Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract
2.2. Animals
2.3. Sample Collection
2.4. Studies on Isolated Perfused Rat Small Intestine
2.5. Total Body Fat Content
2.6. Fasting Total GLP-1 Plasma Concentrations
2.7. RNA Extraction and Gene Expression
2.8. Plasma Metabolic Markers
2.9. Analysis of Hepatic Fat Percentage
2.10. Proteomics Analysis of Liver Tissue
2.11. NMR Spectroscopy
2.12. Metabolomic Analysis of Plasma and Cecal Contents
2.13. SCFA Analysis
2.14. Statistical Analyses
3. Results
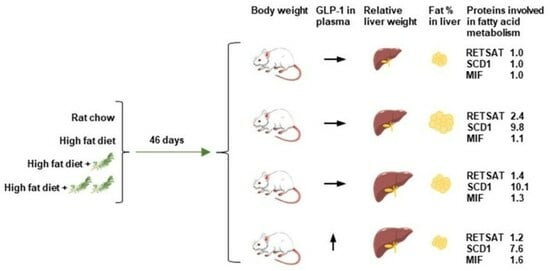
3.1. Body Weight and Feed Intake
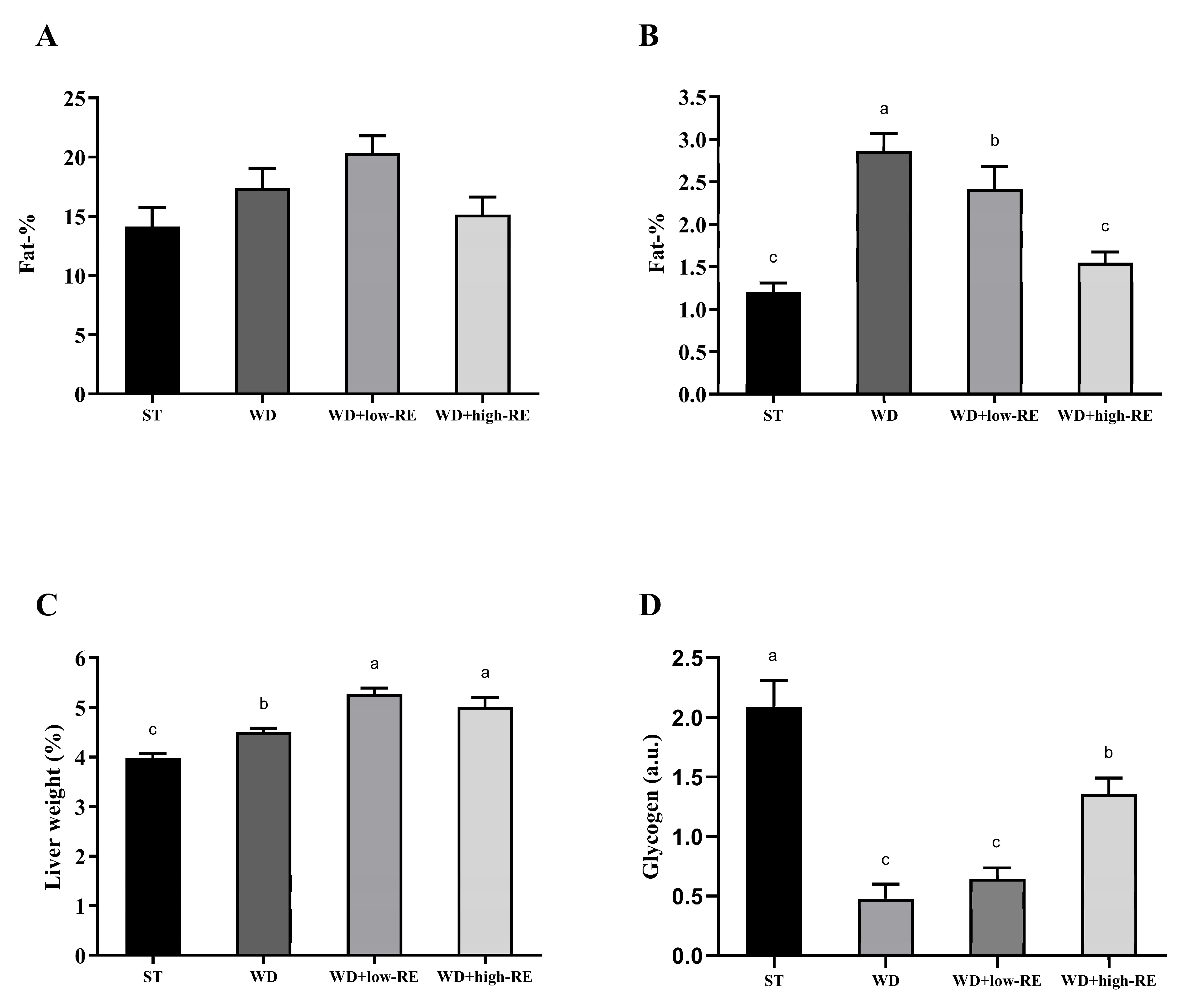
3.2. Liver Weight and Fat Accumulation in the Liver and Whole Body
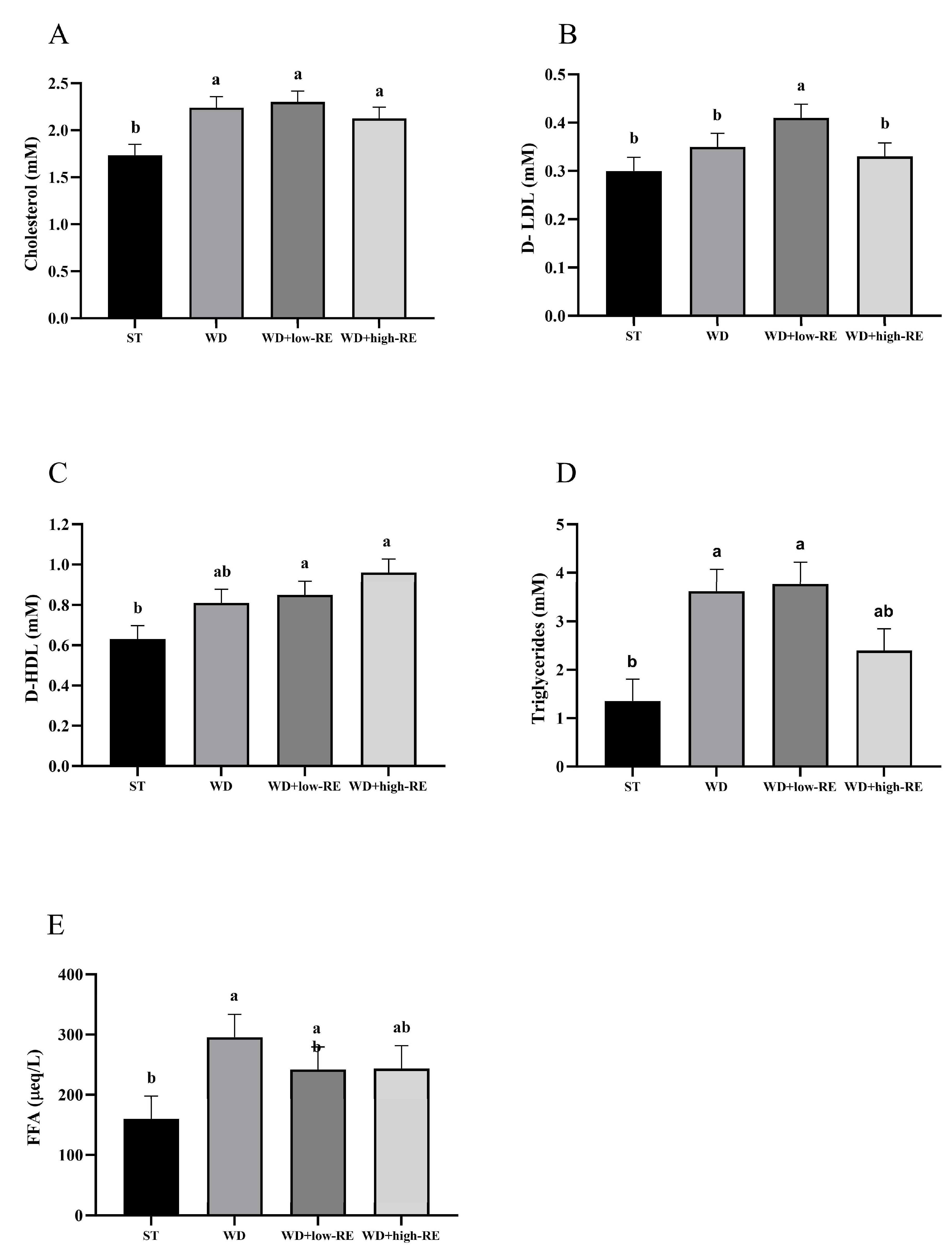
3.3. Plasma Metabolic Markers
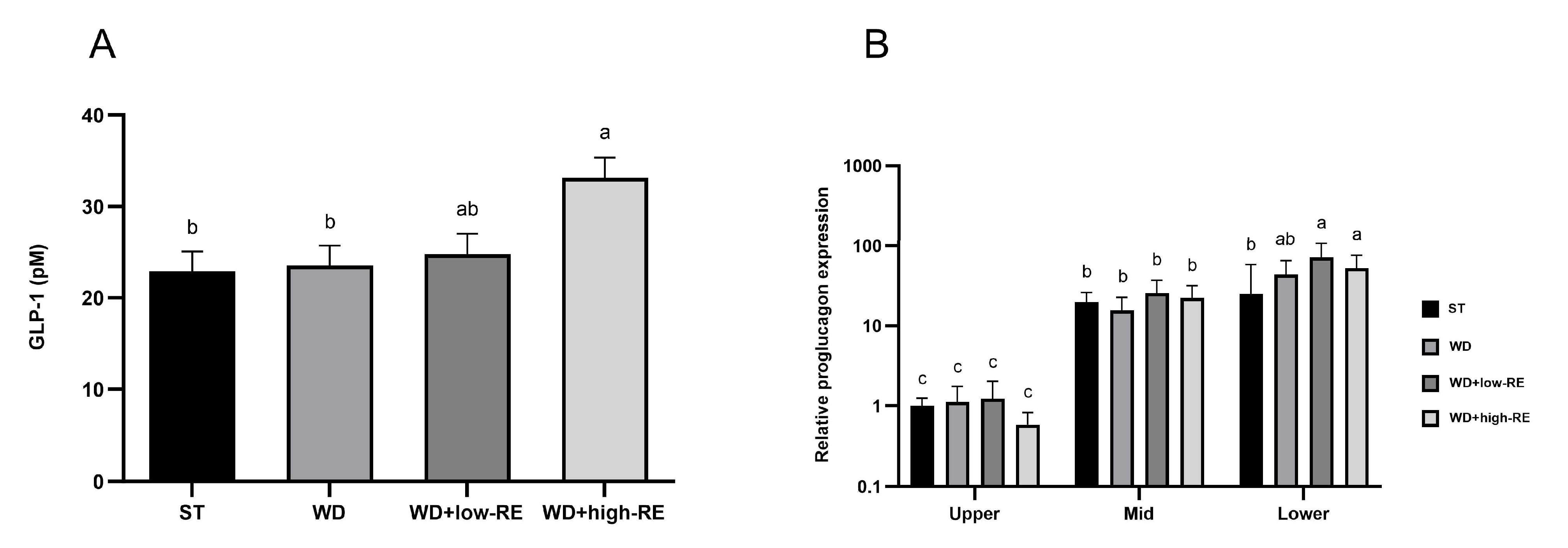
3.4. Fasting GLP-1 Plasma Concentrations and Proglucagon Expression
3.5. Acute Effects of RE on GLP-1 Secretion from Isolated Perfused Rat Small Intestine
3.6. Proteomic Analysis of Liver Tissue
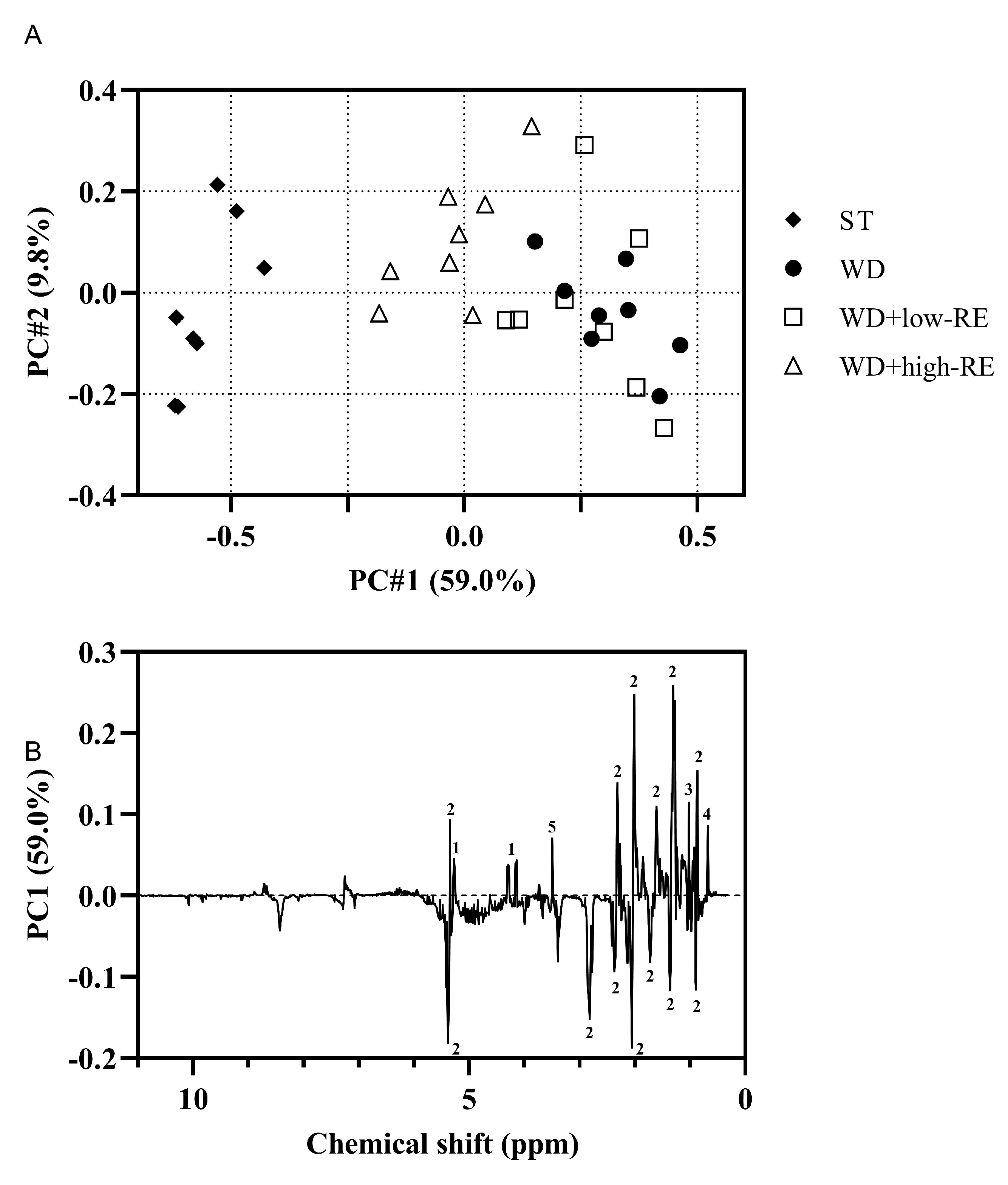
3.7. NMR Analysis of Liver Tissue
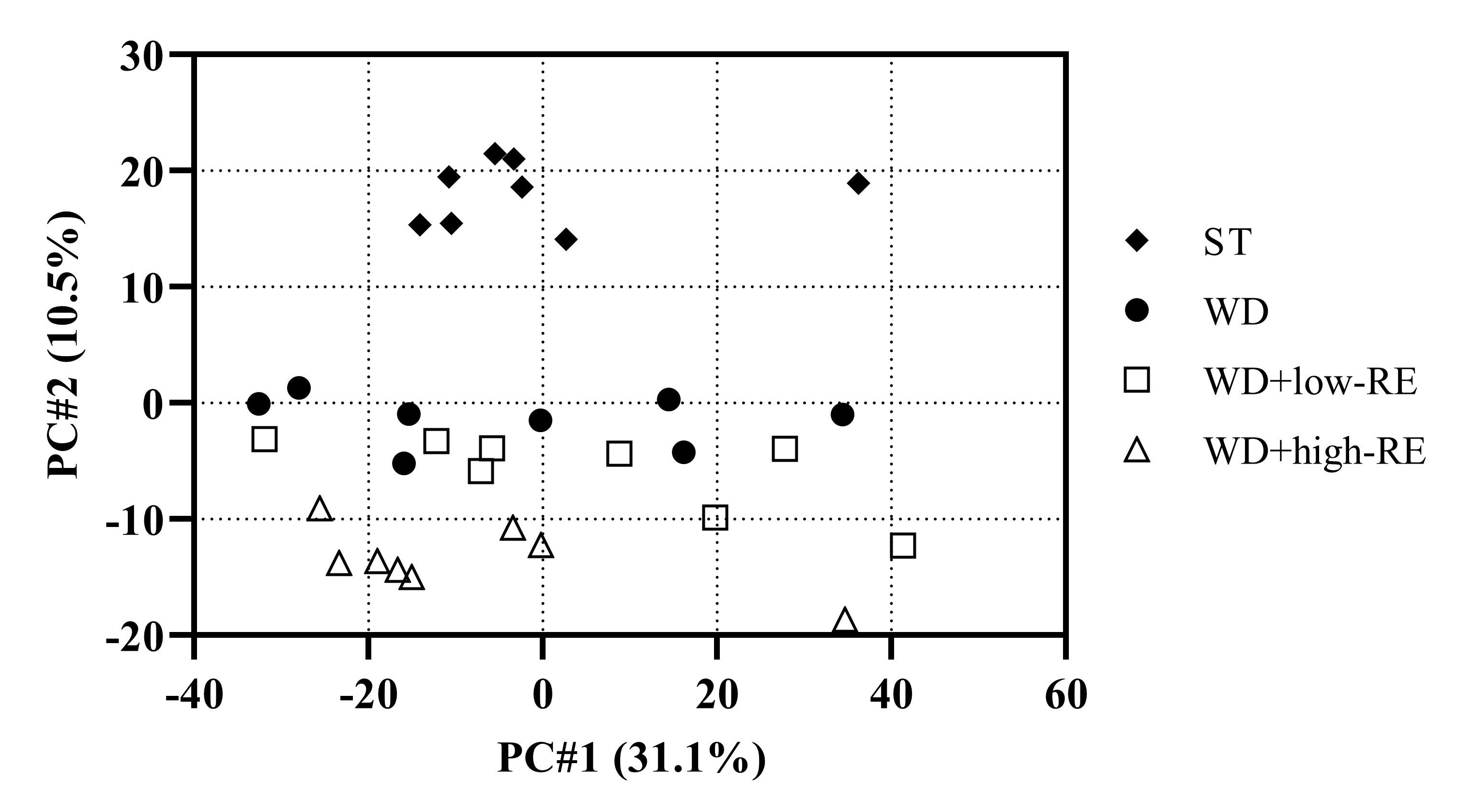
3.8. LC-MS Metabolomics of Plasma and Caecum Content
3.9. Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317s–325s. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol. Nutr. Food Res. 2013, 57, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Hurst, W.J.; Stuart, D.A.; Lambert, J.D. Inhibition of Key Digestive Enzymes by Cocoa Extracts and Procyanidins. J. Agric. Food Chem. 2011, 59, 5305–5311. [Google Scholar] [CrossRef] [PubMed]
- Matsui, N.; Ito, R.; Nishimura, E.; Yoshikawa, M.; Kato, M.; Kamei, M.; Shibata, H.; Matsumoto, I.; Abe, K.; Hashizume, S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition 2005, 21, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshidao, T. Effect of Polyphenol-Rich Extract from Walnut on Diet-Induced Hypertriglyceridemia in Mice via Enhancement of Fatty Acid Oxidation in the Liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Ibarra, A.; Cases, J.; Roller, M.; Chiralt-Boix, A.; Coussaert, A.; Ripoll, C. Carnosic acid-rich rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and improves cholesterol levels and glycaemia in mice on a high-fat diet. Br. J. Nutr. 2011, 106, 1182–1189. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorg. Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Madsen, S.; Hedemann, M.S.; Knudsen, K.E.B.; Sparsø, F.V.; Laursen, A.; Jensen, H.M.; Knudsen, T.A.; Purup, S. Effect of food ingredients on glucagon-like peptide-1 secretion in STC-1 and HuTu-80 cells. Int. J. Food Sci. Technol. 2019, 54, 3149–3155. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Christensen, M.; Junker, A.E.; Knop, F.K.; Gluud, L.L. Effects of glucagon-like peptide-1 receptor agonists on weight loss: Systematic review and meta-analyses of randomised controlled trials. BMJ 2012, 344, d7771. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Metabolic Messengers: Glucagon-like peptide 1. Nat. Metab. 2021, 3, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Knauf, C.; Cani, P.D.; Perrin, C.; Iglesias, M.A.; Maury, J.F.; Bernard, E.; Benhamed, F.; Gremeaux, T.; Drucker, D.J.; Kahn, C.R.; et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J. Clin. Investig. 2005, 115, 3554–3563. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Henriksen, T.I.; Pedersen, B.K.; Solomon, T.P.J. Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS ONE 2012, 7, e44284. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.; Taher, J.; Adeli, K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Ruotolo, G.; Robbins, D.C. Obesity and dyslipidemia. Endocrinol. Metab. Clin. North Am. 2003, 32, 855–867. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Polyphenols to Combat Nonalcoholic Fatty Liver Disease via the Gut–Brain–Liver Axis: A Review of Possible Mechanisms. J. Agric. Food Chem. 2021, 69, 3585–3600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sedighi, R.; Wang, P.; Chen, H.; Zhu, Y.; Sang, S. Carnosic Acid as a Major Bioactive Component in Rosemary Extract Ameliorates High-Fat-Diet-Induced Obesity and Metabolic Syndrome in Mice. J. Agric. Food Chem. 2015, 63, 4843–4852. [Google Scholar] [CrossRef] [PubMed]
- Hedemann, M.S.; Hermansen, K.; Pedersen, S.; Bach Knudsen, K.E. Resistant Starch but Not Enzymatically Modified Waxy Maize Delays Development of Diabetes in Zucker Diabetic Fatty Rats. J. Nutr. 2017, 147, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Frost, C.R.; Svendsen, B.; Holst, J.J. Molecular Mechanisms of Glucose-Stimulated GLP-1 Secretion From Perfused Rat Small Intestine. Diabetes 2015, 64, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Holst, J.J. Mechanisms Underlying Gut Hormone Secretion Using the Isolated Perfused Rat Small Intestine. J. Vis. Exp. 2019, 144, e58533. [Google Scholar] [CrossRef]
- Ørskov, C.; Rabenhøj, L.; Wettergren, A.; Kofod, H.; Holst, J.J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 1994, 43, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Zougman, A.; Selby, P.J.; Banks, R.E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 2014, 14, 1006-1000. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Silva, J.C.; Gorenstein, M.V.; Li, G.-Z.; Vissers, J.P.C.; Geromanos, S.J. Absolute Quantification of Proteins by LCMSE: A Virtue of Parallel ms Acquisition *S. Mol. Cell. Proteom. 2006, 5, 144–156. [Google Scholar] [CrossRef]
- Yde, C.C.; Clausen, M.R.; Ditlev, D.B.; Lillefosse, H.; Madsen, L.; Kristiansen, K.; Liaset, B.; Bertram, H.C. Multi-block PCA and multi-compartmental study of the metabolic responses to intake of hydrolysed versus intact casein in C57BL/6J mice by NMR-based metabolomics. Metabolomics 2014, 10, 938–949. [Google Scholar] [CrossRef]
- Fathi, F.; Brun, A.; Rott, K.H.; Falco Cobra, P.; Tonelli, M.; Eghbalnia, H.R.; Caviedes-Vidal, E.; Karasov, W.H.; Markley, J.L. NMR-Based Identification of Metabolites in Polar and Non-Polar Extracts of Avian Liver. Metabolites 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-C.; Wang, S.-Y.; Kuo, C.-h.; Tseng, Y.J. Distribution-Based Classification Method for Baseline Correction of Metabolomic 1D Proton Nuclear Magnetic Resonance Spectra. Anal. Chem. 2013, 85, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.A.; Magalhães, A.; Ferreira, M.M.C. Optimized bucketing for NMR spectra: Three case studies. Chemom. Intell. Lab. Syst. 2013, 122, 93–102. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Tafintseva, V.; Bendiks, Z.A.; Marco, M.L.; Kohler, A.; Xu, Y.; Nørskov, N.P.; Nygaard Lærke, H.; Bach Knudsen, K.E.; Hedemann, M.S. Obesity-Related Metabolome and Gut Microbiota Profiles of Juvenile Göttingen Minipigs—Long-Term Intake of Fructose and Resistant Starch. Metabolites 2020, 10, 456. [Google Scholar] [CrossRef]
- Curtasu, M.V.; Knudsen, K.E.B.; Callesen, H.; Purup, S.; Stagsted, J.; Hedemann, M.S. Obesity Development in a Miniature Yucatan Pig Model: A Multi-compartmental Metabolomics Study on Cloned and Normal Pigs Fed Restricted or Ad Libitum High-Energy Diets. J. Proteome Res. 2019, 18, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. Microbial-Production of Skatole in the Hind Gut of Pigs Given Different Diets and Its Relation to Skatole Deposition in Backfat. Anim. Sci. 1995, 61, 293–304. [Google Scholar] [CrossRef]
- Harach, T.; Aprikian, O.; Monnard, I.; Moulin, J.; Membrez, M.; Beolor, J.C.; Raab, T.; Mace, K.; Darimont, C. Rosemary (Rosmarinus officinalis L.) Leaf Extract Limits Weight Gain and Liver Steatosis in Mice Fed a High-Fat Diet. Planta Med. 2010, 76, 566–571. [Google Scholar] [CrossRef]
- Song, Z.B.; Xie, W.R.; Chen, S.S.; Strong, J.A.; Print, M.S.; Wang, J.I.; Shareef, A.F.; Ulrich-Lai, Y.M.; Zhang, J.M. High-fat diet increases pain behaviors in rats with or without obesity. Sci. Rep. 2017, 7, 10350. [Google Scholar] [CrossRef]
- Levin, B.E.; DunnMeynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol-Regul. Integr. Comp. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Prud’homme, D.; Blanchet, R.; Giroux, I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.M.; Hernandez, L.M.R.; Berhow, M.A.; de Mejia, E.G. Bioactive Compounds from Culinary Herbs Inhibit a Molecular Target for Type 2 Diabetes Management, Dipeptidyl Peptidase IV. J. Agric. Food Chem. 2014, 62, 6147–6158. [Google Scholar] [CrossRef] [PubMed]
- Wewer Albrechtsen, N.J.; Kuhre, R.E.; Toräng, S.; Holst, J.J. The intestinal distribution pattern of appetite- and glucose regulatory peptides in mice, rats and pigs. BMC Res. Notes 2016, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Kshirsagar, S.; Sutariya, B.; Patel, M.; Patel, H.; Pandey, D.; Patel, D.; Bahekar, R.; Jain, M. Central administration of coagonist of GLP-1 and glucagon receptors improves dyslipidemia. Biomed. Pharmacother. 2018, 98, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Avila, J.A.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; De la Rosa, L.A. Modulation of PPAR Expression and Activity in Response to Polyphenolic Compounds in High Fat Diets. Int. J. Mol. Sci. 2016, 17, 1002. [Google Scholar] [CrossRef] [PubMed]
- Yao-Borengasser, A.; Rassouli, N.; Varma, V.; Bodles, A.M.; Rasouli, N.; Unal, R.; Phanavanh, B.; Ranganathan, G.; McGehee, R.E., Jr.; Kern, P.A. Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-gamma responsiveness. J. Clin. Endocrinol. Metab. 2008, 93, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Schupp, M.; Lefterova, M.I.; Janke, J.; Leitner, K.; Cristancho, A.G.; Mullican, S.E.; Qatanani, M.; Szwergold, N.; Steger, D.J.; Curtin, J.C.; et al. Retinol saturase promotes adipogenesis and is downregulated in obesity. Proc. Natl. Acad. Sci. USA 2009, 106, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Miyazaki, M.; Dobrzyn, A.; Ntambi, J. Stearoyl CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. FASEB J. 2007, 21, A109. [Google Scholar] [CrossRef]
- Chu, K.; Miyazaki, M.; Man, W.C.; Ntambi, J.M. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol. Cell. Biol. 2006, 26, 6786–6798. [Google Scholar] [CrossRef]
- Heidenreich, S.; Witte, N.; Weber, P.; Goehring, I.; Tolkachov, A.; von Loeffelholz, C.; Docke, S.; Bauer, M.; Stockmann, M.; Pfeiffer, A.F.H.; et al. Retinol saturase coordinates liver metabolism by regulating ChREBP activity. Nat. Commun. 2017, 8, 384. [Google Scholar] [CrossRef]
- Silbernagel, G.; Kovarova, M.; Cegan, A.; Machann, J.; Schick, F.; Lehmann, R.; Haring, H.U.; Stefan, N.; Schleicher, E.; Fritsche, A.; et al. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J. Clin. Endocrinol. Metab. 2012, 97, E2288–E2292. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Kim, Y.C.; Gray-Keller, M.P.; Attie, A.D.; Ntambi, J.M. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J. Biol. Chem. 2000, 275, 30132–30138. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.; Berres, M.L.; Coeuru, M.; Knauel, M.; Nellen, A.; Fischer, P.; Philippeit, C.; Bucala, R.; Trautwein, C.; Wasmuth, H.E.; et al. Protective role of macrophage migration inhibitory factor in nonalcoholic steatohepatitis. FASEB J. 2014, 28, 5136–5147. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.K.; Saxena, N.K.; Lin, S.B.; Gupta, N.; Anania, F.A. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 2006, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, J.L.; Griffin, P.S.; Wittmer, C.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Dolman, C.S.; Erickson, M.R.; Napora, J.; Parkes, D.G.; Roth, J.D. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol-Gastrointest. Liver Physiol. 2012, 302, G762–G772. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Saccomanno, S.; Rychlicki, C.; Agostinelli, L.; De Minicis, S.; Candelaresi, C.; Faraci, G.; Pacetti, D.; Vivarelli, M.; Nicolini, D.; et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 2011, 31, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Khound, R.; Taher, J.; Baker, C.; Adeli, K.; Su, Q. GLP-1 Elicits an Intrinsic Gut-Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2252–2259. [Google Scholar] [CrossRef]
- Chang, H.C.; Peng, C.H.; Yeh, D.M.; Kao, E.S.; Wang, C.J. Hibiscus sabdariffa extract inhibits obesity and fat accumulation, and improves liver steatosis in humans. Food Funct. 2014, 5, 734–739. [Google Scholar] [CrossRef]
- Gan, L.; Meng, Z.J.; Xiong, R.B.; Guo, J.Q.; Lu, X.C.; Zheng, Z.W.; Deng, Y.P.; Luo, B.D.; Zou, F.; Li, H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol. Sin. 2015, 36, 597–605. [Google Scholar] [CrossRef]
- Maronpot, R.R.; Yoshizawa, K.; Nyska, A.; Harada, T.; Flake, G.; Mueller, G.; Singh, B.; Ward, J.M. Hepatic Enzyme Induction: Histopathology. Toxicol. Pathol. 2010, 38, 776–795. [Google Scholar] [CrossRef]
- Maierean, S.M.; Serban, M.C.; Sahebkar, A.; Ursoniu, S.; Serban, A.; Penson, P.; Banach, M.; Meta-anal, L.B.P. The effects of cinnamon supplementation on blood lipid concentrations: A systematic review and meta-analysis. J. Clin. Lipidol. 2017, 11, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Vega-Lopez, S.; Kaul, N.; Schonlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Rigby, N.; Sójka, M.; Kołodziejczyk, K.; Mackie, A.; Zduńczyk, Z. Raspberry pomace alters cecal microbial activity and reduces secondary bile acids in rats fed a high-fat diet. J. Nutr. Biochem. 2017, 46, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Haraguchi, T.; Iwanaga, S.; Tomotake, H.; Okazaki, Y.; Mineo, S.; Moriyama, A.; Inoue, J.; Kato, N. Consumption of Some Polyphenols Reduces Fecal Deoxycholic Acid and Lithocholic Acid, the Secondary Bile Acids of Risk Factors of Colon Cancer. J. Agric. Food Chem. 2009, 57, 8587–8590. [Google Scholar] [CrossRef]
- Haraguchi, T.; Kayashima, T.; Okazaki, Y.; Inoue, J.; Mineo, S.; Matsubara, K.; Sakaguchi, E.; Yanaka, N.; Kato, N. Cecal Succinate Elevated by Some Dietary Polyphenols May Inhibit Colon Cancer Cell Proliferation and Angiogenesis. J. Agric. Food Chem. 2014, 62, 5589–5594. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; Selma, M.-V.; Larrosa, M.; Obiol, M.; García-Villalba, R.; González-Barrio, R.; Issaly, N.; Flanagan, J.; Roller, M.; Tomás-Barberán, F.A.; et al. A rosemary extract rich in carnosic acid selectively modulates caecum microbiota and inhibits β-glucosidase activity, altering fiber and short chain fatty acids fecal excretion in lean and obese female rats. PLoS ONE 2014, 9, e94687. [Google Scholar] [CrossRef]
- Fernández-Veledo, S.; Vendrell, J. Gut microbiota-derived succinate: Friend or foe in human metabolic diseases? Rev. Endocr. Metab. Disord. 2019, 20, 439–447. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]



| Protein Name | Gene | WD/ST | WD+Low-RE/ ST | WD+High-RE/ ST |
|---|---|---|---|---|
| Arylacetamide deacetylase | AADAC | 1.7 | 1.6 | 1.7 |
| ATP-binding cassette sub-family D member 3 | ABCD3 | 1.2 | 1.3 | 1.5 |
| Short/branched chain specific acyl-CoA dehydrogenase, mitochondrial | ACADS | 0.5 | 0.6 | 0.5 |
| Aldehyde oxidase 1 | AOX1 | 1.2 | 1.2 | 1.3 |
| CDGSH iron-sulfur domain-containing protein 1 | CISD1 | 0.9 | 0.8 | 0.8 |
| 2,4-dienoyl-CoA reductase, mitochondrial | DECR1 | 2.0 | 1.6 | 1.8 |
| Enoyl-CoA delta isomerase 1, mitochondrial | ECI1 | 2.1 | 1.8 | 2.2 |
| Hydroxyacyl-coenzyme A dehydrogenase, mitochondrial | HADH | 1.3 | 1.0 | 1.0 |
| Trifunctional enzyme subunit alpha, mitochondrial | HADHA | 1.4 | 1.3 | 1.5 |
| Trifunctional enzyme subunit beta, mitochondrial | HADHB | 1.3 | 1.0 | 1.3 |
| Hydroxymethylglutaryl-CoA synthase, mitochondrial | HMGCS2 | 1.4 | 1.2 | 1.2 |
| Corticosteroid 11-beta-dehydrogenase isozyme 1 | HSD11B1 | 0.5 | 0.3 | 0.5 |
| Estradiol 17-beta-dehydrogenase 2 | HSD17B2 | 1.2 | 1.4 | 1.6 |
| MICOS complex subunit Mic60 (Fragment) | IMMT | 0.8 | 1.2 | 1.7 |
| Enoyl-[acyl-carrier-protein] reductase, mitochondrial | MECR | 1.6 | 2.2 | 4.1 |
| Macrophage migration inhibitory factor | MIF | 1.1 | 1.3 | 1.6 |
| 5′-AMP-activated protein kinase catalytic subunit alpha-2 | PRKAA2 | 1.4 | 1.2 | 1.5 |
| All-trans-retinol 13,14-reductase | RETSAT | 2.4 | 1.4 | 1.2 |
| Stearoyl-coenzyme A desaturase 1 | SCD1 | 9.8 | 10.1 | 7.6 |
| ST | WD | WD+Low-RE | WD+High-RE | p-Value | |
|---|---|---|---|---|---|
| Cecal content (g) | 4.64 a [3.69–5.83] | 1.79 c [1.42–2.25] | 2.44 b [1.94–3.07] | 2.62 b [2.01–3.41] | <0.0001 |
| Carboxylic acids, pool size (µmol) | |||||
| Total carboxylic acids | 555 a [445–692] | 84 c [67–104] | 125 b [100–155] | 128 b [96–169] | <0.0001 |
| Acetic acid | 302 a [241–379] | 52.3 b [41.7–65.5] | 69.0 b [55.1–86.5] | 69.5 b [52.3–92.5] | <0.0001 |
| Propionic acid | 58.9 a [44.7–77.5] | 13.2 b [10.0–17.3] | 15.5 b [11.8–20.4] | 12.7 b [9.0–18.0] | <0.0001 |
| Butyric acid | 165 a [128–214] | 6.75 b [5.21–8.74] | 3.15 c [2.43–4.08] | 1.72 d [1.27–2.32] | <0.0001 |
| Succinic acid | 4.03 b [2.09–7.75] | 2.45 b [1.41–4.26] | 31.6 a [18.9–53.1] | 21.8 a [12.0–39.7] | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madsen, S.; Bak, S.Y.; Yde, C.C.; Jensen, H.M.; Knudsen, T.A.; Bæch-Laursen, C.; Holst, J.J.; Laustsen, C.; Hedemann, M.S. Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet. Metabolites 2023, 13, 974. https://doi.org/10.3390/metabo13090974
Madsen S, Bak SY, Yde CC, Jensen HM, Knudsen TA, Bæch-Laursen C, Holst JJ, Laustsen C, Hedemann MS. Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet. Metabolites. 2023; 13(9):974. https://doi.org/10.3390/metabo13090974
Chicago/Turabian StyleMadsen, Sidsel, Steffen Yde Bak, Christian Clement Yde, Henrik Max Jensen, Tine Ahrendt Knudsen, Cecilie Bæch-Laursen, Jens Juul Holst, Christoffer Laustsen, and Mette Skou Hedemann. 2023. "Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet" Metabolites 13, no. 9: 974. https://doi.org/10.3390/metabo13090974
APA StyleMadsen, S., Bak, S. Y., Yde, C. C., Jensen, H. M., Knudsen, T. A., Bæch-Laursen, C., Holst, J. J., Laustsen, C., & Hedemann, M. S. (2023). Unravelling Effects of Rosemary (Rosmarinus officinalis L.) Extract on Hepatic Fat Accumulation and Plasma Lipid Profile in Rats Fed a High-Fat Western-Style Diet. Metabolites, 13(9), 974. https://doi.org/10.3390/metabo13090974