Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms
Abstract
1. Introduction
2. Methods
3. The Effects of HRW on Exercise Performance Enhancement
3.1. Significant Outcomes
3.2. Non-Significant Outcomes
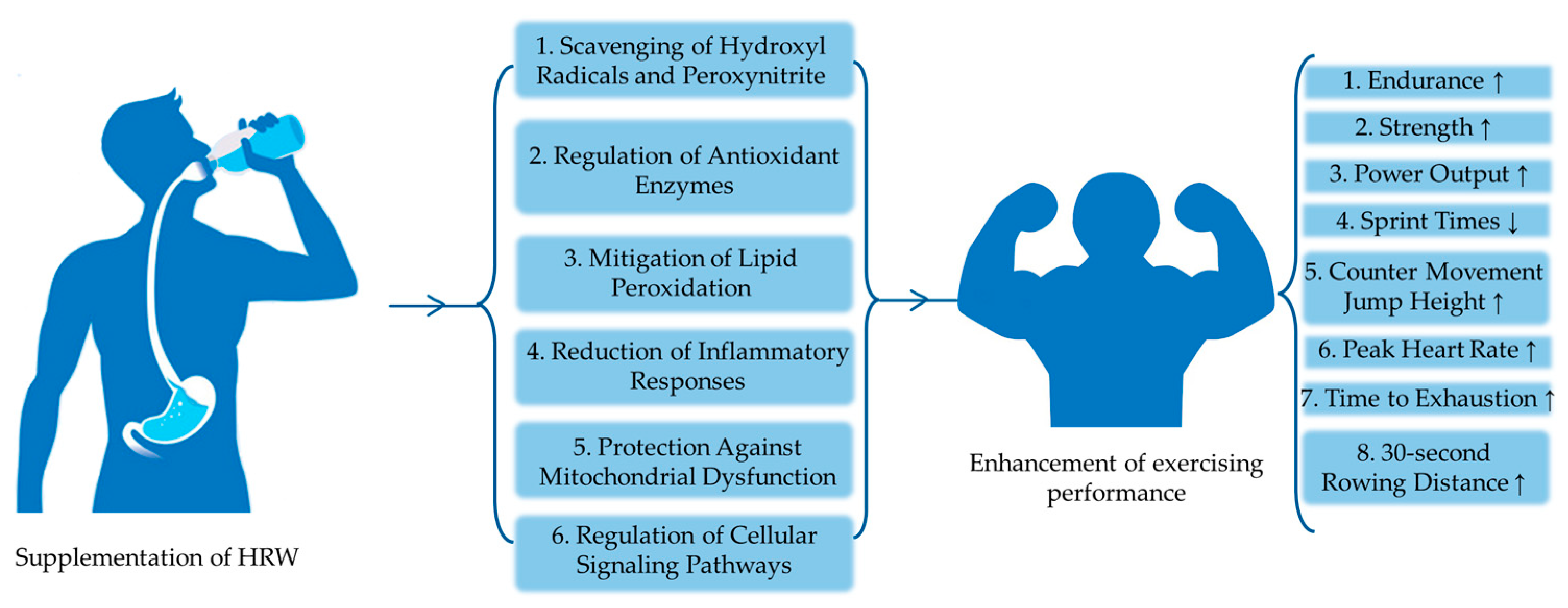
4. Potential Mechanisms of Action
4.1. HRW as a Selective Antioxidant
4.2. Effects of HRW on Exercise through Oxidative Stress Mechanisms
4.2.1. Muscle Fatigue
4.2.2. Inflammation
4.2.3. Performance Decline
4.3. Roles of HRW in Redox Homeostasis
4.3.1. Scavenging of Hydroxyl Radicals and Peroxynitrite
4.3.2. Regulation of Antioxidant Enzymes
4.3.3. Mitigation of Lipid Peroxidation
4.3.4. Reduction of Inflammatory Responses
4.3.5. Protection against Mitochondrial Dysfunction
4.3.6. Regulation of Cellular Signaling Pathways
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhillon, G.; Buddhavarapu, V.; Grewal, H.; Sharma, P.; Verma, R.K.; Munjal, R.; Devadoss, R.; Kashyap, R. Hydrogen Water: Extra Healthy or a Hoax?-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 973. [Google Scholar] [CrossRef] [PubMed]
- Sladeckova, B.; Botek, M.; Krejci, J.; Valenta, M.; McKune, A.; Neuls, F.; Klimesova, I. Hydrogen-rich water supplementation promotes muscle recovery after two strenuous training sessions performed on the same day in elite fin swimmers: Randomized, double-blind, placebo-controlled, crossover trial. Front. Physiol. 2024, 15, 1321160. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Kabayama, S.; Watanabe, Y.; Cui, Y. Health Benefits of Electrolyzed Hydrogen Water: Antioxidant and Anti-Inflammatory Effects in Living Organisms. Antioxidants 2024, 13, 313. [Google Scholar] [CrossRef]
- Dong, G.; Fu, J.; Bao, D.; Zhou, J. Short-Term Consumption of Hydrogen-Rich Water Enhances Power Performance and Heart Rate Recovery in Dragon Boat Athletes: Evidence from a Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 5413. [Google Scholar] [CrossRef]
- Koh, B.; Freeman, L.; Zaslawski, C. Alternative medicine and doping in sports. Australas. Med. J. 2012, 5, 18–25. [Google Scholar] [CrossRef]
- Hancock, J.T.; Russell, G. Downstream Signalling from Molecular Hydrogen. Plants 2021, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef]
- Ruan, Y.; Yuan, P.-P.; Li, P.-Y.; Chen, Y.; Fu, Y.; Gao, L.-Y.; Wei, Y.-X.; Zheng, Y.-J.; Li, S.-F.; Feng, W.-S.; et al. Tingli Dazao Xiefei Decoction ameliorates asthma in vivo and in vitro from lung to intestine by modifying NO–CO metabolic disorder mediated inflammation, immune imbalance, cellular barrier damage, oxidative stress and intestinal bacterial disorders. J. Ethnopharmacol. 2023, 313, 116503. [Google Scholar] [CrossRef]
- Siauciunaite, R.; Foulkes, N.S.; Calabro, V.; Vallone, D. Evolution Shapes the Gene Expression Response to Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 3040. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; He, Q.; Li, S.; Liu, T.; Zhang, J. Hydrogen-Rich Water Mitigates LPS-Induced Chronic Intestinal Inflammatory Response in Rats via Nrf-2 and NF-kappaB Signaling Pathways. Vet. Sci. 2022, 9, 621. [Google Scholar] [CrossRef]
- Lee, J.; Yang, G.; Kim, Y.J.; Tran, Q.H.; Choe, W.; Kang, I.; Kim, S.S.; Ha, J. Hydrogen-rich medium protects mouse embryonic fibroblasts from oxidative stress by activating LKB1-AMPK-FoxO1 signal pathway. Biochem. Biophys. Res. Commun. 2017, 491, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kashio, A.; Sakamoto, T.; Suzukawa, K.; Kakigi, A.; Yamasoba, T. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci. Lett. 2011, 487, 12–16. [Google Scholar] [CrossRef]
- Li, J.; Hong, Z.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-Rich Saline Promotes the Recovery of Renal Function after Ischemia/Reperfusion Injury in Rats via Anti-apoptosis and Anti-inflammation. Front. Pharmacol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Todorovic, N.; Javorac, D.; Stajer, V.; Ostojic, S.M. The Effects of Supersaturated Hydrogen-Rich Water Bathing on Biomarkers of Muscular Damage and Soreness Perception in Young Men Subjected to High-Intensity Eccentric Exercise. J. Sports Med. 2020, 2020, 8836070. [Google Scholar] [CrossRef]
- Botek, M.; Khanna, D.; Krejci, J.; Valenta, M.; McKune, A.; Sladeckova, B.; Klimesova, I. Molecular Hydrogen Mitigates Performance Decrement during Repeated Sprints in Professional Soccer Players. Nutrients 2022, 14, 508. [Google Scholar] [CrossRef]
- Timon, R.; Olcina, G.; Gonzalez-Custodio, A.; Camacho-Cardenosa, M.; Camacho-Cardenosa, A.; Martinez Guardado, I. Effects of 7-day intake of hydrogen-rich water on physical performance of trained and untrained subjects. Biol. Sport 2021, 38, 269–275. [Google Scholar] [CrossRef]
- Farley, J.B.; Stein, J.; Keogh, J.W.L.; Woods, C.T.; Milne, N. The Relationship Between Physical Fitness Qualities and Sport-Specific Technical Skills in Female, Team-Based Ball Players: A Systematic Review. Sports Med. Open 2020, 6, 18. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-rich water reduces inflammatory responses and prevents apoptosis of peripheral blood cells in healthy adults: A randomized, double-blind, controlled trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.; Xue, Y.; Hou, D.; Chen, S.; Xu, Z.; Peng, S.; Zhao, H.; Wang, C.; Liu, C. Timing Matters: Time of Day Impacts the Ergogenic Effects of Caffeine-A Narrative Review. Nutrients 2024, 16, 1421. [Google Scholar] [CrossRef] [PubMed]
- Botek, M.; Krejci, J.; McKune, A.; Valenta, M.; Sladeckova, B. Hydrogen Rich Water Consumption Positively Affects Muscle Performance, Lactate Response, and Alleviates Delayed Onset of Muscle Soreness After Resistance Training. J. Strength Cond. Res. 2022, 36, 2792–2799. [Google Scholar] [CrossRef]
- Da Ponte, A.; Giovanelli, N.; Nigris, D.; Lazzer, S. Effects of hydrogen rich water on prolonged intermittent exercise. J. Sports Med. Phys. Fit. 2018, 58, 612–621. [Google Scholar] [CrossRef]
- Jebabli, N.; Ouerghi, N.; Abassi, W.; Yagin, F.H.; Khlifi, M.; Boujabli, M.; Bouassida, A.; Ben Abderrahman, A.; Ardigo, L.P. Acute effect of hydrogen-rich water on physical, perceptual and cardiac responses during aerobic and anaerobic exercises: A randomized, placebo-controlled, double-blinded cross-over trial. Front. Physiol. 2023, 14, 1240871. [Google Scholar] [CrossRef]
- Valenta, M.; Botek, M.; Krejci, J.; McKune, A.; Sladeckova, B.; Neuls, F.; Bajgar, R.; Klimesova, I. Acute pre-exercise hydrogen rich water intake does not improve running performance at maximal aerobic speed in trained track and field runners: A randomized, double-blind, placebo-controlled crossover study. PLoS ONE 2022, 17, e0279307. [Google Scholar] [CrossRef]
- Botek, M.; Krejci, J.; McKune, A.J.; Sladeckova, B. Hydrogen-Rich Water Supplementation and Up-Hill Running Performance: Effect of Athlete Performance Level. Int. J. Sports Physiol. Perform. 2020, 15, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular Hydrogen Therapy—A Review on Clinical Studies and Outcomes. Molecules 2023, 28, 7785. [Google Scholar] [CrossRef]
- Li, S.Y.; Xue, R.Y.; Wu, H.; Pu, N.; Wei, D.; Zhao, N.; Song, Z.M.; Tao, Y. Novel Role of Molecular Hydrogen: The End of Ophthalmic Diseases? Pharmaceuticals 2023, 16, 1567. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The Key Roles of ROS and RNS as a Signaling Molecule in Plant-Microbe Interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 628157. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- de Almeida, A.; de Oliveira, J.; da Silva Pontes, L.V.; de Souza Junior, J.F.; Goncalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.J.; Hsu, M.K.; Cai, J.R.; Wang, H.Y. A strategy of novel molecular hydrogen-producing antioxidative auxiliary system improves virus production in cell bioreactor. Sci. Rep. 2024, 14, 4092. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-Gonzalez, A.; Madrigal-Santillan, E.O.; Morales-Martinez, M.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I.; Gutierrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life 2021, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Eckl, P. Impact of oxidative stress on exercising skeletal muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef] [PubMed]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef]
- Li, Y.; Shen, C.; Zhou, X.; Zhang, J.; Lai, X.; Zhang, Y. Local Treatment of Hydrogen-Rich Saline Promotes Wound Healing In Vivo by Inhibiting Oxidative Stress via Nrf-2/HO-1 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 2949824. [Google Scholar] [CrossRef]
- Clemente-Suarez, V.J.; Bustamante-Sanchez, A.; Mielgo-Ayuso, J.; Martinez-Guardado, I.; Martin-Rodriguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Liu, Y.; Zhang, Z. Effects of Exercise-Induced ROS on the Pathophysiological Functions of Skeletal Muscle. Oxidative Med. Cell. Longev. 2021, 2021, 3846122. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef]
- Yang, C.F.; Tsai, W.C. Calmodulin: The switch button of calcium signaling. Tzu Chi Med. J. 2022, 34, 15–22. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef] [PubMed]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Kuzmiak-Glancy, S.; Glancy, B.; Kay, M.W. Ischemic damage to every segment of the oxidative phosphorylation cascade elevates ETC driving force and ROS production in cardiac mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H499–H512. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef]
- Leermakers, P.A.; Dybdahl, K.L.T.; Husted, K.S.; Riisager, A.; de Paoli, F.V.; Pinos, T.; Vissing, J.; Krag, T.O.B.; Pedersen, T.H. Depletion of ATP Limits Membrane Excitability of Skeletal Muscle by Increasing Both ClC1-Open Probability and Membrane Conductance. Front. Neurol. 2020, 11, 541. [Google Scholar] [CrossRef]
- Theofilidis, G.; Bogdanis, G.C.; Koutedakis, Y.; Karatzaferi, C. Monitoring Exercise-Induced Muscle Fatigue and Adaptations: Making Sense of Popular or Emerging Indices and Biomarkers. Sports 2018, 6, 153. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediat. Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Gaspar, D.; Flores-Felix, J.D.; Falcao, A.; Alves, G.; Silva, L.R. Effects of Functional Phenolics Dietary Supplementation on Athletes’ Performance and Recovery: A Review. Int. J. Mol. Sci. 2022, 23, 4652. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Su, C.H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef]
- Gallo, J.; Raska, M.; Kriegova, E.; Goodman, S.B. Inflammation and its resolution and the musculoskeletal system. J. Orthop. Transl. 2017, 10, 52–67. [Google Scholar] [CrossRef]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Franzoni, F.; Scarfo, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin; Ali, A. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J. Clin. Biochem. IJCB 2015, 30, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Vilema-Enriquez, G.; Arroyo, A.; Grijalva, M.; Amador-Zafra, R.I.; Camacho, J. Molecular and Cellular Effects of Hydrogen Peroxide on Human Lung Cancer Cells: Potential Therapeutic Implications. Oxidative Med. Cell. Longev. 2016, 2016, 1908164. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.E.; Malki, M.I. DNA Damage/Repair Management in Cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Mao, L.; Zhao, M.; Yang, B.; Tao, X.; Li, Y.; Yin, G. Hydrogen: A Novel Treatment Strategy in Kidney Disease. Kidney Dis. 2022, 8, 126–136. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Heck, D.E.; Shakarjian, M.; Kim, H.D.; Laskin, J.D.; Vetrano, A.M. Mechanisms of oxidant generation by catalase. Ann. N. Y. Acad. Sci. 2010, 1203, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, G.W.; Fong, C.S.; Alic, N.; Higgins, V.J.; Dawes, I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: Oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 2004, 101, 6564–6569. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhang, J. Molecular hydrogen is a promising therapeutic agent for pulmonary disease. J. Zhejiang Univ. Sci. B 2022, 23, 102–122. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.A.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Cho, I.; Jackson, M.R.; Swift, J. Roles of Cross-Membrane Transport and Signaling in the Maintenance of Cellular Homeostasis. Cell. Mol. Bioeng. 2016, 9, 234–246. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid Peroxidation-Derived Aldehydes, 4-Hydroxynonenal and Malondialdehyde in Aging-Related Disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Forcados, G.E.; Muhammad, A.; Oladipo, O.O.; Makama, S.; Meseko, C.A. Metabolic Implications of Oxidative Stress and Inflammatory Process in SARS-CoV-2 Pathogenesis: Therapeutic Potential of Natural Antioxidants. Front. Cell. Infect. Microbiol. 2021, 11, 654813. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.M.; Hou, Y.C.; Liao, M.T.; Tsai, K.W.; Hu, W.C.; Yeh, C.C.; Lu, K.C. Potential role of molecular hydrogen therapy on oxidative stress and redox signaling in chronic kidney disease. Biomed. Pharmacother. Biomed. Pharmacother. 2024, 176, 116802. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Takahara, T.; Que, W.; Fujino, M.; Guo, W.Z.; Hirano, S.I.; Ye, L.P.; Li, X.K. Hydrogen-rich water protects against liver injury in nonalcoholic steatohepatitis through HO-1 enhancement via IL-10 and Sirt 1 signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G450–G463. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Lee, H.J.; Kim, S.H.; Lee, S.K.; Kim, H.R.; Pae, H.O.; Chung, H.T.; Shin, H.I.; Lee, S.K.; Kim, E.C. Hydrogen peroxide induces heme oxygenase-1 and dentin sialophosphoprotein mRNA in human pulp cells. J. Endod. 2008, 34, 983–989. [Google Scholar] [CrossRef]
- Liu, C.; Arnold, R.; Henriques, G.; Djabali, K. Inhibition of JAK-STAT Signaling with Baricitinib Reduces Inflammation and Improves Cellular Homeostasis in Progeria Cells. Cells 2019, 8, 1276. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Osko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczynska, K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Cheng, D.; Long, J.; Zhao, L.; Liu, J. Hydrogen: A Rising Star in Gas Medicine as a Mitochondria-Targeting Nutrient via Activating Keap1-Nrf2 Antioxidant System. Antioxidants 2023, 12, 2062. [Google Scholar] [CrossRef]
- Jia, Q.; Sieburth, D. Mitochondrial hydrogen peroxide positively regulates neuropeptide secretion during diet-induced activation of the oxidative stress response. Nat. Commun. 2021, 12, 2304. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Fernandez-Checa, J.C. Mitochondrial Oxidative Stress and Antioxidants Balance in Fatty Liver Disease. Hepatol. Commun. 2018, 2, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.I.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Clinical Use and Treatment Mechanism of Molecular Hydrogen in the Treatment of Various Kidney Diseases including Diabetic Kidney Disease. Biomedicines 2023, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Diao, S.; Fan, Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem Cell Res. Ther. 2021, 12, 140. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169 (Suppl. S2), 1–8. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Fang, W.; Tang, L.; Wang, G.; Lin, J.; Liao, W.; Pan, W.; Xu, J. Molecular Hydrogen Protects Human Melanocytes from Oxidative Stress by Activating Nrf2 Signaling. J. Investig. Dermatol. 2020, 140, 2230–2241 e2239. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Z.; Wang, F.; Han, Z.; Wang, Y.; Huang, S.; Hu, T.; Guo, M.; Lei, P. Hydrogen exerts neuroprotection by activation of the miR-21/PI3K/AKT/GSK-3beta pathway in an in vitro model of traumatic brain injury. J. Cell. Mol. Med. 2020, 24, 4061–4071. [Google Scholar] [CrossRef]
- Guo, L.; Liu, M.; Duan, T. Hydrogen suppresses oxidative stress by inhibiting the p38 MAPK signaling pathway in preeclampsia. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2023, 32, 357–367. [Google Scholar] [CrossRef]
- Ma, T.; Yang, L.; Zhang, B.; Lv, X.; Gong, F.; Yang, W. Hydrogen inhalation enhances autophagy via the AMPK/mTOR pathway, thereby attenuating doxorubicin-induced cardiac injury. Int. Immunopharmacol. 2023, 119, 110071. [Google Scholar] [CrossRef]
| No. [Ref.] | Number of Subjects | Dosage (mL) | Findings |
|---|---|---|---|
| 1 [17] | 16 | 1260 | The study observed an improvement in sprint times, while the lactate concentration and perceived exertion ratings remained unchanged. |
| 2 [22] | 12 | 1260 | The study observed improvements in lunges and muscle function, a reduction in the lactate response, and the alleviation of delayed onset muscle soreness. |
| 3 [2] | 12 | 2520 | The study observed improvements in countermovement jump height and reductions in creatine kinase blood activity and muscle soreness following training. |
| 4 [23] | 8 | 2000 | The study observed enhancements in mean and peak power output, time to peak power, fatigue index, and total work capacity. |
| 5 [24] | 22 | 500 | The study observed improvements in maximal aerobic speed during the Vameval test, time to exhaustion at the maximal aerobic speed, perceived exertion rate, and peak heart rate. However, no significant changes were found in squat jump, countermovement jump, and five jump test performance. |
| 6 [4] | 18 | 1000 | The study observed improvements in both the maximum and average power during a 30-s rowing test, along with a decrease in maximum heart rate during the test period. Additionally, heart rate dropped significantly after 2 min of recovery. |
| 7 [18] | 37 | 1920–2240 | The study observed performance improvements in trained cyclists, including increased peak and mean power outputs, along with a decreased fatigue index in the anaerobic test. |
| 8 [25] | 24 | 1260 | The study observed improvements in time to exhaustion, post-exercise blood lactate concentration, maximal heart rate, and oxygen uptake. However, no assessed variables were significantly correlated with time to exhaustion. |
| 9 [26] | 16 | 1680 | The study observed no significant changes in race time, average race heart rate, and the rating of perceived exertion immediately after the race. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, H.; Zhang, Y.; Zhao, Y.; Wang, C.; Liu, C. Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms. Metabolites 2024, 14, 537. https://doi.org/10.3390/metabo14100537
Zhou Q, Li H, Zhang Y, Zhao Y, Wang C, Liu C. Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms. Metabolites. 2024; 14(10):537. https://doi.org/10.3390/metabo14100537
Chicago/Turabian StyleZhou, Qiaorui, Huixin Li, Ye Zhang, Yirui Zhao, Can Wang, and Chang Liu. 2024. "Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms" Metabolites 14, no. 10: 537. https://doi.org/10.3390/metabo14100537
APA StyleZhou, Q., Li, H., Zhang, Y., Zhao, Y., Wang, C., & Liu, C. (2024). Hydrogen-Rich Water to Enhance Exercise Performance: A Review of Effects and Mechanisms. Metabolites, 14(10), 537. https://doi.org/10.3390/metabo14100537






