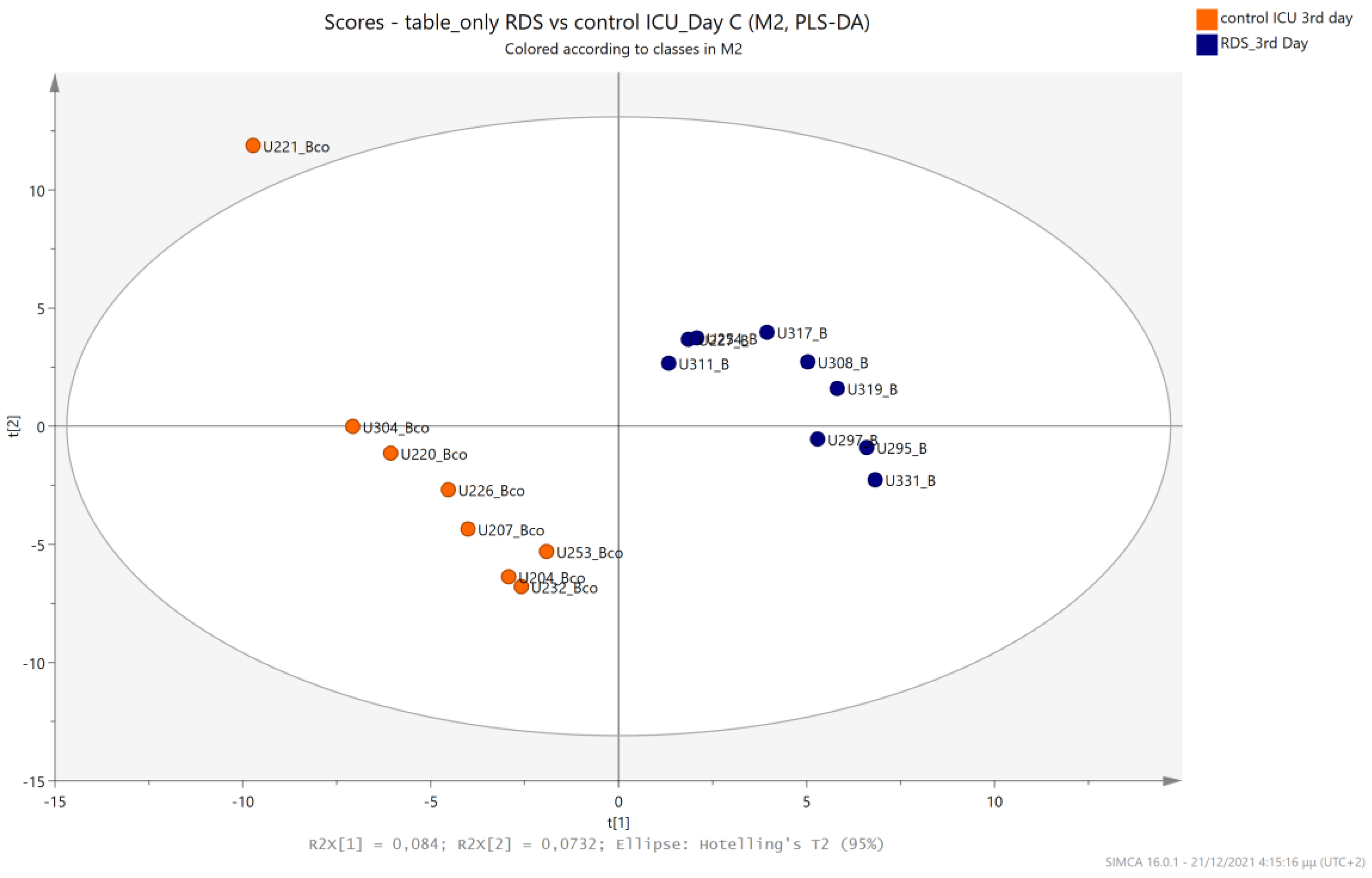
5.2. NICU LPs with RDS vs. NICU LPs without RDS (Control NICU)
5.3. NICU LPs with RDS vs. Healthy LP Neonates
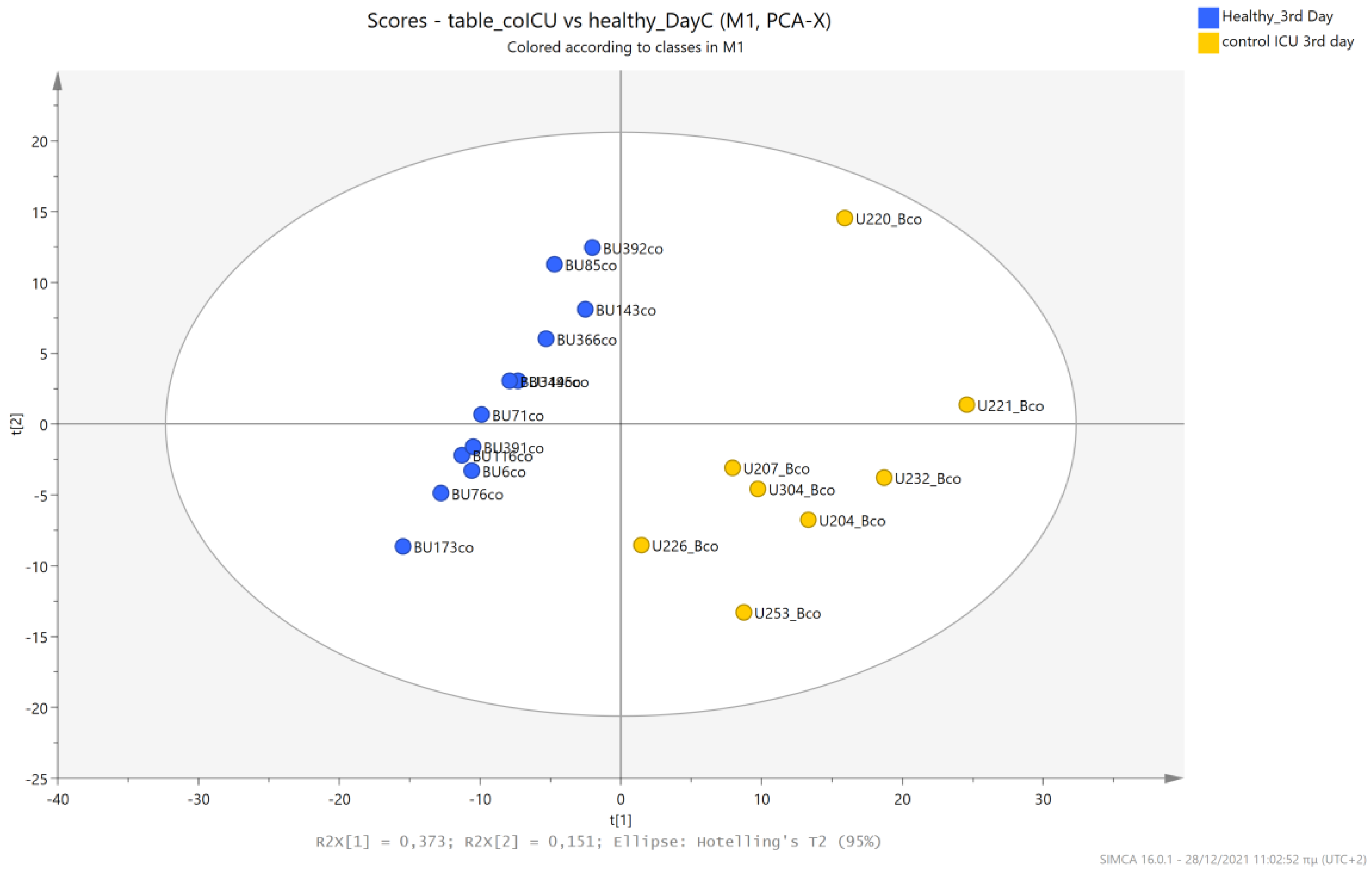
5.4. NICU LPs without RDS (Control NICU) vs. Healthy (Age-Matched) LPs
6. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karnati, S.; Kollikonda, S.; Abu-Shaweesh, J. Late preterm infants—Changing trends and continuing challenges. Int. J. Pediatr. Adolesc. Med. 2020, 7, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Consortium on Safe Labor; Hibbard, J.U.; Wilkins, I.; Sun, L.; Gregory, K.; Haberman, S.; Hoffman, M.; Kominiarek, M.A.; Reddy, U.; Bailit, J.; et al. Respiratory morbidity in late preterm. JAMA 2010, 304, 419–425. [Google Scholar]
- Gibney, M.J.; Walsh, M.; Brennan, L.; Roche, H.M.; German, B.; van Ommen, B. Metabolomics in human nutrition: Opportunities and challenges. Am. J. Clin. Nutr. 2005, 82, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Giallourou, N.; Fardus-Reid, F.; Panic, G.; Veselkov, K.; McCormick, B.J.J.; Olortegui, M.P.; Ahmed, T.; Mduma, E.; Yori, P.P.; Mahfuz, M.; et al. Metabolic maturation in the first 2 years of life in resource-constrained settings and its association with postnatal growth. Sci. Adv. 2020, 6, 5969. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative Omics for Health and Disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Wild, J.; Shanmuganathan, M.; Hayashi, M.; Potter, M.; Britz-Mckibbin, P. Metabolomics for Improved Treatment Monitoring of Phenylketonuria: Urinary Biomarkers for Non-invasive Assessment of Dietary Adherence and Nutritional Deficiencies. Analyst 2019, 144, 6595–6608. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Your Kidneys and How They Work. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work (accessed on 17 May 2019).
- Maurino Alcazar, C.G.; Mazarello Paes, V.; Shao, Y.; Oesser, C.; Miltz, A.; Lawley, T.D.; Brocklehurst, P.; Rodger, A.; Field, N. The association between early-life gut microbiota and childhood respiratory diseases: A systematic review. Lancet Microbe 2022, 3, 867–880. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Trans. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Stiemsma, L.T.; Arrieta, M.C.; Dimitriu, P.A.; Cheng, J.; Thorson, L.; Lefebvre, D.L.; Azad, M.B.; Subbarao, P.; Mandhane, P.; Becker, A.; et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin. Sci. 2016, 130, 2199–2207. [Google Scholar] [CrossRef]
- Vael, C.; Verhulst, S.L.; Nelen, V.; Goossens, H.; Desager, K.N. Intestinal microflora and body mass index during the first 3 years of life: An observational study. Gut Pathog. 2011, 3, 8. [Google Scholar] [CrossRef]
- Alderete, T.L.; Jones, R.B.; Shaffer, J.P.; Holzhausen, E.A.; Patterson, W.B.; Kazemian, E.; Chatzi, L.; Knight, R.; Plows, J.F.; Berger, P.K.; et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes 2021, 13, 1961203. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Hoen, A.G.; Coker, M.O.; Madan, J.C.; Pathmasiri, W.; McRitchie, S.; Dade, E.F.; Doherty, B.T.; Sumner, S.; Karagas, M.R. Association of cesarean delivery and formula supplementation with the stool metabolome of 6-week-old infants. Metabolites 2021, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liang, S.; Chen, Q.; Zhao, L.; Li, B.; Huo, G. Distinct gut microbiota and metabolite profiles induced by delivery mode in healthy Chinese infants. J. Proteom. 2021, 10, 104071. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Huang, Q.; Wei, B.; Chen, Y. Effects of β-lactam antibiotics on gut microbiota colonization and metabolites in late preterm infants. Curr. Microbiol. 2020, 77, 3888–3896. [Google Scholar] [CrossRef]
- Patton, L.; Li, N.; Garrett, T.J.; Ruoss, J.L.; Russell, J.T.; de la Cruz, D.; Bazacliu, C.; Polin, R.A.; Triplett, E.W.; Neu, J. Antibiotics effects on the fecal metabolome in preterm infants. Metabolites 2020, 10, 331. [Google Scholar] [CrossRef]
- Brink, L.R.; Mercer, K.E.; Piccolo, B.D.; Chintapalli, S.V.; Elolimy, A.; Bowlin, A.K.; Matazel, K.S.; Pack, L.; Adams, S.H.; Shankar, K.; et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula. Am. J. Clin. Nutr. 2020, 111, 1190–1202. [Google Scholar] [CrossRef]
- Wald, C. Diagnostics: A flow of information. Nature 2017, 551, 48–50. [Google Scholar] [CrossRef]
- Khamis, M.M.; Adamko, D.J.; El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 2017, 36, 115–134. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P. The human urine metabolome. PLoS ONE 2013, 8, 73076. [Google Scholar] [CrossRef]
- Lv, H.; Hung, C.S.; Chaturvedi, K.S.; Hooton, T.M.; Henderson, J.P. Development of an integrated metabolomic profiling approach for infectious diseases research. Analyst 2011, 136, 4752–4763. [Google Scholar] [CrossRef] [PubMed]
- Salek, R.M.; Maguire, M.L.; Bentley, E.; Rubtsov, D.V.; Hough, T.; Cheeseman, M.; Nunez, D.; Sweatman, B.C.; Haselden, J.N.; Cox, R.D. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genom. 2007, 29, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jiang, C.; Huang, S.; Gong, X.; Wang, S.; Shen, P. Analysis of urinary metabolites for breast cancer patients receiving chemotherapy by CE-MS coupled with on-line concentration. Clin. Biochem. 2013, 46, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Liu, L.F.; Tang, Z.; Zhang, M.; Chua, K.K.; Song, J.X.; Mok, V.C.; Li, M.; Cai, Z. Comprehensive urinary metabolomic profiling and identification of potential noninvasive marker for idiopathic Parkinson’s disease. Sci. Rep. 2015, 5, 13888. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, Y. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev. Proteom. 2015, 12, 623–636. [Google Scholar] [CrossRef]
- Zhang, S.; Nagana Gowda, G.A.; Ye, T.; Raftery, D. Advances in NMR-based biofluid analysis and metabolite profiling. Analyst 2010, 135, 1490–1498. [Google Scholar] [CrossRef]
- Georgakopoulou, I.; Chasapi, S.A.; Bariamis, S.E.; Varvarigou, A.; Spraul, M.; Spyroulias, G.A. Metabolic changes in early neonatal life: NMR analysis of the neonatal metabolic profile to monitor postnatal metabolic adaptations. Metabolomics 2020, 16, 58. [Google Scholar] [CrossRef]
- Georgiopoulou, P.D.; Chasapi, S.A.; Christopoulou, I.; Varvarigou, A.; Spyroulias, G.A. Untargeted 1H-NMR urine metabolomic analysis of preterm infants with neonatal sepsis. Appl. Sci. 2022, 12, 1932. [Google Scholar] [CrossRef]
- Chasapi, S.A.; Karagkouni, E.; Kalavrizioti, D.; Vamvakas, S.; Zompra, A.; Takis, P.G.; Goumenos, D.S.; Spyroulias, G.A. NMR-Based Metabolomics in Differential Diagnosis of Chronic Kidney Disease (CKD) Subtypes. Metabolites 2022, 12, 490. [Google Scholar] [CrossRef]
- Matzarapi, K.; Giannakopoulos, A.; Chasapi, S.A.; Kritikou, D.; Efthymiadou, A.; Chrysis, D.; Spyroulias, G.A. NMR-based metabolic profiling of children with premature adrenarche. Metabolomics 2022, 18, 78. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Groer, M.W.; Luciano, A.A.; Dishaw, L.J.; Ashmeade, T.L.; Miller, E.; Gilbert, J.A. Development of the preterm infant gut microbiome: A research priority. Microbiome 2014, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yan, F.; Wang, N.; Song, Y.; Yue, Y.; Guan, J.; Li, B.; Huo, G. Distinct Gut Microbiota and Metabolite Profiles Induced by Different Feeding Methods in Healthy Chinese Infants. Front. Microbiol. 2020, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748. [Google Scholar] [CrossRef]
- Coscia, A.; Bardanzellu, F.; Caboni, E.; Fanos, V.; Peroni, D.G. When a Neonate Is Born, So Is a Microbiota. Life 2021, 11, 148. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, 724–732. [Google Scholar] [CrossRef]
- Bosch, A.A.T.M.; Levin, E.; van Houten, M.A.; Hasrat, R.; Kalkman, G.; Biesbroek, G.; de Steenhuijsen Piters, W.A.A.; de Groot, P.K.C.M.; Pernet, P.; Keijser, B.J.F.; et al. Development of Upper Respiratory Tract Microbiota in Infancy Is Affected by Mode of Delivery. EBioMedicine 2016, 9, 336–345. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.; Momin, S.R.; Senn, M.K.; Wood, A.C. Metabolomic Insights into the Effects of Breast Milk Versus Formula Milk Feeding in Infants. Curr. Nutr. Rep. 2019, 8, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Shimizu, T. Effect of Human Breast Milk on Biological Metabolism in Infants. Pediatr. Int. 2019, 61, 6–15. [Google Scholar] [CrossRef]
- Roggero, P.; Liotto, N.; Pozzi, C.; Braga, D.; Troisi, J.; Menis, C.; Gianni, M.L.; Canani, R.B.; Paparo, L.; Nocerino, R.; et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei CBA L74-fermented formula. Nat. Commun. 2020, 11, 2703. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, 9076. [Google Scholar] [CrossRef]
- Marino, L.V.; Paulson, S.; Ashton, J.J.; Weeks, C.; Young, A.; Pappachan, J.V.; Swann, J.; Johnson, M.J.; Beattie, R.M. A scoping review: Urinary markers of metabolic maturation in preterm infants and future interventions to improve growth. Nutrients 2022, 14, 3957. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fontanille, P.; Pandey, A.; Larroche, C. Gluconic Acid: Properties, Applications and Microbial Production. Food Technol. Biotechnol. 2006, 44, 185–195. [Google Scholar]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Aldieri, E.; Ghigo, D. The Pentose Phosphate Pathway: An Antioxidant Defense and a Crossroad in Tumor Cell Fate. Free Radic. Biol. Med. 2012, 53, 421–436. [Google Scholar] [CrossRef]
- Psihogios, N.G.; Gazi, I.F.; Elisaf, M.S.; Seferiadis, K.I.; Bairaktari, E.T. Gender-related and age-related urinalysis of healthy subjects by NMR-based metabonomics. NMR Biomed. 2008, 21, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomics marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effects in aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Growth, Reproduction, and Health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Haschke-Becher, E.; Kainz, A.; Bachmann, C. Reference Values of Amino Acids and of Common Clinical Chemistry in Plasma of Healthy Infants Aged 1 and 4 Months. J. Inherit. Metab. Dis. 2016, 39, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Sakuragawa, T.; Hishiki, T.; Ueno, Y.; Ikeda, S.; Soga, T.; Yachie-Kinoshita, A.; Kajimura, M.; Suematsu, M. Hypotaurine is an Energy-Saving Hepatoprotective Compound against Ischemia-Reperfusion Injury of the Rat Liver. J. Clin. Biochem. Nutr. 2010, 46, 126–134. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Yeh, K.W.; Lin, G.; Chiang, M.H.; Yang, S.C.; Chao, W.J.; Yao, T.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; et al. Metabolomics Reveals Dynamic Metabolic Changes Associated with Age in Early Childhood. PLoS ONE 2016, 11, 0149823. [Google Scholar] [CrossRef]
- Palomo-Buitrago, M.E.; Sabater-Masdeu, M.; Moreno-Navarrete, J.M.; Caballano-Infantes, E.; Arnoriaga-Rodríguez, M.; Coll, C.; Ramió, L.; Palomino-Schätzlein, M.; Gutiérrez-Carcedo, P.; Pérez-Brocal, V.; et al. Glutamate interactions with obesity, insulin resistance, cognition and gut microbiota composition. Acta Diabetol. 2019, 56, 569–579. [Google Scholar] [CrossRef]
- Go, Y.M.; Walker, D.I.; Soltow, Q.A.; Uppal, K.; Wachtman, L.M.; Strobel, F.H.; Pennell, K.; Promislow, D.E.; Jones, D.P. Metabolome-wide association study of phenylalanine in plasma of common marmosets. Amino Acids 2015, 47, 589–601. [Google Scholar] [CrossRef]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261–267. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes 2012, 3, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.D.; Haffner, S.M.; Hanley, A.J. Branched-chain amino acids and insulin metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Würtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–997. [Google Scholar] [CrossRef]
- Burrage, L.C.; Nagamani, S.C.S.; Campeau, P.M.; Lee, B.H. Branched-chain amino acid metabolism: From rare Mendelian diseases to more common disorders. Hum. Mol. Genet. 2014, 23, 1–8. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Stevens, A.; Bonshek, C.; Whatmore, A.; Butcher, I.; Hanson, D.; De Leonibus, C.; Shaikh, G.; Brown, M.; O’Shea, E.; Victor, S.; et al. Insights into the pathophysiology of catch-up compared with non-catch-up growth in children born small for gestational age: An integrated analysis of metabolic and transcriptomic data. Pharm. J. 2014, 14, 376–384. [Google Scholar] [CrossRef]
- Carlomagno, G.; De Grazia, S.; Unfer, V.; Manna, F. Myo-inositol in a new pharmaceutical form: A step forward to a broader clinical use. Expert Opin. Drug Deliv. 2012, 9, 267–271. [Google Scholar] [CrossRef]
- Dessì, A.; Marincola, F.C.; Pattumelli, M.G.; Ciccarelli, S.; Corbu, S.; Ossicini, C.; Fanos, V. Investigation of the 1H-NMR based urine metabolomic profiles of IUGR, LGA and AGA newborns on the first day of life. J. Matern. Fetal Neonatal Med. 2014, 27, 13–19. [Google Scholar] [CrossRef]
- Marincola, F.C.; Dessì, A.; Pattumelli, M.G.; Corbu, S.; Ossicini, C.; Ciccarelli, S.; Agostino, R.; Mussap, M.; Fanos, V. (1)H NMR-based urine metabolic profile of IUGR, LGA, and AGA newborns in the first week of life. Clin. Chim. Acta 2015, 451, 28–34. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.X.; Li, X.W.; Fu, W.; Zhang, W.Q. Metabolomic Research on Newborn Infants With Intrauterine Growth Restriction. Medicine 2016, 95, 3564. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.O.; Pinto, J.; Barros, A.S.; Morais, E.; Duarte, D.; Negrão, F.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Spraul, M.; et al. Newborn Urinary Metabolic Signatures of Prematurity and Other Disorders: A Case Control Study. J. Proteome Res. 2015, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Dessì, A.; Atzori, L.; Noto, A.; Visser, G.H.A.; Gazzolo, D.; Zanardo, V.; Barberini, L.; Puddu, M.; Ottonello, G.; Atzei, A.; et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): Urine reveals markers of metabolic syndrome. J. Matern. Neonatal Med. 2011, 24, 35–39. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2010, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.J. Carbohydrate Reserves in the Newborn Infant. BMJ 1964, 1, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Liao, P.; Li, Z.; Li, W.; Li, X.; Wu, Y.; Pei, F. NMR spectroscopic-based metabonomic investigation on the acute biochemical effects induced by Ce(NO3)3 in rats. J. Inorg. Biochem. 2005, 99, 2151–2160. [Google Scholar] [CrossRef]
- Briggs, J.P.; Levitt, M.F.; Abramson, R.G. Renal excretion of allantoin in rats: A micropuncture and clearance study. Am. J. Physiol. 1977, 233, 373–381. [Google Scholar] [CrossRef]
- Saoi, M.; Percival, M.; Nemr, C.; Li, A.; Gibala, M.; Britz-McKibbin, P. Characterization of the Human Skeletal Muscle Metabolome for Elucidating the Mechanisms of Bicarbonate Ingestion on Strenuous Interval Exercise. Anal. Chem. 2019, 91, 4709–4718. [Google Scholar] [CrossRef]
- Siopi, A.; Deda, O.; Manou, V.; Kellis, S.; Kosmidis, I.; Komninou, D.; Raikos, N.; Christoulas, K.; Theodoridis, G.A.; Mougios, V. Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites 2017, 7, 5. [Google Scholar] [CrossRef]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. 1H NMR-Based Metabonomic Investigation of the Effect of Two Different Exercise Sessions on the Metabolic Fingerprint of Human Urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef]
- Stathis, C.G.; Carey, M.F.; Snow, R.J. The influence of allopurinol on urinary purine loss after repeated sprint exercise in man. Metabolism 2005, 54, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Cerasani, J.; Ceroni, F.; De Cosmi, V.; Mazzocchi, A.; Morniroli, D.; Roggero, P.; Mosca, F.; Agostoni, C.; Giannì, M. Human Milk Feeding and Preterm Infants’ Growth and Body Composition: A Literature Review. Nutrients 2020, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Dang, D.; Lv, X.M.; Wang, T.F.; Du, J.F.; Wu, H. Human milk fortifier with high versus standard protein content for promoting growth of preterm infants: A meta-analysis. J. Int. Med. Res. 2015, 43, 279–289. [Google Scholar] [CrossRef]
- Mangili, G.; Garzoli, E. Feeding of preterm infants and fortification of breast milk. Pediatr. Med. Chir. 2017, 39, 158. [Google Scholar] [CrossRef]
- Lin, J.; Tang, Q. Analysis of causes of extrauterine growth restriction in premature infants and the status of nutritional intake. J. Clin. Pediatr. 2016, 34, 712–715. [Google Scholar]
- Fenf, H.; Liu, X. Application of breastfeeding combined with addition of human milk fortifier in the feeding of premature infants with very low birth weight. J. Clin. Med. Pract. 2019, 23, 113–115. [Google Scholar]
- Rigo, J.; Hascoët, J.M.; Billeaud, C.; Picaud, J.C.; Mosca, F.; Rubio, A.; Saliba, E.; Radkë, M.; Simeoni, U.; Guillois, B.; et al. Growth and Nutritional Biomarkers of Preterm Infants Fed a New Powdered Human Milk Fortifier: A Randomized Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 83–93. [Google Scholar] [CrossRef]
- Li, Y.; Chi, C.; Li, C.; Song, J.; Song, Z.; Wang, W.; Sun, J. Efficacy of Donated Milk in Early Nutrition of Preterm Infants: A Meta-Analysis. Nutrients 2022, 14, 1724. [Google Scholar] [CrossRef]
- Napierala, M.; Merritt, T.A.; Miechowicz, I.; Mielnik, K.; Mazela, J.; Florek, E. The effect of maternal tobacco smoking and second-hand tobacco smoke exposure on human milk oxidant-antioxidant status. Environ. Res. 2019, 170, 110–121. [Google Scholar] [CrossRef]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2018, 6, Cd002971. [Google Scholar] [CrossRef]
- Hanson, L.A.; Korotkova, M.; Telemo, E. Breast-feeding, infant formulas, and the immune system. Ann. Allergy Asthma Immunol. 2003, 90, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Bardanzellu, F.; Piras, C.; Atzei, A.; Neroni, P.; Fanos, V. Early Urinary Metabolomics in Patent Ductus Arteriosus Anticipates the Fate: Preliminary Data. Front. Pediatr. 2020, 8, 613749. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Bazzano, G.; Demontis, R.; Chighine, A.; Fanos, V.; d’Aloja, E. Exploring Perinatal Asphyxia by Metabolomics. Metabolites 2020, 10, 141. [Google Scholar] [CrossRef] [PubMed]












| N | % | |
|---|---|---|
| Sex | ||
| Male | 28 | 51.9 |
| Female | 26 | 48.1 |
| IVF | ||
| Yes | 7 | 13.0 |
| No | 47 | 87.0 |
| Delivery mode | ||
| Normal | 7 | 13.0 |
| Cesarean | 47 | 87.0 |
| Parity | ||
| 1st | 29 | 53.7 |
| 2nd | 18 | 33.3 |
| 3rd | 6 | 11.1 |
| 4th | 1 | 1.9 |
| Maternal smoking | ||
| Yes | 2 | 3.7 |
| No | 52 | 96.3 |
| Preeclampsia | ||
| Yes | 5 | 9.3 |
| No | 49 | 90.7 |
| Maternal medications | ||
| None | 16 | 29.63 |
| Betamethasone | 28 | 51.85 |
| Thyroxin | 5 | 9.26 |
| Antibiotics | 4 | 7.41 |
| Atosiban | 3 | 5.55 |
| Thyroxine | 2 | 3.70 |
| Ampicilline-Sulbactam | 1 | 1.85 |
| Amitriptilline | 1 | 1.85 |
| Dexamethasone | 1 | 1.85 |
| Ceftriaxone | 1 | 1.85 |
| Nifedipine | 1 | 1.85 |
| Tinzaparin | 1 | 1.85 |
| Nifedipine | 1 | 1.85 |
| Betamethasone | 1 | 1.85 |
| Maternal diseases | ||
| None | 35 | 64.81 |
| Hypothyroidism | 6 | 11.11 |
| Hashimoto | 2 | 3.70 |
| Group B streptococcus colonisation | 2 | 3.70 |
| B-thalasaemia | 1 | 1.85 |
| Migraine | 1 | 1.85 |
| Mild renal failure | 1 | 1.85 |
| Pericarditis | 1 | 1.85 |
| Myopia | 1 | 1.85 |
| Placenta abruption | 1 | 1.85 |
| Uterine fibroids | 1 | 1.85 |
| Oligamnio | 1 | 1.85 |
| Aortic coarctation | 1 | 1.85 |
| Gestational diabetes | ||
| No | 47 | 87.0 |
| Diet only | 6 | 11.1 |
| Insulin | 1 | 1.9 |
| IUGR | ||
| Yes | 12 | 22.6 |
| No | 41 | 77.4 |
| Multiple gestation | ||
| No | 38 | 70.4 |
| Twins | 15 | 27.8 |
| Triplets | 1 | 1.9 |
| Twin-twin transfusion | ||
| Yes | 0 | 0.0 |
| No | 54 | 100.0 |
| Chorioamnionitis | ||
| Yes | 1 | 1.9 |
| No | 53 | 98.1 |
| Maternal antibiotics | ||
| No | 2 | 3.7 |
| Yes < 4 h | 39 | 72.2 |
| Yes | 13 | 24.1 |
| RDS | ||
| Yes | 17 | 31.5 |
| No | 37 | 68.5 |
| PDA | ||
| Yes | 0 | 0.0 |
| No | 54 | 100.0 |
| IVH | ||
| No | 52 | 96.3 |
| Grade1 | 2 | 3.7 |
| Grade2 | 0 | 0.0 |
| Grade3 | 0 | 0.0 |
| Grade4 | 0 | 0.0 |
| Congenital infection | ||
| Yes | 2 | 3.7 |
| No | 52 | 96.3 |
| Early-onset sepsis | ||
| Νο | 38 | 70.4 |
| Yes (- cult) | 10 | 18.5 |
| Yes (+cult,+gram) | 4 | 7.4 |
| Yes (+cult,-gram) | 0 | 0.0 |
| Yes (+cult, fungus) | 2 | 3.7 |
| Late-onset sepsis | ||
| Νο | 46 | 85.2 |
| Yes (-culture) | 2 | 3.7 |
| Yes (+culture, +gram) | 6 | 11.1 |
| Yes (+culture, -gram) | 0 | 0.0 |
| Yes (+culture, fungus) | 0 | 0.0 |
| NEC | ||
| Yes | 1 | 1.9 |
| No | 53 | 98.1 |
| Jaundice | ||
| No | 33 | 61.1 |
| Phototherapy | 21 | 38.9 |
| Exchange transfusion | 0 | 0.0 |
| Hypocalcemia | ||
| Yes | 5 | 9.3 |
| No | 49 | 90.7 |
| Metabolic diseases | ||
| No | 50 | 96.2 |
| Yes | 2 | 3.8 |
| Other diseases | ||
| None | 38 | 70.37 |
| ASD | 3 | 5.55 |
| Pneumothorax | 3 | 5.55 |
| Choroid cysts | 2 | 3.70 |
| Meconium aspiration | 1 | 1.85 |
| Conexingene | 1 | 1.85 |
| Hypospadias | 1 | 1.85 |
| Polycystic kidneys | 1 | 1.85 |
| Hydronephrosis | 1 | 1.85 |
| Laryngomalacia | 1 | 1.85 |
| Congenital heart disease | 1 | 1.85 |
| Death | ||
| Yes | 0 | 0.0 |
| No | 54 | 100.0 |
| Medications DOL 5 | ||
| None | 3 | 5.55 |
| Ampicillin/gentamicin | 31 | 57.41 |
| Ampicillin/cefotaxine | 6 | 11.11 |
| Ampicillin/gentamicin/teicoplanin | 4 | 7.41 |
| Meropenem/vancomycin | 3 | 5.55 |
| Ampicillin/gentamicin/micafungin | 1 | 1.85 |
| Ampicillin/gentamicin/amphotericin | 1 | 1.85 |
| Ampicillin/gentamicin/fluconazole | 1 | 1.85 |
| Vitamin D | 1 | 1.85 |
| Meropenem/vancomycin/rifampicin | 1 | 1.85 |
| Ampicillin/cefotaxime/teicoplanin | 1 | 1.85 |
| Ampicillin/meropenem/vancomycin | 1 | 1.85 |
| Day | Metabolites | δH (ppm)/Multiplicity | FDR p-Values/Effect in NICU LPs | Levels of NICU LPs |
|---|---|---|---|---|
| 1st | Acetoacetate | 2.25 (s) | 0.001 | ↓ |
| Gluconate | 3.83–3.81 (m) | 2.63 × 10−6 | ↑ | |
| Glycolate | 3.99 (s) | 7.92 × 10−5 | ↑ | |
| Hippurate | 7.83–7.81 (d) | 4.53 × 10−6 | ↓ | |
| Lactose | 4.46–4.43 (d) | 4.83 × 10−5 | ↓ | |
| 3rd | Gluconate | 3.83–3.81 (m) | 1.38 × 10−5 | ↑ |
| Glycolate | 3.99 (s) | 0.0003 | ↑ | |
| Lactose | 4.46–4.43 (d) | 0.007 | ↓ |
| Day | Metabolites | p-Values | Group Levels |
|---|---|---|---|
| 1st | 1-Methylnicotinamide | 0.0013 | control NICU > RDS |
| Glycine | 4.29 × 10−7 | control NICU > RDS | |
| Formate | 9.66 × 10−9 | RDS > control NICU | |
| Alanine | 2.80 × 10−5 | control NICU > RDS | |
| Hippurate | 0.008 | control NICU > RDS | |
| Glucose | 1.74 × 10−5 | control NICU > RDS | |
| Lactose | 0.00017 | RDS > control NICU | |
| 4-Hydroxyproline | 0.0066 | RDS > control NICU | |
| Gluconate | 0.0004 | RDS > control NICU | |
| Dimethylglycine | 1.98 × 10−6 | RDS > control NICU | |
| Myoinositol | 0.0063 | RDS > control NICU | |
| Acetoacetate | 2.42 × 10−6 | RDS > control NICU | |
| Leucine | 3.54 × 10−7 | control ICU > RDS | |
| Allantoin | 0.00107 | RDS > control NICU | |
| Betaine | 2.42 × 10−6 | control ICU > RDS | |
| Creatine | 0.0006 | RDS > control NICU | |
| Tyrosine | 0.007 | control ICU > RDS | |
| 4-Hydroxybenzoate | 0.0004 | control ICU > RDS | |
| Pyruvate | 0.00017 | RDS > control NICU | |
| Dimethylamine | 3.54 × 10−7 | control ICU > RDS | |
| Trimethylamine | 0.006 | control ICU > RDS | |
| Dimethylglycine | 2.85 × 10−5 | RDS > control NICU | |
| Oxoglutarate | 0.00017 | control NICU > RDS | |
| Ethanolamine | 0.0006 | RDS > control ICU | |
| TMAO | 0.0024 | control NICU > RDS | |
| 1-Methylnicotinamide, N1-Methyl-2-pyridone-5-carboxamide | 3.60 × 10−5 | control NICU > RDS | |
| Hypoxanthine | 0.0008 | control NICU > RDS | |
| Trigonelline | 0.0015 | control NICU > RDS | |
| 4-Hydroxyphenyl acetate | 3.64 × 10−6 | control NICU > RDS | |
| 3-Hydroxyisovaleric acid | 0.005 | control NICU > RDS | |
| Indoxyl sulfate | 0.0037 | RDS > control NICU | |
| 3rd | 1-Methylnicotinamide | 4.96 × 10−5 | RDS > control NICU |
| Glycine | 1.04 × 10−7 | RDS > control NICU | |
| Formate | 0.0011 | RDS > control NICU | |
| Alanine | 3.84 × 10−7 | RDS > control NICU | |
| Hippurate | 2.55 × 10−5 | control NICU > RDS | |
| Lactose | 0.0058 | control NICU > RDS | |
| 4-Hydroxyproline | 4.30 × 10−6 | control NICU > RDS | |
| Gluconate | 6.28 × 10−5 | RDS > control ICU | |
| Dimethylglycine | 0.0015 | control NICU > RDS | |
| Myoinositol | 1.24 × 10−5 | control NICU > RDS | |
| Acetoacetate | 0.00044 | RDS > control NICU | |
| Leucine | 0.0092 | RDS > control NICU | |
| Betaine | 1.05 × 10−6 | RDS > control NICU | |
| Tyrosine | 4.32 × 10−5 | control NICU > RDS | |
| 4-Hydroxybenzoate | 0.00015 | control NICU > RDS | |
| Pyruvate | 0.0033 | control NICU > RDS | |
| Dimethylamine | 1.05 × 10−6 | control NICU > RDS | |
| Trimethylamine | 3.84 × 10−7 | RDS > control ICU | |
| Dimethylglycine | 5.04 × 10−5 | control NICU > RDS | |
| Oxoglutarate | 7.32 × 10−5 | control NICU > RDS | |
| Ethanolamine | 5.04 × 10−5 | control NICU > RDS | |
| Taurine | 0.0011 | RDS > control NICU | |
| 1-Methylnicotinamide, N1-Methyl-2-pyridone-5-carboxamide | 5.38 × 10−5 | RDS > control NICU | |
| Hypoxanthine | 1.20 × 10−5 | RDS > control NICU | |
| Trigonelline | 0.0062 | RDS > control NICU | |
| 4-Hydroxyphenyl acetate | 2.31 × 10−5 | control NICU > RDS | |
| Lys/Arg | 3.26 × 10−6 | control NICU > RDS | |
| 3-Hydroxyisovaleric acid | 6.82 × 10−5 | control NICU > RDS | |
| Indoxyl sulfate | 0.0021 | RDS > control NICU |
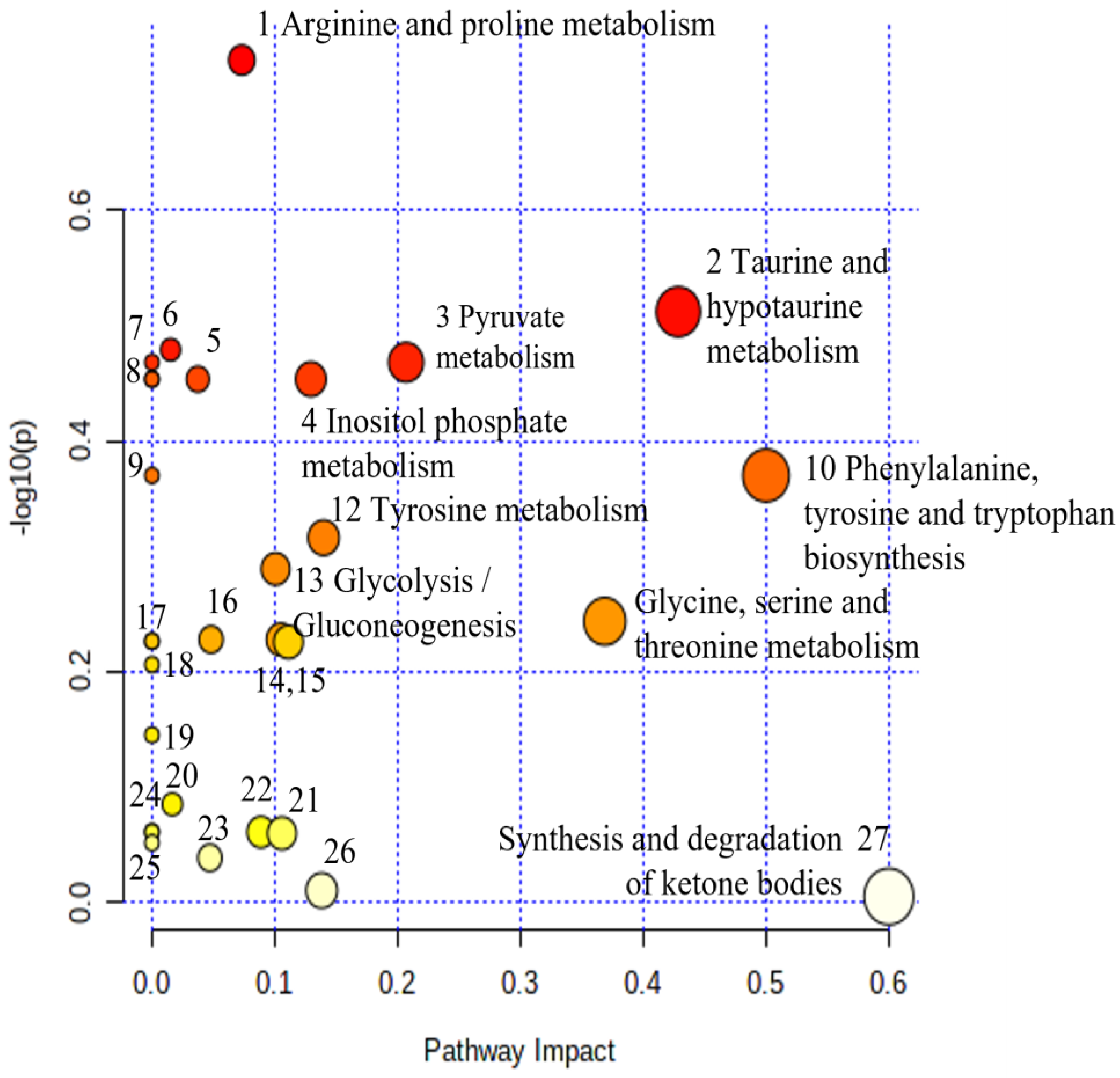
| No. | Biochemical Pathway | Metabolites Measured | Row p Value | Impact |
|---|---|---|---|---|
| 1 | Arginine and proline metabolism | Creatine, Hydroxyproline, Pyruvate | 1.86 × 10−1 | 0.07 |
| 2 | Taurine and hypotaurine metabolism | Taurine | 3.08 × 10−1 | 0.43 |
| 3 | Pyruvate metabolism | Pyruvate | 3.40 × 10−1 | 0.21 |
| 4 | Inositol phosphate metabolism | myo-Inositol | 3.52 × 10−1 | 0.13 |
| 5 | Phosphatidylinositol signaling system | myo-Inositol | 3.52 × 10−1 | 0.04 |
| 6 | Primary bile acid biosynthesis | Glycine, Taurine | 3.32 × 10−1 | 0.02 |
| 7 | Cysteine and methionine metabolism | Pyruvate; | 3.40 × 10−1 | 0.00 |
| 8 | Ascorbate and aldarate metabolism | myo-Inositol | 3.52 × 10−1 | 0.00 |
| 9 | Ubiquinone and other terpenoid-quinone biosynthesis | Tyrosine | 4.26 × 10−1 | 0.00 |
| 10 | Phenylalanine, tyrosine, and tryptophan biosynthesis | Tyrosine | 4.26 × 10−1 | 0.50 |
| 11 | Glycine, serine, and threonine metabolism | Betaine, N, N-Dimethylglycine, Glycine, Creatine, Pyruvate | 5.71 × 10−1 | 0.37 |
| 12 | Tyrosine metabolism | Tyrosine, Pyruvate, Acetoacetate, 4-Hydroxyphenylacetate | 4.83 × 10−1 | 0.14 |
| 13 | Glycolysis/Gluconeogenesis | Pyruvate, beta-Glucose | 5.14 × 10−1 | 0.10 |
| 14 | Butanoate metabolism | Acetoacetate, 2-Oxoglutarate | 5.95 × 10−1 | 0.11 |
| 15 | Citrate cycle (TCA cycle) | 2-Oxoglutarate, pyruvate | 5.92 × 10−1 | 0.10 |
| 16 | Alanine, aspartate, and glutamate metabolism | 2-Oxoglutarate, pyruvate | 5.92 × 10−1 | 0.05 |
| 17 | D-Glutamine and D-glutamate metabolism | 2-Oxoglutarate | 5.94 × 10−1 | 0.00 |
| 18 | Phenylalanine metabolism | Hippurate; Tyrosine | 6.22 × 10−1 | 0.00 |
| 19 | Valine, leucine, and isoleucine biosynthesis | Tyrosine | 7.16 × 10−1 | 0.00 |
| 20 | Purine metabolism | Hypoxanthine | 8.22 × 10−1 | 0.02 |
| 21 | Glyoxylate and dicarboxylate metabolism | Glycine, Pyruvate, Formate | 8.71 × 10−1 | 0.11 |
| 22 | Glutathione metabolism | Glycine | 8.69 × 10−1 | 0.09 |
| 23 | Pentose phosphate pathway | Gluconate | 9.15 × 10−1 | 0.05 |
| 24 | Porphyrin metabolism | Glycine | 8.69 × 10−1 | 0.00 |
| 25 | Valine, leucine, and isoleucine degradation | Acetoacetate, Leucine | 8.88 × 10−1 | 0.00 |
| 26 | Nicotinate and nicotinamide metabolism | 1-Methylnicotinamide | 9.77 × 10−1 | 0.14 |
| 27 | Synthesis and degradation of ketone bodies | Acetoacetate | 9.88 × 10−1 | 0.60 |
| Day | Metabolites | p-Values | Group Levels |
|---|---|---|---|
| 1st | Alanine | 0.047 | RDS > healthy |
| Lactose | 0.016 | Healthy > RDS | |
| Acetoacetate | 0.01 | Healthy > RDS | |
| Leucine | 0.01 | RDS > healthy | |
| Allantoin | 0.015 | Healthy > RDS | |
| 4-Hydroxybenzoate | 0.019 | Healthy > RDS | |
| Pyruvate | 0.05 | Healthy > RDS | |
| Trimethylamine | 0.05 | Healthy > RDS | |
| Oxoglutarate | 0.033 | Healthy > RDS | |
| Taurine | 0.05 | Healthy > RDS | |
| TMAO | 0.01 | RDS > healthy | |
| Gluconate | 0.01 | RDS > healthy | |
| Trigonelline | 0.002 | Healthy > RDS | |
| 3-OH-hydroxyisovalerate | 2.86 × 10−6 | RDS > healthy | |
| Indoxyl sulfate | 0.01 | RDS > healthy | |
| 3rd | Alanine | 0.004 | RDS > healthy |
| Gluconate | 0.002 | RDS > healthy | |
| Leucine | 0.0039 | RDS > healthy | |
| Hypoxanthine | 0.0064 | RDS > healthy | |
| Lys/Arg | 0.0023 | RDS > healthy | |
| 3-OH-hydroxyisovalerate | 0.0049 | RDS > healthy | |
| Indoxyl sulfate | 0.0023 | RDS > healthy |
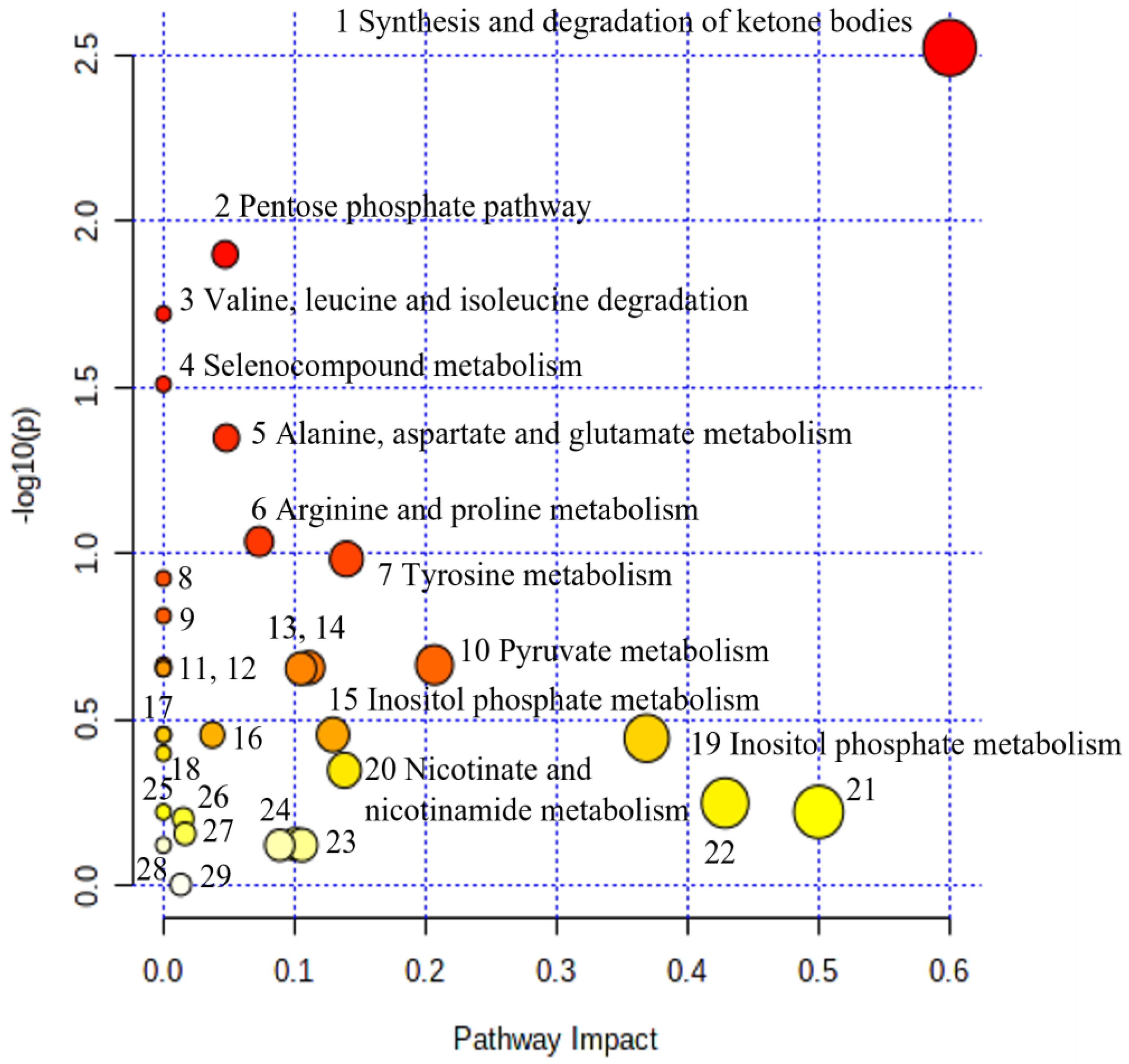
| No | Biochemical Pathway | Metabolites Measured | Row p Value | Impact |
|---|---|---|---|---|
| 1. | Synthesis and degradation of ketone bodies | Acetoacetate | 3.00 × 10−3 | 0.60 |
| 2. | Pentose phosphate pathway | Gluconate | 1.26 × 10−2 | 0.05 |
| 3. | Valine, leucine, and isoleucine degradation | Acetoacetate, Leucine | 1.90 × 10−2 | 0.00 |
| 4. | Selenocompound metabolism | Alanine | 3.09 × 10−2 | 0.00 |
| 5. | Alanine, aspartate, and glutamate metabolism | Alanine, Pyruvate; 2-Oxoglutarate; | 4.48 × 10−2 | 0.05 |
| 6. | Arginine and proline metabolism | Creatine, Hydroxyproline, Pyruvate | 9.19 × 10−2 | 0.07 |
| 7. | Tyrosine metabolism | Tyrosine, Pyruvate; Acetoacetate; 4-Hydroxyphenylacetate; | 1.04 × 10−1 | 0.14 |
| 8. | Aminoacyl-tRNA biosynthesis | Glycine, Alanine, Leucine, Tyrosine | 1.19 × 10−1 | 0.00 |
| 9. | Phenylalanine metabolism | Hippurate; Tyrosine | 1.54 × 10−1 | 0.00 |
| 10. | Pyruvate metabolism | Pyruvate | 2.16 × 10−1 | 0.21 |
| 11. | Cysteine and methionine metabolism | Pyruvate | 2.16 × 10−1 | 0.00 |
| 12. | D-Glutamine and D-glutamate metabolism | 2-Oxoglutarate | 2.22 × 10−1 | 0.00 |
| 13. | Citrate cycle (TCA cycle) | 2-Oxoglutarate; Pyruvate | 2.22 × 10−1 | 0.10 |
| 14. | Butanoate metabolism | Acetoacetate, 2-Oxoglutarate | 2.20 × 10−1 | 0.11 |
| 15. | Inositol phosphate metabolism | myo-Inositol | 3.51 × 10−1 | 0.13 |
| 16. | Phosphatidylinositol signaling system | myo-Inositol | 3.51 × 10−1 | 0.04 |
| 17. | Ascorbate and aldarate metabolism | myo-Inositol | 3.51e−01 | 0.00 |
| 18. | Valine, leucine, and isoleucine biosynthesis | Leucine | 3.99 × 10−1 | 0.00 |
| 19. | Glycine, serine, and threonine metabolism | Betaine, N, N-Dimethylglycine, Glycine, Creatine, Pyruvate | 3.59 × 10−1 | 0.37 |
| 20. | Nicotinate and nicotinamide metabolism | 1-Methylnicotinamide, N1-Methyl-2-pyridone-5-carboxamide | 4.45 × 10−1 | 0.14 |
| 21. | Phenylalanine, tyrosine, and tryptophan biosynthesis | Tyrosine | 5.99 × 10−1 | 0.50 |
| 22. | Taurine and hypotaurine metabolism | Taurine | 5.63 × 10−1 | 0.43 |
| 23. | Glyoxylate and dicarboxylate metabolism | Glycine, Pyruvate, Formate | 7.53 × 10−1 | 0.11 |
| 24. | Glutathione metabolism | Glycine | 7.54 × 10−1 | 0.09 |
| 25. | Ubiquinone and other terpenoid-quinone biosynthesis | Tyrosine; | 5.99 × 10−1 | 0.00 |
| 26. | Primary bile acid biosynthesis | Glycine, Taurine | 6.28 × 10−1 | 0.02 |
| 27. | Purine metabolism | Hypoxanthine | 6.96 × 10−1 | 0.02 |
| 28. | Porphyrin metabolism | Glycine | 7.54 × 10−1 | 0.00 |
| 29 | Glycerophospholipid metabolism | Ethanolamine | 9.90 × 10−1 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulou, I.; Kostopoulou, E.; Matzarapi, K.; Chasapi, S.A.; Spyroulias, G.A.; Varvarigou, A. Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis. Metabolites 2023, 13, 644. https://doi.org/10.3390/metabo13050644
Christopoulou I, Kostopoulou E, Matzarapi K, Chasapi SA, Spyroulias GA, Varvarigou A. Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis. Metabolites. 2023; 13(5):644. https://doi.org/10.3390/metabo13050644
Chicago/Turabian StyleChristopoulou, Irene, Eirini Kostopoulou, Konstantina Matzarapi, Styliani A. Chasapi, Georgios A. Spyroulias, and Anastasia Varvarigou. 2023. "Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis" Metabolites 13, no. 5: 644. https://doi.org/10.3390/metabo13050644
APA StyleChristopoulou, I., Kostopoulou, E., Matzarapi, K., Chasapi, S. A., Spyroulias, G. A., & Varvarigou, A. (2023). Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis. Metabolites, 13(5), 644. https://doi.org/10.3390/metabo13050644








