Mass Spectrometry Detects Sphingolipid Metabolites for Discovery of New Strategy for Cancer Therapy from the Aspect of Programmed Cell Death
Abstract
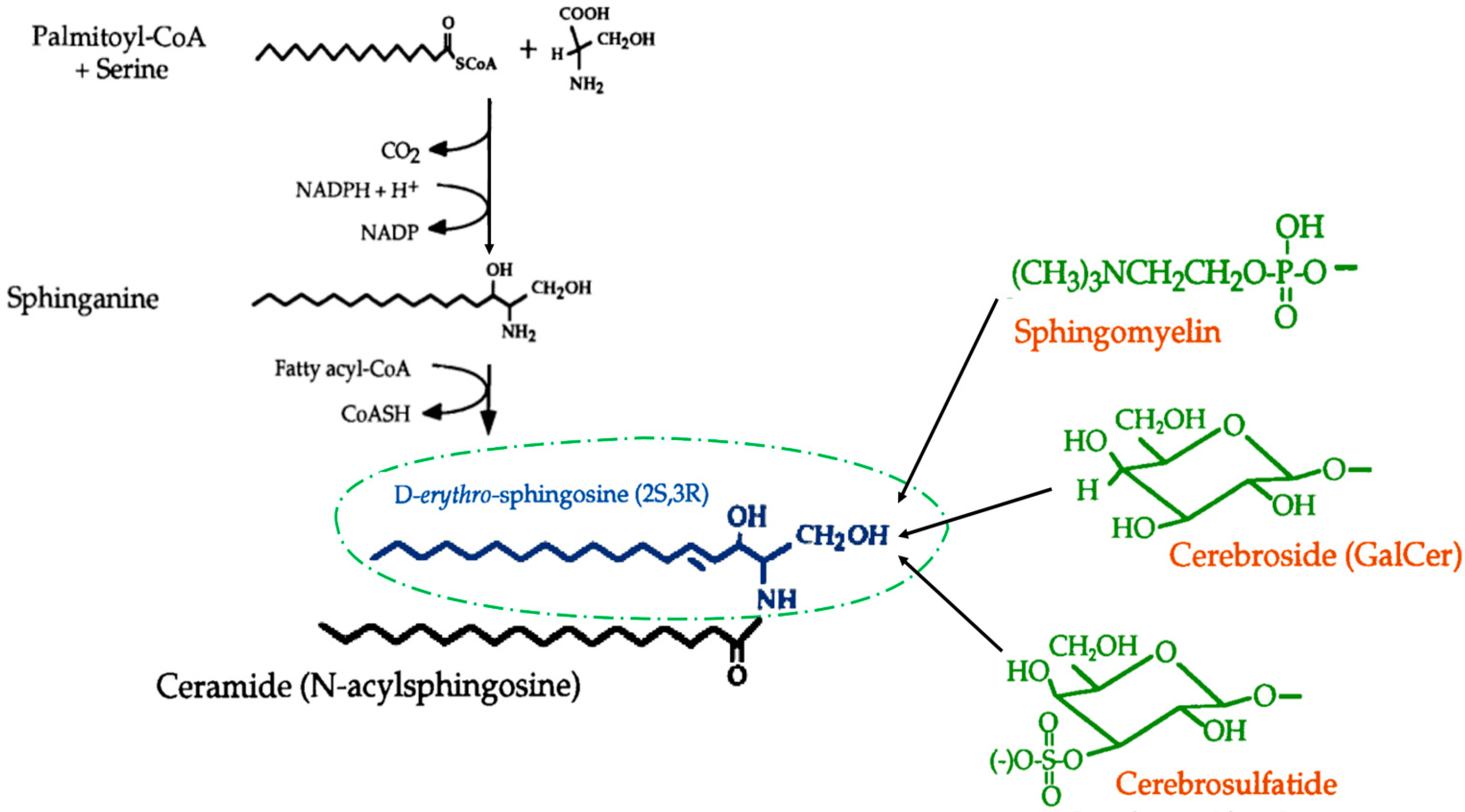
1. Introduction
2. Mass Spectrometry Detected Sphingolipid Biomarkers for Carcinoma
2.1. Electron Ionization Mass Spectrometry
2.2. Secondary Ion Mass Spectrometry
2.3. Electrospray Ionization Mass Spectrometry
2.4. Liquid Chromatography and Electrospray Ionization Mass Spectrometry
2.5. Gas Chromatography Mass Spectrometry
2.6. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
2.7. Matrix-Assisted Laser Desorption Ionization Mass Spectrometry
3. Sphingolipid Metabolism and Programmed Cell Death
3.1. Apoptosis
3.2. Autophagy
3.3. Necroptosis
3.4. Pyroptosis
3.5. Ferroptosis and Cuproptosis
| Mass Spectrometry | Advantages, Disadvantages, and Characteristics | Sphingolipids | Ref. |
|---|---|---|---|
| LC-MS, HPLC-ESI-MS | Detect and analyze a wide variety of sphingolipids metabolites | Ceramide | [40,56,132,133] |
| FTICR-MS, HPLC-ESI-MS | Detect and analyze a wide variety of sphingolipids metabolites | Sphingosine | [40,56] |
| FTICR-MS, LC-MS | Remove the interference of isomers and isotope lipids in sphingolipid analysis | Sphingomyelin | [56] |
| FTICR-MS with LC-MS | Reduce false positive interference and improve detection sensitivity | Dihydroceramide | [134,135] |
| LC-MS | Reduce the interference of isomers, improve the accuracy and sensitivity | Glucosylceramide | [136,137] |
| LC-MS/MS | Reduce the interference of isomers, improve the accuracy and sensitivity | Sphingosine-1-phosphate | [138] |
| LC-MS | Reduce the interference of isomers, improve the accuracy and sensitivity | Phosphatidylinositol | [139] |
| Cell Death Pathway | Sphingolipid | Morphological Feature | Function | Ref. |
|---|---|---|---|---|
| Apoptosis | Ceramide, sphingomyelinase phosphate | Induces the extrinsic pathways of the apoptosis pathway | Adjusted, amplified the signal | [64,65,66,67] |
| Ceramide | Induces the mitochondrial intrinsic apoptosis pathway | Induced | [140] | |
| Dihydroceramide | Interferes with the formation of ceramide channels in mitochondria, significantly reduces the permeability of the outer mitochondrial membrane, and inhibits ceramide-induced apoptosis | Inhibited | [141] | |
| Ceramide synthase 6 (CerS6), C16-Ceramide | Regulates activation of ER stress response | Inhibited | [89] | |
| Glucocerebroside; glycosphingolipid, (GSL); Glucosylceramide Synthase (GCS) | Induces mitochondrial intrinsic apoptosis pathway | Anti- Apoptosis | [82] | |
| S1P | Activates ERK and other signaling pathways | Anti- Apoptosis | [83,84,85] | |
| S1P | Activates pro-apoptotic Bcl-2 proteins Bak and Bax | Pro- apoptosis | [86] | |
| Autophagy | Ceramide | Downregulates nutrient transporters | Promoted | [102,104,105] |
| Induces the release of Beclin-1 by dissociation of the Beclin-1/Bcl-2 complex | Induced, promoted | [101] | ||
| Mediates autophagosomes directly anchor to mitochondria | Promoted | [108] | ||
| Reduces mitochondrial membrane potential and activates BNIP3 transcription | Induced | [107] | ||
| Dihydroceramide desaturase 1, DES1 | ATP synthesis damage activates Ampk, activating unc-51-like kinases to cause autophagosome formation | Promoted | [142] | |
| Sphingomyelin, sphingomyelinase, ceramide kinase, ceramide-1-phosphate | Calcium-dependent liposome fusion, which regulates the fusion of autophagosomes and lysosomes | Promoted | [110] | |
| Sphingosine-1-phosphate phosphohydrolase-1 (SPP1) | Deletion of SPP1 increases the expression of transcriptional regulators C/EBP homologous protein and Grp78/BiP, as well as phosphorylation of eukaryotic translation initiation factor-2 α (eIF2α), induces ER stress. | Induced | [143] | |
| Necroptosis | Ceramide | Mediates MLKL repositioning into lipid rafts | Induced | [115] |
| Ceramide–RIPK1 complex formation leads to disruption of lipid bilayer integrity | Induced | [116] | ||
| Pyroptosis | Sphingosine, SPHK1 | Activates NLRP3 and oligomeric RLRP3 inflammasomes | Induced | [118] |
| Ferroptosis | Ceramide; acid sphingomyelinase, aSMase | Ceramide enrichment membrane plateau formation induces GPX4 autophagy degradation | Induced, promoted | [124] |
| Inhibitor/Drug Name | Target Sphingolipids | Related PCD | Cancer Relevance | Trial Phase | Ref. |
|---|---|---|---|---|---|
| Myriocin | De novo ceramide synthesis | Apoptosis | Increased activity in response to chemotherapy and radiotherapy in breast cancer cells | [144,145,146] | |
| FB1; HDAC1 or HDAC2 | Synthesis of C18 (dihydro)ceramide | Autophagy | Induces mitophagy in head and neck and AML cell lines, mouse xenograft models, and patient-derived AML cells | Preclinical | [147,148,149] |
| FB1 | Synthesis of C16 (dihydro)ceramide | Apoptosis—mitochondria | Induces caspase activation and cell death in lung cancer cells; preserves ER and Golgi integrity in head and neck cancer cells; elevated in breast tumour tissues; protects from GVHD in a mouse model of leukaemia | Preclinical | [89,150,151,152,153,154,155,156] |
| Fenretinide; ABC294640; C8-CPC | Ceramide synthesis | Apoptosis | Induces cell cycle arrest in neuroblastoma cells | Preclinical | [157] |
| Tri-cyclic anti-depressants | Ceramide generation | Apoptosis | Induces apoptosis in lymphoblasts; promotes haematogenous tumour metastasis in mouse models | [158,159,160] | |
| GW4869 | Ceramide generation | Apoptosis | Mediates cell cycle arrest in breast cancer cells; exosome release | [161,162,163] | |
| THI | S1P breakdown | Apoptosis | Induces ceramide accumulation and colon cancer cell death | [164,165] | |
| CHC | Ceramide transport from ER to Golgi | Autophagy | Inhibits pro-apoptotic ceramide signalling in breast cancer cells and tumours in mouse models | Preclinical | [166,167,168] |
| NVP-231 | C1P generation | Apoptosis | Induces breast cancer cell survival in culture and mouse models | Preclinical | [169,170,171] |
| PPMP; PDMP | GlcCer synthesis | Apoptosis—mitochondria | Mediates drug resistance in patients with oral cancer and in breast cancer cells and xenografts | [172,173,174,175,176] | |
| LCL-521 | Ceramide cleavage | Apoptosis—mitochondria | Mediates resistance to cell death in prostate cancer cells and xenografts and elevated in tumours from patients | Preclinical | [177,178,179,180] |
| PF543 | S1P generation | Apoptosis | Mediates pro-survival signalling and metastasis in bladder cancer, lung cancer, and melanoma cells in culture and in mouse models | Preclinical | [181,182,183] |
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Breslow, D.K. Sphingolipid homeostasis in the endoplasmic reticulum and beyond. Cold Spring Harb. Perspect. Biol. 2013, 5, a013326. [Google Scholar] [CrossRef] [PubMed]
- Zahumensky, J.; Mota Fernandes, C.; Vesela, P.; Del Poeta, M.; Konopka, J.B.; Malinsky, J. Microdomain Protein Nce102 Is a Local Sensor of Plasma Membrane Sphingolipid Balance. Microbiol. Spectr. 2022, 10, e0196122. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Merrill, A.H., Jr.; Liotta, D.C.; Riley, R.T. Fumonisins: Fungal toxins that shed light on sphingolipid function. Trends Cell Biol. 1996, 6, 218–223. [Google Scholar] [CrossRef]
- Jiang, Y.; DiVittore, N.A.; Young, M.M.; Jia, Z.; Xie, K.; Ritty, T.M.; Kester, M.; Fox, T.E. Altered sphingolipid metabolism in patients with metastatic pancreatic cancer. Biomolecules 2013, 3, 435–448. [Google Scholar] [CrossRef]
- Faedo, R.R.; da Silva, G.; da Silva, R.M.; Ushida, T.R.; da Silva, R.R.; Lacchini, R.; Matos, L.L.; Kowalski, L.P.; Lopes, N.P.; Leopoldino, A.M. Sphingolipids signature in plasma and tissue as diagnostic and prognostic tools in oral squamous cell carcinoma. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159057. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, B.; Bhadwal, P.; Sharma, P.; Agnihotri, N. Chapter 3—Lipids as nutraceuticals: A shift in paradigm In Therapeutic Foods; Academic Press: Cambridge, MA, USA, 2018; p. 51. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; He, M.; Wang, Y.; Zhao, X.; He, Y.; Shi, B. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp. Ther. Med. 2019, 18, 3603–3614. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suzuki, M.; Ito, E.; Goto-Inoue, N.; Miseki, K.; Iida, J.; Yamazaki, Y.; Yamada, M.; Suzuki, A. Convenient structural analysis of glycosphingolipids using MALDI-QIT-TOF mass spectrometry with increased laser power and cooling gas flow. J. Biochem. 2006, 139, 771–777. [Google Scholar] [CrossRef]
- Chaurasia, B.; Ying, L.; Talbot, C.L.; Maschek, J.A.; Cox, J.; Schuchman, E.H.; Hirabayashi, Y.; Holland, W.L.; Summers, S.A. Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes. Mol. Metab. 2021, 45, 101145. [Google Scholar] [CrossRef] [PubMed]
- Fenger, M.; Linneberg, A.; Jeppesen, J. Network-based analysis of the sphingolipid metabolism in hypertension. Front. Genet. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Saba, J.D. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: The fat’s in the fire. Transl. Cancer Res. 2015, 4, 469–483. [Google Scholar] [CrossRef]
- De Wit, N.M.; Mol, K.; Rodriguez-Lorenzo, S.; de Vries, H.E.; Kooij, G. The Role of Sphingolipids and Specialized Pro-Resolving Mediators in Alzheimer’s Disease. Front. Immunol. 2020, 11, 620348. [Google Scholar] [CrossRef]
- Mingo-Casas, P.; Sanchez-Cespedes, J.; Blazquez, A.B.; Casas, J.; Balsera-Manzanero, M.; Herrero, L.; Vazquez, A.; Pachon, J.; Aguilar-Guisado, M.; Cisneros, J.M.; et al. Lipid signatures of West Nile virus infection unveil alterations of sphingolipid metabolism providing novel biomarkers. Emerg. Microbes Infect. 2023, 12, 2231556. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Wang, H.R.; Bai, Y.F.; Sun, J.P.; Wang, W.S.; Xu, Y.; Zhang, M.S.; Liu, J. Substance P promotes epidural fibrosis via induction of type 2 macrophages. Neural Regen. Res. 2023, 18, 2252–2259. [Google Scholar] [CrossRef]
- Ejsing, C.S.; Moehring, T.; Bahr, U.; Duchoslav, E.; Karas, M.; Simons, K.; Shevchenko, A. Collision-induced dissociation pathways of yeast sphingolipids and their molecular profiling in total lipid extracts: A study by quadrupole TOF and linear ion trap-orbitrap mass spectrometry. J. Mass. Spectrom. 2006, 41, 372–389. [Google Scholar] [CrossRef]
- Haynes, C.A.; Allegood, J.C.; Park, H.; Sullards, M.C. Sphingolipidomics: Methods for the comprehensive analysis of sphingolipids. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2696–2708. [Google Scholar] [CrossRef]
- Capolupo, L.; Khven, I.; Lederer, A.R.; Mazzeo, L.; Glousker, G.; Ho, S.; Russo, F.; Montoya, J.P.; Bhandari, D.R.; Bowman, A.P.; et al. Sphingolipids control dermal fibroblast heterogeneity. Science 2022, 376, eabh1623. [Google Scholar] [CrossRef]
- Poisson, L.M.; Suhail, H.; Singh, J.; Datta, I.; Denic, A.; Labuzek, K.; Hoda, M.N.; Shankar, A.; Kumar, A.; Cerghet, M.; et al. Untargeted Plasma Metabolomics Identifies Endogenous Metabolite with Drug-like Properties in Chronic Animal Model of Multiple Sclerosis. J. Biol. Chem. 2015, 290, 30697–30712. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Qi, D.; Huo, H.; Dong, X.; Tian, L.; Liu, C.; Cao, Y. Metabolomic and transcriptomic analyses highlight the influence of lipid changes on the post-harvest softening of Pyrus ussurian Max. ‘Zaoshu Shanli’. Genomics 2021, 113, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ruan, Q.; Li, Y.; Ye, G.; Lu, X.; Lin, X.; Xu, G. A novel approach to transforming a non-targeted metabolic profiling method to a pseudo-targeted method using the retention time locking gas chromatography/mass spectrometry-selected ions monitoring. J. Chromatogr. A 2012, 1255, 228–236. [Google Scholar] [CrossRef]
- Zahoor, I.; Suhail, H.; Datta, I.; Ahmed, M.E.; Poisson, L.M.; Waters, J.; Rashid, F.; Bin, R.; Singh, J.; Cerghet, M.; et al. Blood-based untargeted metabolomics in relapsing-remitting multiple sclerosis revealed the testable therapeutic target. Proc. Natl. Acad. Sci. USA 2022, 119, e2123265119. [Google Scholar] [CrossRef]
- Petit, C.S.; Lee, J.J.; Boland, S.; Swarup, S.; Christiano, R.; Lai, Z.W.; Mejhert, N.; Elliott, S.D.; McFall, D.; Haque, S.; et al. Inhibition of sphingolipid synthesis improves outcomes and survival in GARP mutant wobbler mice, a model of motor neuron degeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 10565–10574. [Google Scholar] [CrossRef]
- Chen, S.; Kong, H.; Lu, X.; Li, Y.; Yin, P.; Zeng, Z.; Xu, G. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal. Chem. 2013, 85, 8326–8333. [Google Scholar] [CrossRef]
- Lv, W.; Wang, L.; Xuan, Q.; Zhao, X.; Liu, X.; Shi, X.; Xu, G. Pseudotargeted Method Based on Parallel Column Two-Dimensional Liquid Chromatography-Mass Spectrometry for Broad Coverage of Metabolome and Lipidome. Anal. Chem. 2020, 92, 6043–6050. [Google Scholar] [CrossRef]
- Vargas Medina, D.A.; Burato, J.; Borsatto, J.V.B.; Lancas, F.M. Porous layer open tubular nano liquid chromatography directly coupled to electron ionization mass spectrometry. J. Chromatogr. A 2022, 1674, 463143. [Google Scholar] [CrossRef] [PubMed]
- Hammarstrom, S.; Samuelsson, B. On the biosynthesis of cerebrosides from 2-hydroxy acid ceramides: Use of deuterium labeled substrate and multiple ion detector. Biochem. Biophys. Res. Commun. 1970, 41, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, K.; Sameulsson, B. Gas chromatographic and mass spectrometric studies of synthetic and naturally occurring ceramides. Chem. Phys. Lipids 1970, 5, 44–79. [Google Scholar] [CrossRef]
- Samuelsson, B.; Samuelsson, K. Gas--liquid chromatography-mass spectrometry of synthetic ceramides. J. Lipid Res. 1969, 10, 41–46. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Tanaka, W.; Senoo, Y.; Arita, M.; Arita, M. Comprehensive identification of sphingolipid species by in silico retention time and tandem mass spectral library. J. Cheminform. 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Verkhoturov, D.S.; Crulhas, B.P.; Eller, M.J.; Han, Y.D.; Verkhoturov, S.V.; Bisrat, Y.; Revzin, A.; Schweikert, E.A. Nanoprojectile Secondary Ion Mass Spectrometry for Analysis of Extracellular Vesicles. Anal. Chem. 2021, 93, 7481–7490. [Google Scholar] [CrossRef] [PubMed]
- Aldossari, S.; McMahon, G.; Lockyer, N.P.; Moore, K.L. Microdistribution and quantification of the boron neutron capture therapy drug BPA in primary cell cultures of human glioblastoma tumour by NanoSIMS. Analyst 2019, 144, 6214–6224. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D. The state of the art in thin-layer chromatography-mass spectrometry: A critical appraisal. J. Chromatogr. A 1999, 856, 429–442. [Google Scholar] [CrossRef]
- Cahoon, R.E.; Solis, A.G.; Markham, J.E.; Cahoon, E.B. Mass Spectrometry-Based Profiling of Plant Sphingolipids from Typical and Aberrant Metabolism. Methods Mol. Biol. 2021, 2295, 157–177. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, H.; Qian, C.; Li, H.; Chen, D.D.Y. Characterization of interaction between Bcl-2 oncogene promoter I-Motif DNA and flavonoids using electrospray ionization mass spectrometry and pressure-assisted capillary electrophoresis frontal analysis. Talanta 2020, 215, 120885. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Cheng, H.; Ye, H.; Gross, R.W. Toward fingerprinting cellular lipidomes directly from biological samples by two-dimensional electrospray ionization mass spectrometry. Anal. Biochem. 2004, 330, 317–331. [Google Scholar] [CrossRef]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Sullards, M.C. Analysis of sphingomyelin, glucosylceramide, ceramide, sphingosine, and sphingosine 1-phosphate by tandem mass spectrometry. Methods Enzymol. 2000, 312, 32–45. [Google Scholar] [CrossRef]
- Sullards, M.C.; Merrill, A.H., Jr. Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Sci. STKE 2001, 2001, pl1. [Google Scholar] [CrossRef]
- Sakai, E.; Kurano, M.; Morita, Y.; Aoki, J.; Yatomi, Y. Establishment of a Measurement System for Sphingolipids in the Cerebrospinal Fluid Based on Liquid Chromatography-Tandem Mass Spectrometry, and Its Application in the Diagnosis of Carcinomatous Meningitis. J. Appl. Lab. Med. 2020, 5, 656–670. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, X.; Shi, X.; Li, N.; Gao, S.; Yang, J.; Dong, X. Quantification of alpha-hydroxy ceramides in mice serum by LC-MS/MS: Application to sepsis study. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2023, 1225, 123764. [Google Scholar] [CrossRef] [PubMed]
- Ge, K.; Zheng, D.; Wang, J.; Jia, W.; Zhao, A. A method for quantifying hepatic and intestinal ceramides on mice by UPLC-MS/MS. Anal. Biochem. 2023, 661, 114982. [Google Scholar] [CrossRef] [PubMed]
- Pacetti, D.; Boselli, E.; Hulan, H.W.; Frega, N.G. High performance liquid chromatography-tandem mass spectrometry of phospholipid molecular species in eggs from hens fed diets enriched in seal blubber oil. J. Chromatogr. A 2005, 1097, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kaga, N.; Kazuno, S.; Taka, H.; Iwabuchi, K.; Murayama, K. Isolation and mass spectrometry characterization of molecular species of lactosylceramides using liquid chromatography-electrospray ion trap mass spectrometry. Anal. Biochem. 2005, 337, 316–324. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.H.; Yoo, J.S. Analysis of ceramides in cosmetics by reversed-phase liquid chromatography/electrospray ionization mass spectrometry with collision-induced dissociation. Rapid Commun. Mass. Spectrom. 2003, 17, 64–75. [Google Scholar] [CrossRef]
- Zanfini, A.; Dreassi, E.; Berardi, A.; Piomboni, P.; Costantino-Ceccarini, E.; Luddi, A. GC-EI-MS analysis of fatty acid composition in brain and serum of twitcher mouse. Lipids 2014, 49, 1115–1125. [Google Scholar] [CrossRef]
- Son, H.H.; Moon, J.Y.; Seo, H.S.; Kim, H.H.; Chung, B.C.; Choi, M.H. High-temperature GC-MS-based serum cholesterol signatures may reveal sex differences in vasospastic angina. J. Lipid Res. 2014, 55, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.G.; Hendrickson, C.L. High-resolution mass spectrometers. Annu. Rev. Anal. Chem. 2008, 1, 579–599. [Google Scholar] [CrossRef]
- McFarland, M.A.; Marshall, A.G.; Hendrickson, C.L.; Nilsson, C.L.; Fredman, P.; Mansson, J.E. Structural characterization of the GM1 ganglioside by infrared multiphoton dissociation, electron capture dissociation, and electron detachment dissociation electrospray ionization FT-ICR MS/MS. J. Am. Soc. Mass. Spectrom. 2005, 16, 752–762. [Google Scholar] [CrossRef]
- Jones, J.J.; Stump, M.J.; Fleming, R.C.; Lay, J.O., Jr.; Wilkins, C.L. Strategies and data analysis techniques for lipid and phospholipid chemistry elucidation by intact cell MALDI-FTMS. J. Am. Soc. Mass. Spectrom. 2004, 15, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Wan, L.; Xu, Z.; Haro, J.M.; Li, B.; Jones, J.W. Lithium Hydroxide Hydrolysis Combined with MALDI TOF Mass Spectrometry for Rapid Sphingolipid Detection. J. Am. Soc. Mass. Spectrom. 2021, 32, 289–300. [Google Scholar] [CrossRef]
- Zhao, C.; Xie, P.; Yong, T.; Wang, H.; Chung, A.C.K.; Cai, Z. MALDI-MS Imaging Reveals Asymmetric Spatial Distribution of Lipid Metabolites from Bisphenol S-Induced Nephrotoxicity. Anal. Chem. 2018, 90, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.P.; Bogie, J.F.J.; Hendriks, J.J.A.; Haidar, M.; Belov, M.; Heeren, R.M.A.; Ellis, S.R. Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation. Anal. Bioanal. Chem. 2020, 412, 2277–2289. [Google Scholar] [CrossRef]
- Haynes, C.A.; Allegood, J.C.; Sims, K.; Wang, E.W.; Sullards, M.C.; Merrill, A.H., Jr. Quantitation of fatty acyl-coenzyme As in mammalian cells by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Lipid Res. 2008, 49, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Slotte, J.P. Separation and purification of sphingomyelin diastereomers by high-performance liquid chromatography. Anal. Biochem. 2000, 282, 245–249. [Google Scholar] [CrossRef]
- Muthing, J.; Unland, F. Improved separation of isomeric gangliosides by anion-exchange high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 1994, 658, 39–45. [Google Scholar] [CrossRef]
- Hacker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–17. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Hengartner, M.O. Apoptosis: Corralling the corpses. Cell 2001, 104, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death receptors: Signaling and modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, C.R.; Teichgraber, V.; Gulbins, E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta 2005, 1746, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Grassme, H.; Cremesti, A.; Kolesnick, R.; Gulbins, E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 2003, 22, 5457–5470. [Google Scholar] [CrossRef] [PubMed]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Kolesnick, R. Raft ceramide in molecular medicine. Oncogene 2003, 22, 7070–7077. [Google Scholar] [CrossRef]
- Schutze, S.; Potthoff, K.; Machleidt, T.; Berkovic, D.; Wiegmann, K.; Kronke, M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 1992, 71, 765–776. [Google Scholar] [CrossRef]
- Wiegmann, K.; Schutze, S.; Machleidt, T.; Witte, D.; Kronke, M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell 1994, 78, 1005–1015. [Google Scholar] [CrossRef]
- Liu, P.; Anderson, R.G. Compartmentalized production of ceramide at the cell surface. J. Biol. Chem. 1995, 270, 27179–27185. [Google Scholar] [CrossRef]
- Cifone, M.G.; De Maria, R.; Roncaioli, P.; Rippo, M.R.; Azuma, M.; Lanier, L.L.; Santoni, A.; Testi, R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J. Exp. Med. 1994, 180, 1547–1552. [Google Scholar] [CrossRef]
- Patwardhan, G.A.; Beverly, L.J.; Siskind, L.J. Sphingolipids and mitochondrial apoptosis. J. Bioenerg. Biomembr. 2016, 48, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Moldoveanu, T.; Llambi, F.; Parsons, M.J.; Green, D.R. The BCL-2 family reunion. Mol. Cell 2010, 37, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Morosi, L.; Pozzi, C.; Forcella, M.; Tettamanti, G.; Venerando, B.; Monti, E.; Fusi, P. Human sialidase NEU4 long and short are extrinsic proteins bound to outer mitochondrial membrane and the endoplasmic reticulum, respectively. Glycobiology 2010, 20, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Bionda, C.; Portoukalian, J.; Schmitt, D.; Rodriguez-Lafrasse, C.; Ardail, D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 2004, 382, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Hata, K.; Koseki, K.; Shiozaki, K.; Akita, H.; Wada, T.; Moriya, S.; Miyagi, T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem. J. 2005, 390, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L., Jr.; Matsko, C.M.; Lotze, M.T.; Amoscato, A.A. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J. Biol. Chem. 1999, 274, 30580–30588. [Google Scholar] [CrossRef]
- Kroesen, B.J.; Pettus, B.; Luberto, C.; Busman, M.; Sietsma, H.; de Leij, L.; Hannun, Y.A. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J. Biol. Chem. 2001, 276, 13606–13614. [Google Scholar] [CrossRef]
- Rodriguez-Lafrasse, C.; Alphonse, G.; Broquet, P.; Aloy, M.T.; Louisot, P.; Rousson, R. Temporal relationships between ceramide production, caspase activation and mitochondrial dysfunction in cell lines with varying sensitivity to anti-Fas-induced apoptosis. Biochem. J. 2001, 357, 407–416. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Colell, A.; Mari, M.; Morales, A.; Fernandez-Checa, J.C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef]
- Geilen, C.C.; Bektas, M.; Wieder, T.; Kodelja, V.; Goerdt, S.; Orfanos, C.E. 1alpha,25-dihydroxyvitamin D3 induces sphingomyelin hydrolysis in HaCaT cells via tumor necrosis factor alpha. J. Biol. Chem. 1997, 272, 8997–9001. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, J.Y.; Yin, D.; Patwardhan, G.A.; Gupta, V.; Hirabayashi, Y.; Holleran, W.M.; Giuliano, A.E.; Jazwinski, S.M.; Gouaze-Andersson, V.; et al. A role for ceramide in driving cancer cell resistance to doxorubicin. FASEB J. 2008, 22, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.E.; Takabe, K.; Nagahashi, M.; Milstien, S.; Spiegel, S. Targeting sphingosine-1-phosphate in hematologic malignancies. Anticancer. Agents Med. Chem. 2011, 11, 794–798. [Google Scholar] [CrossRef]
- Takabe, K.; Spiegel, S. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 2014, 55, 1839–1846. [Google Scholar] [CrossRef]
- Chipuk, J.E.; McStay, G.P.; Bharti, A.; Kuwana, T.; Clarke, C.J.; Siskind, L.J.; Obeid, L.M.; Green, D.R. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 2012, 148, 988–1000. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Senkal, C.E.; Ponnusamy, S.; Manevich, Y.; Meyers-Needham, M.; Saddoughi, S.A.; Mukhopadyay, A.; Dent, P.; Bielawski, J.; Ogretmen, B. Alteration of ceramide synthase 6/C16-ceramide induces activating transcription factor 6-mediated endoplasmic reticulum (ER) stress and apoptosis via perturbation of cellular Ca2+ and ER/Golgi membrane network. J. Biol. Chem. 2011, 286, 42446–42458. [Google Scholar] [CrossRef] [PubMed]
- Mesicek, J.; Lee, H.; Feldman, T.; Jiang, X.; Skobeleva, A.; Berdyshev, E.V.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010, 22, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Spiess, C.; Meyer, A.S.; Reissmann, S.; Frydman, J. Mechanism of the eukaryotic chaperonin: Protein folding in the chamber of secrets. Trends Cell Biol. 2004, 14, 598–604. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Klionsky, D.J. Autophagosome formation: Core machinery and adaptations. Nat. Cell Biol. 2007, 9, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef]
- Jung, C.H.; Jun, C.B.; Ro, S.H.; Kim, Y.M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 2010, 22, 132–139. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Obara, K.; Ohsumi, Y. PtdIns 3-Kinase Orchestrates Autophagosome Formation in Yeast. J. Lipids 2011, 2011, 498768. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Pattingre, S.; Espert, L.; Biard-Piechaczyk, M.; Codogno, P. Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 2008, 90, 313–323. [Google Scholar] [CrossRef]
- Guenther, G.G.; Peralta, E.R.; Rosales, K.R.; Wong, S.Y.; Siskind, L.J.; Edinger, A.L. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 17402–17407. [Google Scholar] [CrossRef]
- Romero Rosales, K.; Singh, G.; Wu, K.; Chen, J.; Janes, M.R.; Lilly, M.B.; Peralta, E.R.; Siskind, L.J.; Bennett, M.J.; Fruman, D.A.; et al. Sphingolipid-based drugs selectively kill cancer cells by down-regulating nutrient transporter proteins. Biochem. J. 2011, 439, 299–311. [Google Scholar] [CrossRef]
- Peralta, E.R.; Edinger, A.L. Ceramide-induced starvation triggers homeostatic autophagy. Autophagy 2009, 5, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L. Starvation in the midst of plenty: Making sense of ceramide-induced autophagy by analysing nutrient transporter expression. Biochem. Soc. Trans. 2009, 37, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Scorrano, L. Mitochondrial morphology in mitophagy and macroautophagy. Biochim. Biophys. Acta 2013, 1833, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Daido, S.; Kanzawa, T.; Yamamoto, A.; Takeuchi, H.; Kondo, Y.; Kondo, S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004, 64, 4286–4293. [Google Scholar] [CrossRef]
- Sentelle, R.D.; Senkal, C.E.; Jiang, W.; Ponnusamy, S.; Gencer, S.; Selvam, S.P.; Ramshesh, V.K.; Peterson, Y.K.; Lemasters, J.J.; Szulc, Z.M.; et al. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 2012, 8, 831–838. [Google Scholar] [CrossRef]
- Russo, S.B.; Baicu, C.F.; Van Laer, A.; Geng, T.; Kasiganesan, H.; Zile, M.R.; Cowart, L.A. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Investig. 2012, 122, 3919–3930. [Google Scholar] [CrossRef]
- Hinkovska-Galcheva, V.T.; Boxer, L.A.; Mansfield, P.J.; Harsh, D.; Blackwood, A.; Shayman, J.A. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J. Biol. Chem. 1998, 273, 33203–33209. [Google Scholar] [CrossRef]
- Chen, J.; Kos, R.; Garssen, J.; Redegeld, F. Molecular Insights into the Mechanism of Necroptosis: The Necrosome As a Potential Therapeutic Target. Cells 2019, 8, 1486. [Google Scholar] [CrossRef]
- Dondelinger, Y.; Declercq, W.; Montessuit, S.; Roelandt, R.; Goncalves, A.; Bruggeman, I.; Hulpiau, P.; Weber, K.; Sehon, C.A.; Marquis, R.W.; et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014, 7, 971–981. [Google Scholar] [CrossRef]
- Quarato, G.; Guy, C.S.; Grace, C.R.; Llambi, F.; Nourse, A.; Rodriguez, D.A.; Wakefield, R.; Frase, S.; Moldoveanu, T.; Green, D.R. Sequential Engagement of Distinct MLKL Phosphatidylinositol-Binding Sites Executes Necroptosis. Mol. Cell 2016, 61, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Matsuda, M.; Yaegashi, N.; Nabe, T.; Kitatani, K. Regulation of Necroptosis by Phospholipids and Sphingolipids. Cells 2020, 9, 627. [Google Scholar] [CrossRef]
- Zhang, X.; Kitatani, K.; Toyoshima, M.; Ishibashi, M.; Usui, T.; Minato, J.; Egiz, M.; Shigeta, S.; Fox, T.; Deering, T.; et al. Ceramide Nanoliposomes as a MLKL-Dependent, Necroptosis-Inducing, Chemotherapeutic Reagent in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Nganga, R.; Oleinik, N.; Kim, J.; Selvam, S.P.; De Palma, R.; Johnson, K.A.; Parikh, R.Y.; Gangaraju, V.; Peterson, Y.; Dany, M.; et al. Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA-dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis. J. Biol. Chem. 2019, 294, 502–519. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Vanaja, S.K.; Fitzgerald, K.A. Regulation of inflammasome signaling. Nat. Immunol. 2012, 13, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Cai, W.Q.; Lu, Y. The Relationship of Sphingosine Kinase 1 With Pyroptosis Provides a New Strategy for Tumor Therapy. Front. Immunol. 2020, 11, 574990. [Google Scholar] [CrossRef]
- Taha, T.A.; El-Alwani, M.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase-1 is cleaved by cathepsin B in vitro: Identification of the initial cleavage sites for the protease. FEBS Lett. 2006, 580, 6047–6054. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Tumor suppressive functions of ceramide: Evidence and mechanisms. Apoptosis 2015, 20, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Thayyullathil, F.; Cheratta, A.R.; Alakkal, A.; Subburayan, K.; Pallichankandy, S.; Hannun, Y.A.; Galadari, S. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Edmunds, T.; Baker-Malcolm, J.; Karey, K.P.; Estes, S.; Schwarz, C.; Hughes, H.; Van Patten, S.M. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J. Biol. Chem. 2003, 278, 32744–32752. [Google Scholar] [CrossRef]
- Wu, Z.; Geng, Y.; Lu, X.; Shi, Y.; Wu, G.; Zhang, M.; Shan, B.; Pan, H.; Yuan, J. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Rana, S.V. Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol. 2008, 22, 262–284. [Google Scholar] [CrossRef]
- Lang, F.; Ullrich, S.; Gulbins, E. Ceramide formation as a target in beta-cell survival and function. Expert. Opin. Ther. Targets 2011, 15, 1061–1071. [Google Scholar] [CrossRef]
- Triebl, A.; Weissengruber, S.; Trotzmuller, M.; Lankmayr, E.; Kofeler, H. Quantitative analysis of N-acylphosphatidylethanolamine molecular species in rat brain using solid-phase extraction combined with reversed-phase chromatography and tandem mass spectrometry. J. Sep. Sci. 2016, 39, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB network: An emerging modulator of nervous system injury and repair. Prog. Neurobiol. 2019, 180, 101643. [Google Scholar] [CrossRef]
- Ivanova, P.T.; Cerda, B.A.; Horn, D.M.; Cohen, J.S.; McLafferty, F.W.; Brown, H.A. Electrospray ionization mass spectrometry analysis of changes in phospholipids in RBL-2H3 mastocytoma cells during degranulation. Proc. Natl. Acad. Sci. USA 2001, 98, 7152–7157. [Google Scholar] [CrossRef]
- Schuhmann, K.; Almeida, R.; Baumert, M.; Herzog, R.; Bornstein, S.R.; Shevchenko, A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J. Mass. Spectrom. 2012, 47, 96–104. [Google Scholar] [CrossRef]
- Yang, K.; Dilthey, B.G.; Gross, R.W. Identification and quantitation of fatty acid double bond positional isomers: A shotgun lipidomics approach using charge-switch derivatization. Anal. Chem. 2013, 85, 9742–9750. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rashid, K.; Ibrahim, K.; Wong, J.H.D.; Mohd Ramli, N. Lipid Alterations in Glioma: A Systematic Review. Metabolites 2022, 12, 1280. [Google Scholar] [CrossRef]
- Rosen, H.; Stevens, R.C.; Hanson, M.; Roberts, E.; Oldstone, M.B. Sphingosine-1-phosphate and its receptors: Structure, signaling, and influence. Annu. Rev. Biochem. 2013, 82, 637–662. [Google Scholar] [CrossRef] [PubMed]
- Kilaru, A.; Isaac, G.; Tamura, P.; Baxter, D.; Duncan, S.R.; Venables, B.J.; Welti, R.; Koulen, P.; Chapman, K.D. Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids 2010, 45, 863–875. [Google Scholar] [CrossRef]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef]
- Stiban, J.; Fistere, D.; Colombini, M. Dihydroceramide hinders ceramide channel formation: Implications on apoptosis. Apoptosis 2006, 11, 773–780. [Google Scholar] [CrossRef]
- Siddique, M.M.; Li, Y.; Wang, L.; Ching, J.; Mal, M.; Ilkayeva, O.; Wu, Y.J.; Bay, B.H.; Summers, S.A. Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol. Cell Biol. 2013, 33, 2353–2369. [Google Scholar] [CrossRef]
- Lepine, S.; Allegood, J.C.; Park, M.; Dent, P.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. 2011, 18, 350–361. [Google Scholar] [CrossRef]
- Han, G.; Gupta, S.D.; Gable, K.; Niranjanakumari, S.; Moitra, P.; Eichler, F.; Brown, R.H., Jr.; Harmon, J.M.; Dunn, T.M. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA 2009, 106, 8186–8191. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.; Bourquin, F.; Suriyanarayanan, S.; Wei, Y.; Alecu, I.; Othman, A.; Von Eckardstein, A.; Hornemann, T. HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum. Mol. Genet. 2016, 25, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.; Bielawski, J.; Kistner-Griffin, E.; Othman, A.; Alecu, I.; Ernst, D.; Kornhauser, D.; Hornemann, T.; Spassieva, S. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J. 2015, 29, 4461–4472. [Google Scholar] [CrossRef] [PubMed]
- Meyers-Needham, M.; Ponnusamy, S.; Gencer, S.; Jiang, W.; Thomas, R.J.; Senkal, C.E.; Ogretmen, B. Concerted functions of HDAC1 and microRNA-574-5p repress alternatively spliced ceramide synthase 1 expression in human cancer cells. EMBO Mol. Med. 2012, 4, 78–92. [Google Scholar] [CrossRef]
- Koybasi, S.; Senkal, C.E.; Sundararaj, K.; Spassieva, S.; Bielawski, J.; Osta, W.; Day, T.A.; Jiang, J.C.; Jazwinski, S.M.; Hannun, Y.A.; et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J. Biol. Chem. 2004, 279, 44311–44319. [Google Scholar] [CrossRef]
- Thomas, R.J.; Oleinik, N.; Panneer Selvam, S.; Vaena, S.G.; Dany, M.; Nganga, R.N.; Depalma, R.; Baron, K.D.; Kim, J.; Szulc, Z.M.; et al. HPV/E7 induces chemotherapy-mediated tumor suppression by ceramide-dependent mitophagy. EMBO Mol. Med. 2017, 9, 1030–1051. [Google Scholar] [CrossRef]
- Fekry, B.; Jeffries, K.A.; Esmaeilniakooshkghazi, A.; Ogretmen, B.; Krupenko, S.A.; Krupenko, N.I. CerS6 Is a Novel Transcriptional Target of p53 Protein Activated by Non-genotoxic Stress. J. Biol. Chem. 2016, 291, 16586–16596. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Mullen, T.; Senkal, C.; Lu, P.; Ogretmen, B.; Obeid, L.; Voelkel-Johnson, C. Ceramide synthase 6 modulates TRAIL sensitivity and nuclear translocation of active caspase-3 in colon cancer cells. Oncogene 2009, 28, 1132–1141. [Google Scholar] [CrossRef]
- Lee, H.; Rotolo, J.A.; Mesicek, J.; Penate-Medina, T.; Rimner, A.; Liao, W.C.; Yin, X.; Ragupathi, G.; Ehleiter, D.; Gulbins, E.; et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS ONE 2011, 6, e19783. [Google Scholar] [CrossRef]
- Jensen, S.A.; Calvert, A.E.; Volpert, G.; Kouri, F.M.; Hurley, L.A.; Luciano, J.P.; Wu, Y.; Chalastanis, A.; Futerman, A.H.; Stegh, A.H. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5682–5687. [Google Scholar] [CrossRef]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef]
- Schiffmann, S.; Sandner, J.; Birod, K.; Wobst, I.; Angioni, C.; Ruckhaberle, E.; Kaufmann, M.; Ackermann, H.; Lotsch, J.; Schmidt, H.; et al. Ceramide synthases and ceramide levels are increased in breast cancer tissue. Carcinogenesis 2009, 30, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.H.; Heinrichs, J.; Dany, M.; Nguyen, H.; Dai, M.; Bastian, D.; Schutt, S.; Wu, Y.; Daenthanasanmak, A.; Gencer, S.; et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight 2017, 2, e91701. [Google Scholar] [CrossRef] [PubMed]
- Rahmaniyan, M.; Curley, R.W., Jr.; Obeid, L.M.; Hannun, Y.A.; Kraveka, J.M. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 2011, 286, 24754–24764. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Illes, K.; Heinz, L.X.; Superti-Furga, G.; Nagar, B. Crystal structure of mammalian acid sphingomyelinase. Nat. Commun. 2016, 7, 12196. [Google Scholar] [CrossRef]
- Santana, P.; Pena, L.A.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef]
- Carpinteiro, A.; Becker, K.A.; Japtok, L.; Hessler, G.; Keitsch, S.; Pozgajova, M.; Schmid, K.W.; Adams, C.; Muller, S.; Kleuser, B.; et al. Regulation of hematogenous tumor metastasis by acid sphingomyelinase. EMBO Mol. Med. 2015, 7, 714–734. [Google Scholar] [CrossRef]
- Airola, M.V.; Shanbhogue, P.; Shamseddine, A.A.; Guja, K.E.; Senkal, C.E.; Maini, R.; Bartke, N.; Wu, B.X.; Obeid, L.M.; Garcia-Diaz, M.; et al. Structure of human nSMase2 reveals an interdomain allosteric activation mechanism for ceramide generation. Proc. Natl. Acad. Sci. USA 2017, 114, E5549–E5558. [Google Scholar] [CrossRef]
- Shamseddine, A.A.; Clarke, C.J.; Carroll, B.; Airola, M.V.; Mohammed, S.; Rella, A.; Obeid, L.M.; Hannun, Y.A. P53-dependent upregulation of neutral sphingomyelinase-2: Role in doxorubicin-induced growth arrest. Cell Death Dis. 2015, 6, e1947. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Degagne, E.; Pandurangan, A.; Bandhuvula, P.; Kumar, A.; Eltanawy, A.; Zhang, M.; Yoshinaga, Y.; Nefedov, M.; de Jong, P.J.; Fong, L.G.; et al. Sphingosine-1-phosphate lyase downregulation promotes colon carcinogenesis through STAT3-activated microRNAs. J. Clin. Investig. 2014, 124, 5368–5384. [Google Scholar] [CrossRef] [PubMed]
- Oskouian, B.; Sooriyakumaran, P.; Borowsky, A.D.; Crans, A.; Dillard-Telm, L.; Tam, Y.Y.; Bandhuvula, P.; Saba, J.D. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 17384–17389. [Google Scholar] [CrossRef]
- Heering, J.; Weis, N.; Holeiter, M.; Neugart, F.; Staebler, A.; Fehm, T.N.; Bischoff, A.; Schiller, J.; Duss, S.; Schmid, S.; et al. Loss of the ceramide transfer protein augments EGF receptor signaling in breast cancer. Cancer Res. 2012, 72, 2855–2866. [Google Scholar] [CrossRef]
- Lee, A.J.; Roylance, R.; Sander, J.; Gorman, P.; Endesfelder, D.; Kschischo, M.; Jones, N.P.; East, P.; Nicke, B.; Spassieva, S.; et al. CERT depletion predicts chemotherapy benefit and mediates cytotoxic and polyploid-specific cancer cell death through autophagy induction. J. Pathol. 2012, 226, 482–494. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Tomishige, N.; Sakai, S.; Ishitsuka, R.; Ishii, K.; Makino, A.; Greimel, P.; Abe, M.; Laviad, E.L.; Lagarde, M.; et al. Limonoid compounds inhibit sphingomyelin biosynthesis by preventing CERT protein-dependent extraction of ceramides from the endoplasmic reticulum. J. Biol. Chem. 2012, 287, 24397–24411. [Google Scholar] [CrossRef]
- Wijesinghe, D.S.; Brentnall, M.; Mietla, J.A.; Hoeferlin, L.A.; Diegelmann, R.F.; Boise, L.H.; Chalfant, C.E. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. 2014, 55, 1298–1309. [Google Scholar] [CrossRef]
- Payne, A.W.; Pant, D.K.; Pan, T.C.; Chodosh, L.A. Ceramide kinase promotes tumor cell survival and mammary tumor recurrence. Cancer Res. 2014, 74, 6352–6363. [Google Scholar] [CrossRef]
- Pastukhov, O.; Schwalm, S.; Zangemeister-Wittke, U.; Fabbro, D.; Bornancin, F.; Japtok, L.; Kleuser, B.; Pfeilschifter, J.; Huwiler, A. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br. J. Pharmacol. 2014, 171, 5829–5844. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, Y.; Roh, J.L.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Prognostic value of glucosylceramide synthase and P-glycoprotein expression in oral cavity cancer. Int. J. Clin. Oncol. 2016, 21, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W. Inhibition of Glucosylceramide Synthase Sensitizes Head and Neck Cancer to Cisplatin. Mol. Cancer Ther. 2015, 14, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, M.; Tutusaus, A.; Martinez-Nieto, G.A.; Barcena, C.; de Gregorio, E.; Moutinho, C.; Barbero-Camps, E.; Villanueva, A.; Colell, A.; Mari, M.; et al. Targeting glucosylceramide synthase upregulation reverts sorafenib resistance in experimental hepatocellular carcinoma. Oncotarget 2016, 7, 8253–8267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Patwardhan, G.A.; Bhinge, K.; Gupta, V.; Gu, X.; Jazwinski, S.M. Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Res. 2011, 71, 2276–2285. [Google Scholar] [CrossRef]
- Gupta, V.; Bhinge, K.N.; Hosain, S.B.; Xiong, K.; Gu, X.; Shi, R.; Ho, M.Y.; Khoo, K.H.; Li, S.C.; Li, Y.T.; et al. Ceramide glycosylation by glucosylceramide synthase selectively maintains the properties of breast cancer stem cells. J. Biol. Chem. 2012, 287, 37195–37205. [Google Scholar] [CrossRef]
- Eliyahu, E.; Park, J.H.; Shtraizent, N.; He, X.; Schuchman, E.H. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007, 21, 1403–1409. [Google Scholar] [CrossRef]
- Cheng, J.C.; Bai, A.; Beckham, T.H.; Marrison, S.T.; Yount, C.L.; Young, K.; Lu, P.; Bartlett, A.M.; Wu, B.X.; Keane, B.J.; et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Investig. 2013, 123, 4344–4358. [Google Scholar] [CrossRef]
- Beckham, T.H.; Cheng, J.C.; Lu, P.; Shao, Y.; Troyer, D.; Lance, R.; Marrison, S.T.; Norris, J.S.; Liu, X. Acid ceramidase induces sphingosine kinase 1/S1P receptor 2-mediated activation of oncogenic Akt signaling. Oncogenesis 2013, 2, e49. [Google Scholar] [CrossRef]
- Tirodkar, T.S.; Lu, P.; Bai, A.; Scheffel, M.J.; Gencer, S.; Garrett-Mayer, E.; Bielawska, A.; Ogretmen, B.; Voelkel-Johnson, C. Expression of Ceramide Synthase 6 Transcriptionally Activates Acid Ceramidase in a c-Jun N-terminal Kinase (JNK)-dependent Manner. J. Biol. Chem. 2015, 290, 13157–13167. [Google Scholar] [CrossRef]
- Wang, Z.; Min, X.; Xiao, S.H.; Johnstone, S.; Romanow, W.; Meininger, D.; Xu, H.; Liu, J.; Dai, J.; An, S.; et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 2013, 21, 798–809. [Google Scholar] [CrossRef]
- Kawamori, T.; Kaneshiro, T.; Okumura, M.; Maalouf, S.; Uflacker, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009, 23, 405–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wan, Z.; Liu, S.; Cao, Y.; Zeng, Z. Sphingosine kinase 1 and cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e90362. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Gutierrez, M.E.; Maurer, B.J.; Reynolds, C.P.; Kang, M.; Singh, H.; Crandon, S.; Murgo, A.J.; Doroshow, J.H. Phase I trial of fenretinide lym-x-sorb oral powder in adults with solid tumors and lymphomas. Anticancer. Res. 2011, 31, 961–966. [Google Scholar] [PubMed]
- Rao, R.D.; Cobleigh, M.A.; Gray, R.; Graham, M.L., 2nd; Norton, L.; Martino, S.; Budd, G.T.; Ingle, J.N.; Wood, W.C. Phase III double-blind, placebo-controlled, prospective randomized trial of adjuvant tamoxifen vs. tamoxifen and fenretinide in postmenopausal women with positive receptors (EB193): An intergroup trial coordinated by the Eastern Cooperative Oncology Group. Med. Oncol. 2011, 28 (Suppl. S1), S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Mariani, L.; Decensi, A.; Formelli, F.; Camerini, T.; Miceli, R.; Di Mauro, M.G.; Costa, A.; Marubini, E.; Sporn, M.B.; et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann. Oncol. 2006, 17, 1065–1071. [Google Scholar] [CrossRef]
- Vaishampayan, U.; Heilbrun, L.K.; Parchment, R.E.; Jain, V.; Zwiebel, J.; Boinpally, R.R.; LoRusso, P.; Hussain, M. Phase II trial of fenretinide in advanced renal carcinoma. Investig. New Drugs 2005, 23, 179–185. [Google Scholar] [CrossRef]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharmacol. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Yu, H. Targeting sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell carcinoma cell growth. Biochem. Biophys. Res. Commun. 2018, 497, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Costa-Pinheiro, P.; Patterson, L.; Drews, K.; Spiegel, S.; Kester, M. Novel Sphingolipid-Based Cancer Therapeutics in the Personalized Medicine Era. Adv. Cancer Res. 2018, 140, 327–366. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A. The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer. Mediators Inflamm. 2017, 2017, 4806541. [Google Scholar] [CrossRef] [PubMed]
- Tabasinezhad, M.; Samadi, N.; Ghanbari, P.; Mohseni, M.; Saei, A.A.; Sharifi, S.; Saeedi, N.; Pourhassan, A. Sphingosin 1-phosphate contributes in tumor progression. J. Cancer Res. Ther. 2013, 9, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Gellhaus, A.; Winterhager, E.; Gulbins, E. Ceramide-enriched membrane domains in infectious biology and development. Subcell. Biochem. 2008, 49, 523–538. [Google Scholar] [CrossRef]
- Dany, M.; Gencer, S.; Nganga, R.; Thomas, R.J.; Oleinik, N.; Baron, K.D.; Szulc, Z.M.; Ruvolo, P.; Kornblau, S.; Andreeff, M.; et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 2016, 128, 1944–1958. [Google Scholar] [CrossRef]
- Parham, K.A.; Zebol, J.R.; Tooley, K.L.; Sun, W.Y.; Moldenhauer, L.M.; Cockshell, M.P.; Gliddon, B.L.; Moretti, P.A.; Tigyi, G.; Pitson, S.M.; et al. Sphingosine 1-phosphate is a ligand for peroxisome proliferator-activated receptor-gamma that regulates neoangiogenesis. FASEB J. 2015, 29, 3638–3653. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Tang, C.; Wu, J.-x.; Ji, B.-w.; Gong, B.-m.; Wu, X.-h.; Wang, X. Mass Spectrometry Detects Sphingolipid Metabolites for Discovery of New Strategy for Cancer Therapy from the Aspect of Programmed Cell Death. Metabolites 2023, 13, 867. https://doi.org/10.3390/metabo13070867
Shi M, Tang C, Wu J-x, Ji B-w, Gong B-m, Wu X-h, Wang X. Mass Spectrometry Detects Sphingolipid Metabolites for Discovery of New Strategy for Cancer Therapy from the Aspect of Programmed Cell Death. Metabolites. 2023; 13(7):867. https://doi.org/10.3390/metabo13070867
Chicago/Turabian StyleShi, Ming, Chao Tang, Jia-xing Wu, Bao-wei Ji, Bao-ming Gong, Xiao-hui Wu, and Xue Wang. 2023. "Mass Spectrometry Detects Sphingolipid Metabolites for Discovery of New Strategy for Cancer Therapy from the Aspect of Programmed Cell Death" Metabolites 13, no. 7: 867. https://doi.org/10.3390/metabo13070867
APA StyleShi, M., Tang, C., Wu, J.-x., Ji, B.-w., Gong, B.-m., Wu, X.-h., & Wang, X. (2023). Mass Spectrometry Detects Sphingolipid Metabolites for Discovery of New Strategy for Cancer Therapy from the Aspect of Programmed Cell Death. Metabolites, 13(7), 867. https://doi.org/10.3390/metabo13070867








