Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. flavicarpa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Material
2.3. Obtaining Passion Fruit Peel Flour
2.4. Color
2.5. Proximate Composition and Starch Content
2.6. Fourier Transform Infrared (FTIR) Spectroscopy
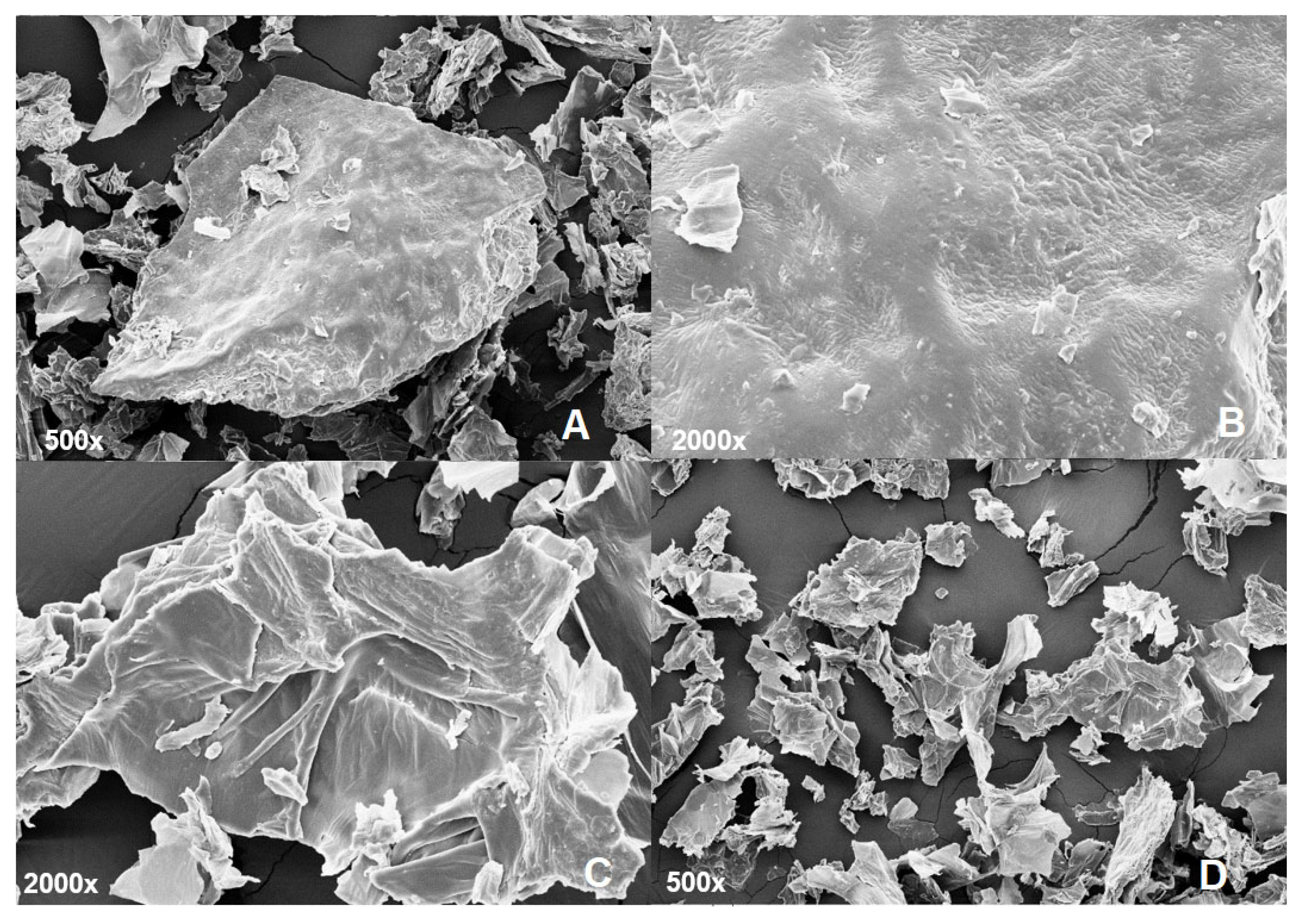
2.7. Scanning Electron Microscopy (SEM)
2.8. Granulometry
2.9. Preparation of PFPF Extracts
2.10. Total Phenolic Compounds and Antioxidant Capacity
2.11. Carotenoids
2.12. Determination of the Profile of Phenolic Compounds
2.13. Chemical Profile
3. Results and Discussion
3.1. Characterization of Passion Fruit Peel Flour
3.1.1. Color
3.1.2. Proximate Composition and Starch Content
3.1.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.1.4. Scanning Electron Microscopy (SEM)
3.1.5. Granulometry
3.1.6. Total Phenolic Compounds (TPC) and Antioxidant Capacity
3.1.7. Carotenoids
3.1.8. Determination of the Profile of Phenolic Compounds
3.1.9. Chemical Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delvar, A.; de Caro, P.; Caro, Y.; Shum Cheong Sing, A.; Thomas, R.; Raynaud, C. Semi-Siccative Oils and Bioactive Fractions Isolated from Reunion Island Fruit Co-Product: Two Case Studies. Eur. J. Lipid Sci. Technol. 2019, 121, 1800391. [Google Scholar] [CrossRef]
- Figueiredo, D.A.F.; Pordeus, L.C.M.; Paulo, L.L.; Braga, R.M.; Fonsêca, D.V.; Sousa, B.S.; Costa, M.J.C.; Gonçalves, M.C.R.; Oliveira, K.H.D. Effects of Bark Flour of Passiflora Edulis on Food Intake, Body Weight and Behavioral Response of Rats. Braz. J. Pharmacogn. 2016, 26, 595–600. [Google Scholar] [CrossRef]
- Maia, S.M.P.C.; Pontes, D.F.; Garruti, D.D.S.; de Oliveira, M.N.; Arcanjo, S.R.S.; Chinelate, G.C.B. Farinha de Maracujá Na Elaboração de Bolo de Milho. Rev. Verde Agroecol. e Desenvolv. Sustentável 2018, 13, 328. [Google Scholar] [CrossRef]
- Claro, M.D.L.; Péres-Rodrigues, G.; Teixeira, S.A. Propriedades Funcionais Da Casca Do Maracujá (Passiflora Edulis) Na Síndrome Metabólica. DEMETRA Aliment. Nutr. Saúde 2018, 13, 181–194. [Google Scholar] [CrossRef]
- do Socorro Fernandes Marques, S.; Libonati, R.M.F.; Sabaa-Srur, A.U.O.; Luo, R.; Shejwalkar, P.; Hara, K.; Dobbs, T.; Smith, R.E. Evaluation of the Effects of Passion Fruit Peel Flour (Passiflora Edulis Fo. Flavicarpa) on Metabolic Changes in HIV Patients with Lipodystrophy Syndrome Secondary to Antiretroviral Therapy. Rev. Bras. Farmacogn. 2016, 26, 420–426. [Google Scholar] [CrossRef]
- Cauz-Santos, L.A.; Munhoz, C.F.; Rodde, N.; Cauet, S.; Santos, A.A.; Penha, H.A.; Dornelas, M.C.; Varani, A.M.; Oliveira, G.C.X.; Bergès, H.; et al. The Chloroplast Genome of Passiflora Edulis (Passifloraceae) Assembled from Long Sequence Reads: Structural Organization and Phylogenomic Studies in Malpighiales. Front. Plant Sci. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Santos, V.A.d.; Ramos, J.D.; Laredo, R.R.; Silva, F.O.d.R.; Chagas, E.A.; Pasqual, M. Produção e Qualidade de Frutos de Maracujazeiro-Amarelo Provenientes Do Cultivo Com Mudas Em Diferentes Idades. Rev. Ciências Agroveterinárias 2017, 16, 33–40. [Google Scholar] [CrossRef]
- IBGE. IBGE—Instituto Brasileiro de Geografia e Estatística; IBGE: São Paulo, Brazil, 2023. [Google Scholar]
- De Oliveira, C.F.; Giordani, D.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of Pectin from Passion Fruit Peel Using Moderate Electric Field and Conventional Heating Extraction Methods. Innov. Food Sci. Emerg. Technol. 2015, 29, 201–208. [Google Scholar] [CrossRef]
- Wijeratnam, S.W. Passion Fruit. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 230–234. [Google Scholar]
- Weng, M.; Li, Y.; Wu, L.; Zheng, H.; Lai, P.; Tang, B.; Luo, X. Effects of Passion Fruit Peel Flour as a Dietary Fibre Resource on Biscuit Quality. Food Sci. Technol. 2021, 41, 65–73. [Google Scholar] [CrossRef]
- Santos, R.O.; Silva, M.V.F.; Nascimento, K.O.; Batista, A.L.D.; Moraes, J.; Andrade, M.M.; Andrade, L.G.Z.S.; Khosravi-Darani, K.; Freitas, M.Q.; Raices, R.S.L.; et al. Prebiotic Flours in Dairy Food Processing: Technological and Sensory Implications. Int. J. Dairy Technol. 2018, 71, 1–10. [Google Scholar] [CrossRef]
- da Silva Alves, P.L.; Berrios Jose, J.D.J.; Pan, J.; Ramirez Ascheri, J.L. Passion Fruit Shell Flour and Rice Blends Processed into Fiber-Rich Expanded Extrudates. CYTA-J. Food 2018, 16, 901–908. [Google Scholar] [CrossRef]
- Coelho, E.M.; Gomes, R.G.; Machado, B.A.S.; Oliveira, R.S.; dos Santos Lima, M.; de Azêvedo, L.C.; Guez, M.A.U. Passion Fruit Peel Flour—Technological Properties and Application in Food Products. Food Hydrocoll. 2017, 62, 158–164. [Google Scholar] [CrossRef]
- Do Rosário, R.C.; Soares, S.D.; Martins, M.G.; Do Nascimento, F.D.C.A.; Silva Junior, J.O.C.; Teixeira-Costa, B.E.; de Souza Figueira, M.; Dos Santos, O.V. Bioactive, Technological-Functional Potential and Morphological Structures of Passion Fruit Albedo (Passiflora Edulis). Food Sci. Technol. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Da Silva, J.K.; Colomeu, T.C.; Zollner, R.L.; Maróstica Junior, M.R. Antioxidant Capacity and Chemical Composition of Passion Fruit Peel (Passifl Ora Edulis | Capacidade Antioxidante e Composição Química Da Casca de Maracujá (Passifl Ora Edulis). Cienc. Rural 2014, 44, 1699–1704. [Google Scholar] [CrossRef]
- Nguyen, N.M.P.; Le, T.T.; Vissenaekens, H.; Gonzales, G.B.; Van Camp, J.; Smagghe, G.; Raes, K. In Vitro Antioxidant Activity and Phenolic Profiles of Tropical Fruit By-products. Int. J. Food Sci. Technol. 2019, 54, 1169–1178. [Google Scholar] [CrossRef]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical Characterization, Antioxidant Capacity, Total Phenolic and Proanthocyanidin Content of Flours Prepared from Pequi (Caryocar Brasilense Camb.) Fruit by-Products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Horwitz, W., Ed.; AOAC: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Martino, H.S.D.; Tomazi, P.A.; Moraes, É.A.; da Conceição, L.L.; da Silva Oliveira, D.; Queiroz, V.A.V.; Rodrigues, J.A.S.; Pirozi, M.R.; Helena Maria, P.-S.; Ribeiro, S.M.R. Chemical Characterization and Size Distribution of Sorghum Genotypes for Human Consumption Caracterização Química e Distribuição Granulométrica de Genótipos de Sorgo Para Alimentação Humana. Rev. Inst. Adolfo. Lutz. 2012, 71, 337–344. [Google Scholar]
- Rufino, M.d.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Sahan, Y.; Cansev, A.; Gulen, H. Effect of Processing Techniques on Antioxidative Enzyme Activities, Antioxidant Capacity, Phenolic Compounds, and Fatty Acids of Table Olives. Food Sci. Biotechnol. 2013, 22, 613–620. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Assessment of the Provitamin A Contents of Foods - The Brazilian Experience. J. Food Compos. Anal. 1996, 9, 196–230. [Google Scholar] [CrossRef]
- Eça, K.S.; Machado, M.T.C.; Hubinger, M.D.; Menegalli, F.C. Development of Active Films From Pectin and Fruit Extracts: Light Protection, Antioxidant Capacity, and Compounds Stability. J. Food Sci. 2015, 80, C2389–C2396. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Freitas, L.; Souza, A.; Araújo, R.; Lacerda, I.; Pereira, H.; Augusti, R.; Melo, J. Antioxidant Activity and Metabolomic Analysis of Cagaitas (Eugenia Dysenterica) Using Paper Spray Mass Spectrometry. J. Braz. Chem. Soc. 2019, 30, 1034–1044. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Characterization of Orange Passion Fruit Peel Flour and Its Use as an Ingredient in Bakery Products. J. Culin. Sci. Technol. 2020, 18, 214–230. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of Ultrasound-Assisted Extraction Conditions for Recovery of Phenolic Compounds and Antioxidant Capacity from Banana (Musa Cavendish) Peel. J. Food Process. Preserv. 2016, 41, e13148. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Evaluation of Physicochemical, Technological and Morphological Characteristics of Powdered Yellow Passion Fruit Peel. Int. Food Res. J. 2016, 23, 1653–1662. [Google Scholar]
- Brazilian Health Regulatory Agency—ANVISA. Aprova o Regulamento Técnico Para Produtos de Cereais, Amidos, Farinhas e Farelo; Resolução RDC no. 263, de 22 de Setembro de; ANVISA: São Paulo, Brazil, 2005; pp. 1–368. [Google Scholar]
- Garcia, M.V.; Milani, M.S.; Ries, E.F. Production Optimization of Passion Fruit Peel Flour and Its Incorporation into Dietary Food. Food Sci. Technol. Int. 2020, 26, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santos, B.; de los Ángeles Vivar-Vera, M.; Rodríguez-Miranda, J.; Herman-Lara, E.; Torruco-Uco, J.G.; Acevedo-Vendrell, O.; Martínez-Sánchez, C.E. Dietary Fibre and Antioxidant Compounds in Passion Fruit (Passiflora Edulis f. Flavicarpa) Peel and Depectinised Peel Waste. Int. J. Food Sci. Technol. 2015, 50, 268–274. [Google Scholar] [CrossRef]
- Silva, E.C.O.d.; Silva, W.P.d.; Silva, E.T.d.; Lopes, J.D.; Gusmão, R.P. de Obtenção e Caracterização Da Farinha Do Albedo de Maracujá (Passiflora Edulis f. Flavicarpa) Para Uso Alimentício. Rev. Verde Agroecol. e Desenvolv. Sustentável 2016, 11, 69. [Google Scholar] [CrossRef]
- Bussolo de Souza, C.; Jonathan, M.; Isay Saad, S.M.; Schols, H.A.; Venema, K. Characterization and in Vitro Digestibility of Byproducts from Brazilian Food Industry: Cassava Bagasse, Orange Bagasse and Passion Fruit Peel. Bioact. Carbohydr. Diet. Fibre 2018, 16, 90–99. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. ATR-FTIR Spectroscopy to Determine Cell Wall Composition: Application on a Large Diversity of Fruits and Vegetables. Carbohydr. Polym. 2019, 212, 186–196. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the Use of Banana Peels for Oil Spill Removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier Transform Infrared (FTIR ) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Flores-morales, A.; Jiménez-estrada, M.; Mora-escobedo, R. Determination of the Structural Changes by FT-IR, Raman, and CP/MAS 13 C NMR Spectroscopy on Retrograded Starch of Maize Tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of Pectin Extraction from Banana Peels with Citric Acid by Using Response Surface Methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Umoren, S.A.; Obot, I.B.; Madhankumar, A.; Gasem, Z.M. Performance Evaluation of Pectin as Ecofriendly Corrosion Inhibitor for X60 Pipeline Steel in Acid Medium: Experimental and Theoretical Approaches. Carbohydr. Polym. 2015, 124, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Quênia, P.; Bezerra, M.; Filgueiras, M.; De Matos, R.; Graça, I.; Magalhães-guedes, K.T.; Izabel, J.; Alberto, J.; Costa, V. Innovative Functional Nanodispersion: Combination of Carotenoid from Spirulina and Yellow Passion Fruit Albedo. Food Chem. 2019, 285, 397–405. [Google Scholar] [CrossRef]
- Montalvo Andia, J.; Larrea, A.; Salcedo, J.; Reyes, J.; Lopez, L.; Yokoyama, L. Synthesis and Characterization of Chemically Activated Carbon from Passiflora Ligularis, Inga Feuilleei and Native Plants of South America. J. Environ. Chem. Eng. 2020, 8, 103892. [Google Scholar] [CrossRef]
- Pelissari, F.M.; do Amaral Sobral, P.J.; Menegalli, F.C. Isolation and Characterization of Cellulose Nanofibers from Banana Peels. Cellulose 2014, 21, 417–432. [Google Scholar] [CrossRef]
- Chao, H.P.; Chang, C.C.; Nieva, A. Biosorption of Heavy Metals on Citrus Maxima Peel, Passion Fruit Shell, and Sugarcane Bagasse in a Fixed-Bed Column. J. Ind. Eng. Chem. 2014, 20, 3408–3414. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Saeid Hosseini, S.; Hojjati, M. Pistachio Green Hull Pectin: Optimization of Microwave-Assisted Extraction and Evaluation of Its Physicochemical, Structural and Functional Properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef]
- da Sliva Alves, P.L.; Ascheri, J.L.R. Análise Por Imagem e Microscopia Electrônica Por Varredura Das Farinhas Extrudadas de Arroz e Maracujá. Hig. Aliment 2016, 30, 144–148. [Google Scholar]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential Ultrasound-Microwave Assisted Acid Extraction (UMAE) of Pectin from Pomelo Peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-Chemical Properties of Commercial Fibres from Different Sources: A Comparative Approach. Food Res. Int. 2009, 42, 176–184. [Google Scholar] [CrossRef]
- Leoro, M.G.V.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Evaluation of the in Vitro Glycemic Index of a Fiber-Rich Extruded Breakfast Cereal Produced with Organic Passion Fruit Fiber and Corn Flour. Ciência e Tecnol. Aliment. 2010, 30, 964–968. [Google Scholar] [CrossRef]
- Coelho, E.M.; de Azevêdo, L.C.; Viana, A.C.; Ramos, I.G.; Gomes, R.G.; dos S Lima, M.; Umsza-Guez, M.A. Physicochemical Properties, Rheology and Degree of Esterification of Passion Fruit (Passiflora edulis f. flavicarpa) Peel Flour. J. Sci. Food Agric. 2018, 98, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.F.; Santos, J.F.N.; de Lima, M.A.; Pinto, L.Í.F.; Borges, J.M.; Silva, R.A. da Análise Dos Parâmetros Físico-Químicos e de Composição Centesimal Da Farinha Do Bagaço Da Cana-de-Açúcar (Saccharum officinarum L.). Res. Soc. Dev. 2020, 9, e773974689. [Google Scholar] [CrossRef]
- Brito, T.B.; Carrajola, J.F.; Gonçalves, E.C.B.A.; Martelli-Tosi, M.; Ferreira, M.S.L. Fruit and Vegetable Residues Flours with Different Granulometry Range as Raw Material for Pectin-Enriched Biodegradable Film Preparation. Food Res. Int. 2019, 121, 412–421. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Schmidt, E.M.; Bonafe, E.G.; Eberlin, M.N.; Sawaya, A.C.H.F.; Visentainer, J.V. Antioxidant Activity, Phenolics and UPLC-ESI(-)-MS of Extracts from Different Tropical Fruits Parts and Processed Peels. Food Res. Int. 2015, 77, 392–399. [Google Scholar] [CrossRef]
- Do Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; De Carvalho, C.W.P.; Cárcel, J.A. Effects of High-Intensity Ultrasound on Drying Kinetics and Antioxidant Properties of Passion Fruit Peel. J. Food Eng. 2016, 170, 108–118. [Google Scholar] [CrossRef]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic Compound Contents and Antioxidant Activity in Plants with Nutritional and/or Medicinal Properties from the Peruvian Andean Region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-álvarez, J.A.; Viuda-martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Arquelau, P.; Silva, M.; Augusti, R.; Melo, J.; Fante, C. Use of Paper Spray-Mass Spectrometry to Determine the Chemical Profile of Ripe Banana Peel Flour and Evaluation of Its Physicochemical and Antioxidant Properties. Quim. Nova 2020, 43, 579–585. [Google Scholar] [CrossRef]
- Dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora Edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Hartanto, S.; Lister, I.N.E.; Fachrial, E. A Comparative Study of Peel and Seed Extract of Passion Fruit (Passiflora Edulis) as Anti Collagenase. Am. Sci. Res. J. Eng. Technol. Sci. 2019, 54, 42–48. [Google Scholar]
- Chutia, H.; Mahanta, C.L. Green Ultrasound and Microwave Extraction of Carotenoids from Passion Fruit Peel Using Vegetable Oils as a Solvent: Optimization, Comparison, Kinetics, and Thermodynamic Studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Elik, A.; Koçak, D.; Gö, F. Microwave-Assisted Extraction of Carotenoids from Carrot Juice Processing Waste Using Fl Axseed Oil as a Solvent. LWT-Food Sci. Technol. 2020, 123. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids Bioavailability from Foods: From Plant Pigments to Efficient Biological Activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Gadioli, I.L.; da Cunha, M.d.S.B.; de Carvalho, M.V.O.; Costa, A.M.; de Oliveira Pineli, L.d.L. A Systematic Review on Phenolic Compounds in Passiflora Plants: Exploring Biodiversity for Food, Nutrition, and Popular Medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Konta, E.M.; Almeida, M.R.; Lira, C. Evaluation of the Antihypertensive Properties of Yellow Passion Fruit Pulp (Passiflora Edulis Sims f. Flavicarpa Deg.) in Spontaneously Hypertensive Rats. Phyther. Res. 2014, 32, 28–32. [Google Scholar] [CrossRef]
- Abu-reidah, I.M.; Ali-shtayeh, M.S.; Jamous, R.M.; Arráez-román, D.; Segura-carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus Coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and Characterization of Phenolic Compounds in Hydromethanolic Extracts of Sorghum Wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of Chlorogenic Acids and Related Cinnamic Acid Derivatives from Nicotiana Tabacum Tissues with the Aid of UPLC-QTOF-MS/MS Based on the in-Source Collision-Induced Dissociation Method. Chem. Cent. J. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Guo, Y.; Gu, Z.; Liu, X.; Liu, J.; Ma, M.; Chen, B.; Wang, L. Rapid Analysis of Corni Fructus Using Paper Spray-Mass Spectrometry. Phytochem. Anal. 2017, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R.; Schmidt, T.J. Analysis of Chemical Constituents of Melastoma Dodecandrum Lour. By UPLC-ESI-Q-Exactive Focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, X.; Chantapakul, T.; Wang, D.; Zhang, S.; Ma, X.; Ding, T.; Ye, X.; Liu, D. Acoustic Cavitation Assisted Extraction of Pectin from Waste Grapefruit Peels: A Green Two-Stage Approach and Its General Mechanism. Food Res. Int. 2017, 102, 101–110. [Google Scholar] [CrossRef]
- Otify, A.; George, C.; Elsayed, A.; Farag, M.A. Function Mechanistic Evidence of Passiflora Edulis (Passifloraceae) Anxiolytic Activity in Relation to Its Metabolite Fi Ngerprint as Revealed via LC-MS And. Food Funct. 2015, 6, 3807–3817. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Manap, N.W.A.; Bhuyar, P.; Azelee, N.I.W. Passion Fruit (Passiflora Edulis) Peel Powder Extract and Its Application towards Antibacterial and Antioxidant Activity on the Preserved Meat Products. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- da Veiga Correia, V.T.; D’Angelis, D.F.; Macedo, M.C.C.; Ramos, A.L.C.C.; Vieira, A.L.S.; Queiroz, V.A.V.; Augusti, R.; Ferreira, A.A.; Fante, C.A.; Melo, J.O.F. Perfil Químico Da Farinha Extrusada de Sorgo Do Genótipo BRS 305 Por Paper Spray. Res. Soc. Dev. 2021, 10, e40710111414. [Google Scholar] [CrossRef]
- Jia, C.; Zhu, A.Y.; Zhang, J.; Yang, J.; Xu, C.; Mao, D. Identification of Glycoside Compounds from Tobacco by High Performance Liquid Chromatography/Electrospray Ionization Linear Ion-Trap Tandem Mass Spectrometry Coupled with Electrospray Ionization Orbitrap Mass Spectrometry. J. Braz. Chem. Soc. 2017, 28, 629–640. [Google Scholar] [CrossRef]



| Components | Content * (%) |
|---|---|
| Lipid | 1.24 ± 0.20 |
| Protein | 2.13 ± 0.16 |
| Ash | 7.28 ± 0.11 |
| Total carbohydrates | 89.33 ± 2.66 |
| Starch | 3.15 ± 0.02 |
| Compound | PFPF Content * |
|---|---|
| Gallic acid | 12.37 ± 0.01 |
| Caffeic acid | 2.32 ± 0.02 |
| Ellagic acid | 0.61 ± 0.02 |
| Quercetin | 0.34 ± 0.01 |
| Chlorogenic acid | ND |
| Catechin | ND |
| Identification | m/z | MS/MS | Reference |
|---|---|---|---|
| Organic acid | |||
| Malic acid | 133 | 115 | [56,65] |
| Phenolics acid | |||
| Caffeic acid | 179 | 135 | [66] |
| Caftaric acid | 311 | 133 | [65] |
| Chlorogenic acid | 353 | 191 | [67] |
| 3,5-Di-O-caffeoylquinic acid methyl ester | 535 | 445 | [56] |
| Phenylpropanoid Glycerides | |||
| 1-O-dihydrocaffeoyl glycerol | 255 | 163 | [56,66] |
| Sugar | |||
| Hexose | 215 | 89, 179 | [56,68] |
| Flavonoids | |||
| Luteolin-7-glucoside (flavone) | 447 | 227 | [69] |
| Kaempferol-3-O-rutinoside | 593 | 285, 447 | [56,70] |
| Rutin (quercetin 3-rutinoside) | 609 | 341 | [56,70] |
| Fatty acids | |||
| Oleic acid | 281 | 237 | [69] |
| Stearic acid | 283 | 265 | [69] |
| Quinone | |||
| 4,9-Dihydroxy-6,7-dimethoxynaphtho(2,3-d)-1,3-dioxole-5,8-dione | 293 | 249 | [69] |
| Tentative Identification | m/z | MS/MS | Reference |
|---|---|---|---|
| Flavonoids | |||
| Diosmetin | 301 | 258 | [71] |
| 3-O-methylquercetin | 317 | 245, 273, 247 | [73] |
| quercetin-3-malonylglucoside | 551 | 303 | [66] |
| Sugars | |||
| Sucrose | 381 | 219 | [21] |
| Morroniside | 429 | 267 | [74] |
| Fatty acid | |||
| Cnidioside Methyl Ester | 413 | 413 | [74] |
| Terpene | |||
| Deacetylforskolin | 369 | 253 | [64] |
| Vomifoliol β-D-glucopyranoside | 409 | 394, 391, 247 | [74] |
| Amino acids | |||
| L-arginin | 175 | 129 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, M.C.C.; Correia, V.T.d.V.; Silva, V.D.M.; Pereira, D.T.V.; Augusti, R.; Melo, J.O.F.; Pires, C.V.; de Paula, A.C.C.F.F.; Fante, C.A. Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. flavicarpa). Metabolites 2023, 13, 684. https://doi.org/10.3390/metabo13060684
Macedo MCC, Correia VTdV, Silva VDM, Pereira DTV, Augusti R, Melo JOF, Pires CV, de Paula ACCFF, Fante CA. Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. flavicarpa). Metabolites. 2023; 13(6):684. https://doi.org/10.3390/metabo13060684
Chicago/Turabian StyleMacedo, Maria Clara Coutinho, Vinícius Tadeu da Veiga Correia, Viviane Dias Medeiros Silva, Débora Tamires Vitor Pereira, Rodinei Augusti, Júlio Onésio Ferreira Melo, Christiano Vieira Pires, Ana Cardoso Clemente Filha Ferreira de Paula, and Camila Argenta Fante. 2023. "Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. flavicarpa)" Metabolites 13, no. 6: 684. https://doi.org/10.3390/metabo13060684
APA StyleMacedo, M. C. C., Correia, V. T. d. V., Silva, V. D. M., Pereira, D. T. V., Augusti, R., Melo, J. O. F., Pires, C. V., de Paula, A. C. C. F. F., & Fante, C. A. (2023). Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. flavicarpa). Metabolites, 13(6), 684. https://doi.org/10.3390/metabo13060684








