A Proposal for a Noxious Stimuli-Free, Moderate-Intensity Treadmill Running Protocol to Improve Aerobic Performance in Experimental Research on Rats
Abstract
1. Introduction
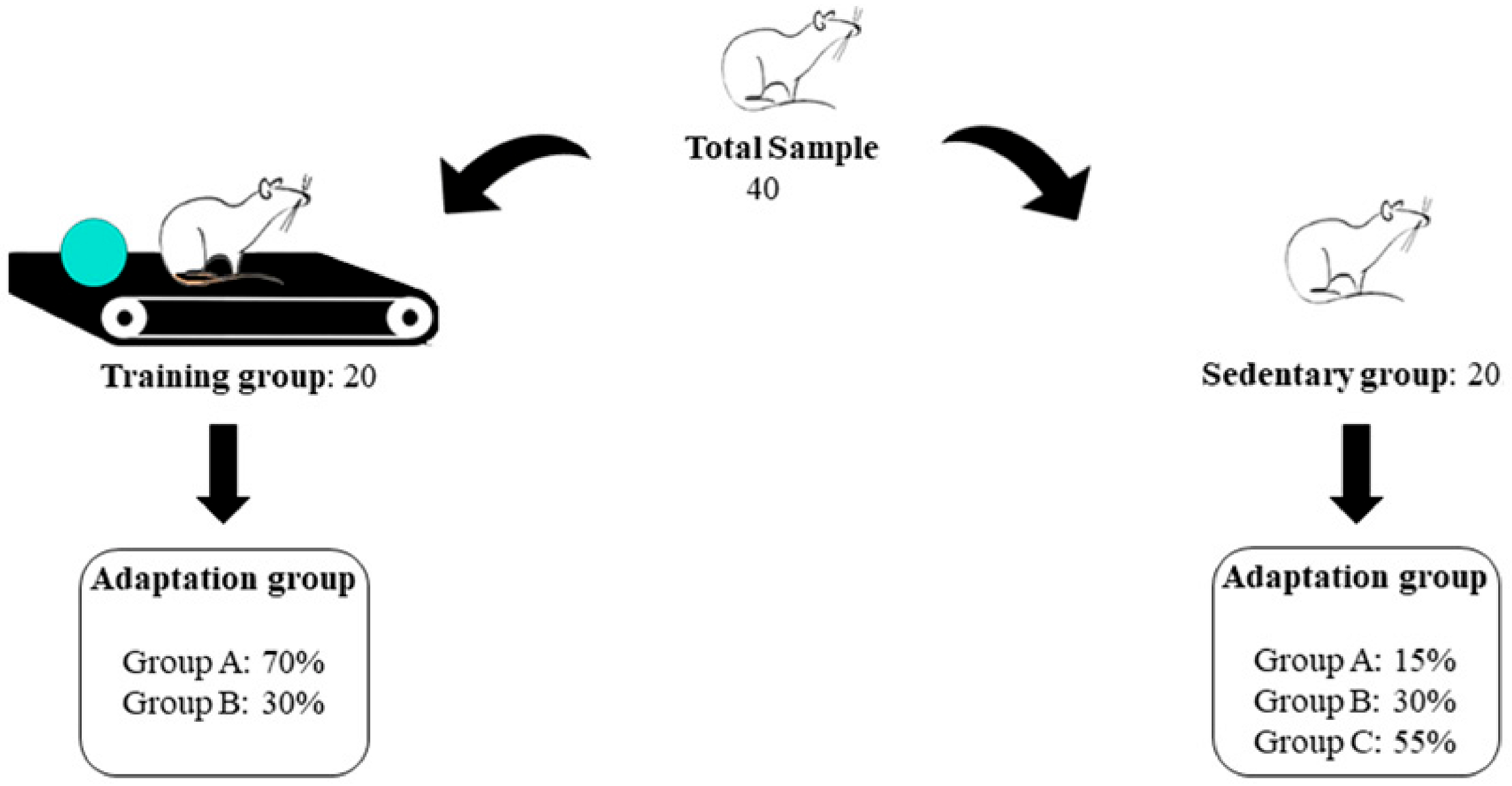
2. Materials and Methods
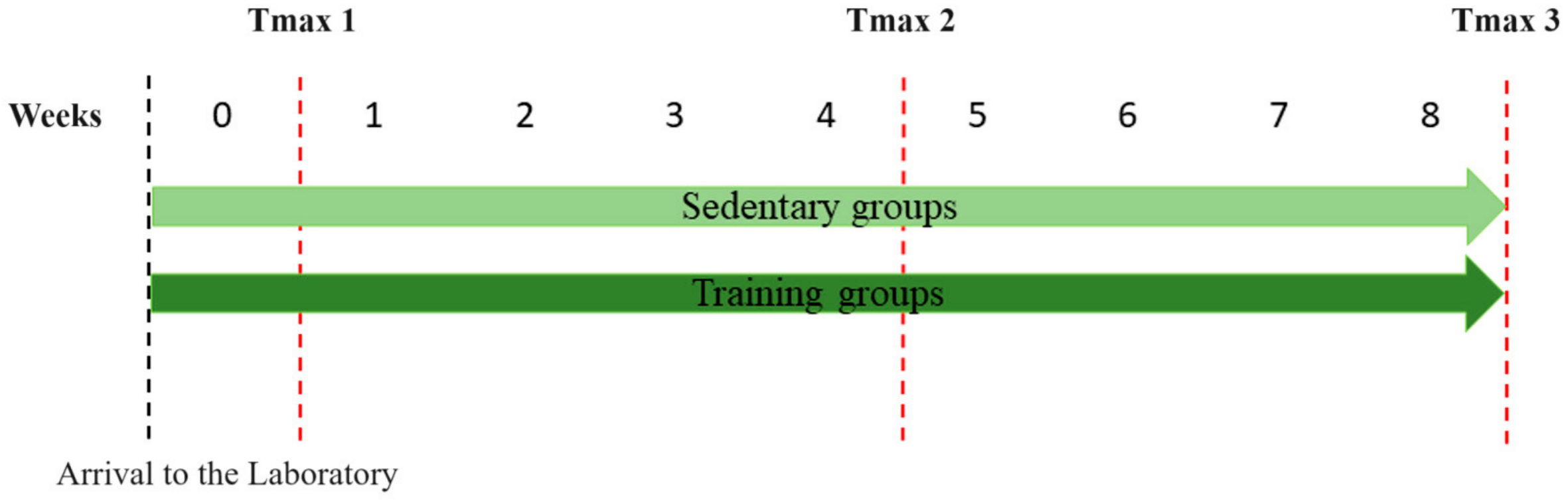
2.1. Tmax—The Treadmill Running Speed Performance Test
- Evaluator 1: Group A—Tmax1, Tmax2, Tmax3; Group B—Tmax1
- Evaluator 2: Group B—Tmax2, Tmax3; Group C—Tmax1, Tmax2, Tmax3
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bei, Y.; Wang, L.; Ding, R.; Che, L.; Fan, Z.; Gao, W.; Liang, Q.; Lin, S.; Liu, S.; Lu, X. Animal exercise studies in cardiovascular research: Current knowledge and optimal design—A position paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J. Sport. Health Sci. 2021, 10, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Oliva, A.; Hernández-Ávalos, I.; Martínez-Burnes, J.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The importance of animal models in biomedical research: Current insights and applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.D. From mice to men: What can we learn about personality from animal research? Psychol. Bull. 2001, 127, 45. [Google Scholar] [CrossRef]
- Angelis, K.D.; Rodrigues, B.; Zanesco, A.; Oliveira, E.M.d.; Evangelista, F.d.S.; Coelho, H.J.; Andreia Delbin, M.; Chakur Brum, P.; Ramires, P.R.; Soares, P.P. The importance of animal studies in Exercise Science. Mot. Rev. Educ. Física 2017, 23, e101625. [Google Scholar] [CrossRef]
- Ríos-Kristjánsson, J.G.; Rizo-Roca, D.; Kristjánsdóttir, K.M.; Núñez-Espinosa, C.A.; Torrella, J.R.; Pages, T.; Viscor, G. A three-criteria performance score for rats exercising on a running treadmill. PLoS ONE 2019, 14, e0219167. [Google Scholar] [CrossRef]
- Garrigos, D.; Martinez-Morga, M.; Toval, A.; Kutsenko, Y.; Barreda, A.; Do Couto, B.R.; Navarro-Mateu, F.; Ferran, J.L. A handful of details to ensure the experimental reproducibility on the FORCED running wheel in rodents: A systematic review. Front. Endocrinol. 2021, 12, 638261. [Google Scholar] [CrossRef]
- Arbus, S.B.; Pirtle, J.M.; Pankey, C.L. A Chronic High-Intensity Interval Training and Diet-Induced Obesity Model to Maximize Exercise Effort and Induce Physiologic Changes in Rats. JoVE (J. Vis. Exp.) 2023, e64447. [Google Scholar] [CrossRef]
- Dao, A.T.; Zagaar, M.A.; Alkadhi, K.A. Moderate treadmill exercise protects synaptic plasticity of the dentate gyrus and related signaling cascade in a rat model of Alzheimer’s disease. Mol. Neurobiol. 2015, 52, 1067–1076. [Google Scholar] [CrossRef]
- Radák, Z.; Silye, G.; Bartha, C.; Jakus, J.; Stefanovits-Bányai, É.; Atalay, M.; Marton, O.; Koltai, E. The effects of cocoa supplementation, caloric restriction, and regular exercise, on oxidative stress markers of brain and memory in the rat model. Food Chem. Toxicol. 2013, 61, 36–41. [Google Scholar] [CrossRef]
- Tomik, W.J.; Brannon, F.J.; Kelley, D.L. Treadmill running performance by rats at near maximal speeds. Res. Q. Am. Assoc. Health Phys. Educ. Recreat. 1968, 39, 822–824. [Google Scholar] [CrossRef]
- Sadri, I.; Nikookheslat, S.D.; Karimi, P.; Khani, M.; Nadimi, S. Aerobic exercise training improves memory function through modulation of brain-derived neurotrophic factor and synaptic proteins in the hippocampus and prefrontal cortex of type 2 diabetic rats. J. Diabetes Metab. Disord. 2024, 23, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, P.R.; Fulk, L.J.; Hand, G.A.; Wilson, M.A. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004, 1019, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Viru, A.; Viru, M. Cortisol-essential adaptation hormone in exercise. Int. J. Sports Med. 2004, 25, 461–464. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J. Cortisol and depression: Three questions for psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef]
- Li-Tempel, T.; Larra, M.F.; Winnikes, U.; Tempel, T.; DeRijk, R.H.; Schulz, A.; Schächinger, H.; Meyer, J.; Schote, A.B. Polymorphisms of genes related to the hypothalamic-pituitary-adrenal axis influence the cortisol awakening response as well as self-perceived stress. Biol. Psychol. 2016, 119, 112–121. [Google Scholar] [CrossRef]
- de Assis, G.G.; Gasanov, E.V. BDNF and cortisol integrative system–plasticity vs. degeneration: Implications of the Val66Met polymorphism. Front. Neuroendocrinol. 2019, 55, 100784. [Google Scholar] [CrossRef]
- Azkona Mendoza, G. Implementing the 3Rs in Laboratory Animal Research—From Theory to Practice. Animals 2023, 13, 3063. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U. The ARRIVE guidelines 2.0, Updated guidelines for reporting animal research. J. Cereb. Blood Flow. Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed]
- Bayne, K.; Turner, P.V. Animal welfare standards and international collaborations. ILAR J. 2019, 60, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Zeeb, S.; Heyne, E.; Koch, L.G.; Britton, S.L.; Doenst, T. High aerobic exercise capacity predicts increased mitochondrial response to exercise training. Eur. Heart J. 2020, 41, ehaa946-3607. [Google Scholar] [CrossRef]
- Tannenbaum, J. Ethics and pain research in animals. ILAR J. 1999, 40, 97–110. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Franczak, E.; Maurer, A.; Drummond, V.C.; Kugler, B.A.; Wells, E.; Wenger, M.; Peelor, F.F., III; Crosswhite, A.; McCoin, C.S.; Koch, L.G. Divergence in aerobic capacity and energy expenditure influence metabolic tissue mitochondrial protein synthesis rates in aged rats. Geroscience 2024, 46, 2207–2222. [Google Scholar] [CrossRef]
- Mäkinen, E.; Wikgren, J.; Pekkala, S.; Koch, L.G.; Britton, S.L.; Nokia, M.S.; Lensu, S. Genotype determining aerobic exercise capacity associates with behavioral plasticity in middle-aged rats. Behav. Brain Res. 2023, 443, 114331. [Google Scholar] [CrossRef]
- Biesiadecki, B.J.; Brotto, M.A.; Brotto, L.S.; Koch, L.G.; Britton, S.L.; Nosek, T.M.; Jin, J.-P. Rats genetically selected for low and high aerobic capacity exhibit altered soleus muscle myofilament functions. Am. J. Physiol.-Cell Physiol. 2020, 318, C422–C429. [Google Scholar] [CrossRef]
- Jones, J.H. Resource book for the design of animal exercise protocols. Am. J. Vet. Res. 2007, 68, 583. [Google Scholar] [CrossRef]
- Bittencourt, M.A.; Wanner, S.P.; Kunstetter, A.C.; Barbosa, N.H.S.; Walker, P.C.L.; Andrade, P.V.R.; Turnes, T.; Guglielmo, L.G.A. Comparative effects of two heat acclimation protocols consisting of high-intensity interval training in the heat on aerobic performance and thermoregulatory responses in exercising rats. PLoS ONE 2020, 15, e0229335. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67. [Google Scholar]
- Cohen, J. Statistical Power for the Behavioural Sciences; Lawrence Erlbaum: Hilsdale, NY, USA, 1988; Volume 58, pp. 7–19. [Google Scholar]
- Wisløff, U.; Helgerud, J.; Kemi, O.J.; Ellingsen, Ø. Intensity-controlled treadmill running in rats: V̇ o 2 max and cardiac hypertrophy. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H1301–H1310. [Google Scholar] [CrossRef]
- Teixeira-Coelho, F.; Fonseca, C.G.; Barbosa, N.H.S.; Vaz, F.F.; Cordeiro, L.M.d.S.; Coimbra, C.C.; Pires, W.; Soares, D.D.; Wanner, S.P. Effects of manipulating the duration and intensity of aerobic training sessions on the physical performance of rats. PLoS ONE 2017, 12, e0183763. [Google Scholar] [CrossRef]
- Terjung, R.L. Muscle fiber involvement during training of different intensities and durations. Am. J. Physiol.-Leg. Content 1976, 230, 946–950. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Smith, J.L.; Simpson, D.R. Muscle fibre type populations of human leg muscles. Histochem. J. 1975, 7, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Dudley, G.A.; Abraham, W.M.; Terjung, R.L. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J. Appl. Physiol. 1982, 53, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M. Invited review: The speed-duration relationship across the animal kingdom. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2023, 279, 111387. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, M.H.; Armstrong, R.B. Rat muscle blood flows as a function of time during prolonged slow treadmill exercise. Am. J. Physiol.-Heart Circ. Physiol. 1983, 244, H814–H824. [Google Scholar] [CrossRef]
- Menezes, H.S.; Coracini, J.C.D.; Kepler, K.C.; Frantz, E.; Abegg, M.P.; Correa, C.A.; Cirino, S.L.M.B. Ácido Láctico como indicativo de aptidão física em ratos. Rev. Bras. Med. Esporte 2010, 16, 210–214. [Google Scholar] [CrossRef]
| (a) First four weeks of treadmill running for Rattus norvegicus | ||||||||
| Week 1 | Week 2 | Week 3 | Week 4 | |||||
| % Speed | Time (min) | % Speed | Time (min) | % Speed | Time (min) | % Speed | Time (min) | |
| Day 1 | 40% | 2 | 40% | 4 | 40% | 5 | 40% | 5 |
| 50% | 3 | 50% | 12 | 50% | 15 | 50% | 13 | |
| 60% | 5 | 60% | 19 | 60% | 20 | 60% | 24 | |
| 30% | 2 | 30% | 5 | 30% | 5 | 30% | 3 | |
| Day 2 | 40% | 2 | 40% | 5 | 40% | 5 | 40% | 5 |
| 50% | 8 | 50% | 17 | 50% | 20 | 50% | 20 | |
| 60% | 7 | 60% | 21 | 60% | 32 | 60% | 30 | |
| 30% | 3 | 30% | 5 | 30% | 3 | 30% | 5 | |
| Day 3 | 40% | 3 | 40% | 2 | 40% | 4 | 40% | 5 |
| 50% | 6 | 50% | 13 | 50% | 13 | 50% | 20 | |
| 60% | 13 | 60% | 20 | 60% | 24 | 60% | 30 | |
| 30% | 3 | 30% | 5 | 30% | 4 | 30% | 5 | |
| Day 4 | 40% | 3 | 40% | 3 | 40% | 3 | 40% | 4 |
| 50% | 9 | 50% | 21 | 50% | 21 | 50% | 10 | |
| 60% | 15 | 60% | 26 | 60% | 33 | 60% | 20 | |
| 30% | 3 | 30% | 5 | 30% | 3 | 30% | 2 | |
| Day 5 | 40% | 3 | 40% | 4 | 40% | 3 | Tmax2 | |
| 50% | 11 | 50% | 23 | 50% | 19 | |||
| 60% | 18 | 60% | 28 | 60% | 35 | |||
| 30% | 4 | 30% | 5 | 30% | 3 | |||
| (b) Second four weeks of treadmill running for Rattus norvegicus | ||||||||
| Week 5 | Week 6 | Week 7 | Week 8 | |||||
| % Speed | Time (min) | % Speed | Time (min) | % Speed | Time (min) | % Speed | Time (min) | |
| Day 1 | 24% | 2 | 24% | 2 | 32% | 2 | 32% | 3 |
| 32% | 2 | 32% | 2 | 40% | 3 | 44% | 3 | |
| 40% | 5 | 44% | 8 | 44% | 3 | 52% | 6 | |
| 44% | 8 | 52% | 12 | 52% | 12 | 60% | 32 | |
| 52% | 9 | 60% | 15 | 60% | 20 | 32% | 4 | |
| 60% | 2 | 32% | 6 | 64% | 2 | - | - | |
| 44% | 12 | - | - | 32% | 3 | - | - | |
| 24% | 5 | - | - | - | - | - | - | |
| Day 2 | 32% | 5 | 32% | 3 | 24% | 3 | 32% | 3 |
| 40% | 7 | 40% | 3 | 36% | 3 | 44% | 3 | |
| 44% | 19 | 44% | 6 | 44% | 3 | 52% | 12 | |
| 52% | 23 | 52% | 18 | 52% | 14 | 60% | 36 | |
| 32% | 6 | 60% | 22 | 60% | 30 | 32% | 4 | |
| - | - | 32% | 5 | 32% | 6 | - | - | |
| - | - | - | - | - | - | - | - | |
| Day 3 | 24% | 2 | 32% | 3 | 32% | 3 | 32% | 3 |
| 32% | 3 | 40% | 3 | 44% | 3 | 44% | 3 | |
| 44% | 5 | 44% | 3 | 52% | 6 | 52% | 6 | |
| 52% | 20 | 52% | 10 | 60% | 26 | 60% | 10 | |
| 60% | 5 | 60% | 21 | 64% | 4 | 64% | 2 | |
| 40% | 5 | 32% | 5 | 32% | 3 | 68% | 2 | |
| 32% | 5 | - | - | - | - | 60% | 10 | |
| - | - | - | - | - | - | 32% | 4 | |
| Day 4 | 32% | 5 | 32% | 3 | 24% | 3 | 32% | 3 |
| 40% | 5 | 40% | 3 | 36% | 3 | 40% | 5 | |
| 44% | 8 | 44% | 3 | 44% | 3 | 44% | 8 | |
| 52% | 12 | 52% | 10 | 52% | 8 | 52% | 17 | |
| 60% | 10 | 60% | 28 | 60% | 38 | 60% | 22 | |
| 32% | 5 | 32% | 6 | 32% | 5 | 32% | 5 | |
| - | - | - | - | - | - | - | - | |
| Day 5 | 32% | 2 | 32% | 3 | 32% | 3 | Tmax3 | |
| 40% | 3 | 40% | 3 | 40% | 3 | |||
| 44% | 5 | 44% | 3 | 44% | 3 | |||
| 52% | 20 | 52% | 10 | 52% | 9 | |||
| 60% | 15 | 60% | 30 | 60% | 30 | |||
| 32% | 5 | 32% | 6 | 64% | 6 | |||
| - | - | - | - | 32% | 3 | |||
| Variables | Training (n = 20) | Sedentary (n = 20) | p-Value |
|---|---|---|---|
| Adaptation sessions (n) | 6.5 ± 2.3 | 5.8 ± 1.8 | 0.291 |
| Weight (g)—Tmax1 | 261.0 ± 13.1 | 256.0 ± 13.6 | 0.244 |
| Weight (g)—Tmax2 | 378.0 ± 14.0 | 357.0 ± 19.8 | 0.0004 |
| Weight (g)—Tmax3 | 422.0 ± 15.9 | 402.0 ± 24.5 | 0.004 |
| Variable | Time: | Tmax1 | Tmax2 | Tmax3 | General Linear Model (Effects) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition: | Training | Sedentary | Training | Sedentary | Training | Sedentary | Time | Condition | Interaction | |
| Mean ± SD | p-Value (η2p) | |||||||||
| Treadmill Time (s) | 529.0 ± 88.8 | 523.0 ± 78.1 | 831.0 ± 1146 †⸸ | 625.0 ± 133.3 † | 754.0 ± 91.1 †⸸ | 608.0 ± 94.2 † | <0.001 (0.430) | <0.001 (0.266) | <0.001 (0.152) | |
| Workload (J) | 202.0 ± 65.9 | 191.8 ± 55.7 | 687.5 ± 184.0 †⸸ | 375.6 ± 151.7 | 651.9 ± 166.7 †⸸ | 399.3 ± 121.7 | <0.001 (0.591) | <0.001 (0.351) | <0.001 (0.201) | |
| Relative Workload (J/kg) | 0.8 ± 0.3 | 0.7 ± 0.2 | 1.8 ± 0.5 †⸸ | 1.0 ± 0.4 † | 1.5 ± 0.4 †⸸ | 1.0 ± 0.3 † | <0.001 (0.389) | <0.001 (0.282) | <0.001 (0.159) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Assis, G.G.; de Souza, E.O.N.; de Almeida-Neto, P.F.; Ceylan, H.İ.; Bragazzi, N.L. A Proposal for a Noxious Stimuli-Free, Moderate-Intensity Treadmill Running Protocol to Improve Aerobic Performance in Experimental Research on Rats. Metabolites 2024, 14, 534. https://doi.org/10.3390/metabo14100534
de Assis GG, de Souza EON, de Almeida-Neto PF, Ceylan Hİ, Bragazzi NL. A Proposal for a Noxious Stimuli-Free, Moderate-Intensity Treadmill Running Protocol to Improve Aerobic Performance in Experimental Research on Rats. Metabolites. 2024; 14(10):534. https://doi.org/10.3390/metabo14100534
Chicago/Turabian Stylede Assis, Gilmara Gomes, Elda Olivia Nobre de Souza, Paulo Francisco de Almeida-Neto, Halil İbrahim Ceylan, and Nicola Luigi Bragazzi. 2024. "A Proposal for a Noxious Stimuli-Free, Moderate-Intensity Treadmill Running Protocol to Improve Aerobic Performance in Experimental Research on Rats" Metabolites 14, no. 10: 534. https://doi.org/10.3390/metabo14100534
APA Stylede Assis, G. G., de Souza, E. O. N., de Almeida-Neto, P. F., Ceylan, H. İ., & Bragazzi, N. L. (2024). A Proposal for a Noxious Stimuli-Free, Moderate-Intensity Treadmill Running Protocol to Improve Aerobic Performance in Experimental Research on Rats. Metabolites, 14(10), 534. https://doi.org/10.3390/metabo14100534









