Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes
Abstract
1. Introduction
2. Materials and Methods
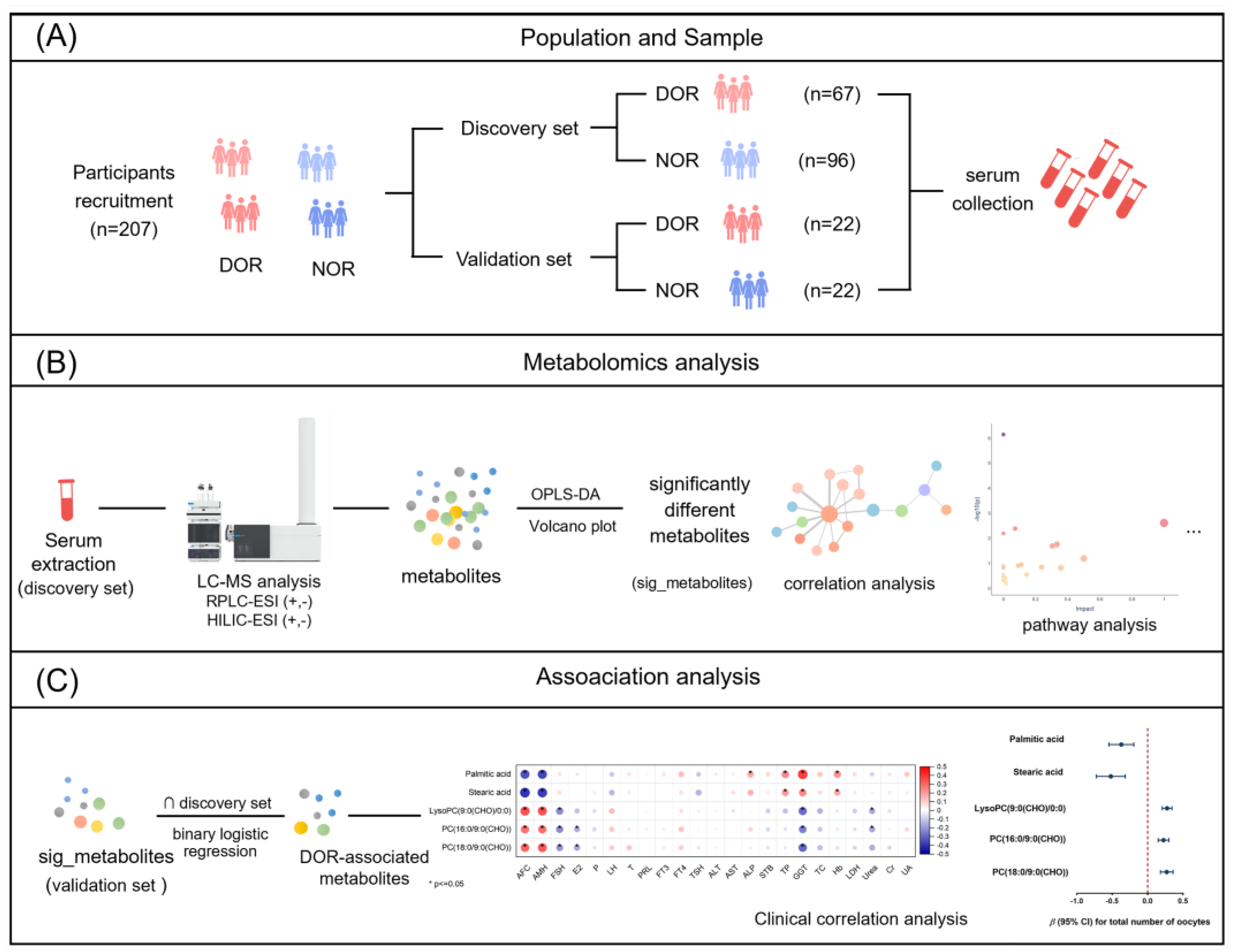
2.1. Participants
2.2. Chemicals and Reagents
2.3. Serum Sample Collection and Preparation
2.4. LC-MS Analysis
2.5. Data Processing
2.6. IVF Outcome Assessment
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Metabolomic Profiling
3.3. Screening and Annotation of Significantly DOR-Associated Metabolites
3.4. Correlation Analysis of Significantly Different Metabolites
3.5. Pathway Analysis and Enrichment Analysis for DOR-Associated Metabolites
3.6. Development of a DOR-Associated Metabolite Panel
3.7. Associations with IVF Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiware, T.M.; Vermeulen, N.; Blondeel, K.; Farquharson, R.; Kiarie, J.; Lundin, K.; Matsaseng, T.C.; Ombelet, W.; Toskin, I. IVF and other ART in low- and middle-income countries: A systematic landscape analysis. Hum. Reprod. Update 2021, 27, 213–228. [Google Scholar] [CrossRef]
- Hershko Klement, A.; Oron, G.; Bentov, Y. Editorial: The expansion of female fertility. Front. Reprod. Health 2021, 3, 781019. [Google Scholar] [CrossRef]
- Dong, L.; Xin, X.; Chang, H.M.; Leung, P.C.K.; Yu, C.; Lian, F.; Wu, H. Expression of long noncoding RNAs in the ovarian granulosa cells of women with diminished ovarian reserve using high-throughput sequencing. J. Ovarian Res. 2022, 15, 119. [Google Scholar] [CrossRef]
- Feng, X.; Luo, J.; Wang, X.; Xie, W.; Jiao, J.; Wu, X.; Fan, L.; Qin, G. Association of exposure to ambient air pollution with ovarian reserve among women in Shanxi province of north China. Environ. Pollut. 2021, 278, 116868. [Google Scholar] [CrossRef]
- Choi, R.; Park, W.; Chun, G.; Lee, S.G.; Lee, E.H. Investigation of the prevalence of diminished ovarian reserve in korean women of reproductive age. J. Clin. Med. 2023, 12, 5099. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, Y.O.; Silachev, D.N.; Nazarenko, T.A.; Birukova, A.M.; Vishnyakova, P.A.; Sukhikh, G.T. Stem-cell-derived extracellular vesicles: Unlocking new possibilities for treating diminished ovarian reserve and premature ovarian insufficiency. Life 2023, 13, 2247. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, Y.; Li, X.; Jiang, F.; Zhang, Y.; Ma, J.; Song, Y.; Ma, J.; Fu, W.; Pang, R.; et al. A Lancet Commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet 2021, 397, 2497–2536. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, D.; Wu, H.; Li, R.; Xu, S.; Kang, Y.; Cao, Y.; Chen, X.; Zhu, Y.; Xu, S.; et al. Epidemiology of infertility in China: A population-based study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Wild, R.A.; Hansen, K.R. Diminished ovarian reserve: Risk for preeclampsia in in vitro fertilization pregnancies. Fertil. Steril. 2023, 119, 802–803. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.M.; Dyer, S.; Zegers-Hochschild, F.; de Mouzon, J.; Ishihara, O.; Banker, M.; Mansour, R.; Kupka, M.S.; Adamson, G.D. International committee for monitoring assisted reproductive technologies world report: Assisted reproductive technology, 2014. Hum. Reprod. 2021, 36, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Zegers-Hochschild, F.; Dyer, S.; Chambers, G.; de Mouzon, J.; Ishihara, O.; Kupka, M.; Banker, M.; Jwa, S.C.; Elgindy, E.; et al. International Committee for Monitoring Assisted Reproductive Technology: World Report on Assisted Reproductive Technology. 2018. Available online: https://www.icmartivf.org/reports-publications/ (accessed on 1 February 2020).
- Génard-Walton, M.; Warembourg, C.; Duros, S.; Mercier, F.; Lefebvre, T.; Guivarc’h-Levêque, A.; Le Martelot, M.T.; Le Bot, B.; Jacquemin, B.; Chevrier, C.; et al. Serum persistent organic pollutants and diminished ovarian reserve: A single-exposure and mixture exposure approach from a French case–control study. Hum. Reprod. 2023, 38, 701–715. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Ma, J.; Ma, H.; Liang, X. Potential factors result in diminished ovarian reserve: A comprehensive review. J. Ovarian Res. 2023, 16, 208. [Google Scholar] [CrossRef]
- Lu, N.; Gao, Y.; Hu, Y.; Jiang, C.; Diao, F.; Liu, J. Cumulative outcomes of minimal stimulation IVF for severe diminished ovarian reserve women: A retrospective cohort study. Authorea 2023. [Google Scholar] [CrossRef]
- Bancsi, L.F.; Broekmans, F.J.; Eijkemans, M.J.; de Jong, F.H.; Habbema, J.D.F.; te Velde, E.R. Predictors of poor ovarian response in in vitro fertilization: A prospective study comparing basal markers of ovarian reserve. Fertil. Steril. 2002, 77, 328–336. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, Q.; Zhu, Q.; He, R.; Li, Y.; Liu, C.; Wang, J.; Liang, X. Decreased fatty acids induced granulosa cell apoptosis in patients with diminished ovarian reserve. J. Assist. Reprod. Gen. 2022, 39, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Shah, D. Fertility with early reduction of ovarian reserve: The last straw that breaks the Camel’s back. Fertil. Res. Pract. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, B.; Jin, L. Perinatal outcome in young patients with diminished ovarian reserve undergoing assisted reproductive technology. Fertil. Steril. 2020, 114, 118–124.e1. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, F.; Yang, Y.; Yuan, Z.; Sun, C.; Zou, H.; Chen, H.; Yi, H.; Gao, S.H.; Zhang, S.; et al. The effect of growth hormone on the metabolome of follicular fluid in patients with diminished ovarian reserve. Reprod. Biol. Endocrinol. 2023, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Abhari, S.; Lu, J.; Hipp, H.S.; Petritis, B.; Gerkowicz, S.A.; Katler, Q.S.; Yen, H.H.; Mao, Y.; Tang, H.; Shang, W.; et al. A case-control study of follicular fluid cytokine profiles in women with diminished ovarian reserve. Reprod. Sci. 2022, 29, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Devine, K.; Mumford, S.L.; Wu, M.; DeCherney, A.H.; Hill, M.J.; Propst, A. Diminished ovarian reserve in the United States assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil. Steril. 2015, 104, 612–619.e3. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Yan, L.; Xu, X.; Chen, D.; Zhao, Y.; Qiao, J. Synthetic phenolic antioxidants and their metabolites in follicular fluid and association with diminished ovarian reserve: A case–control study. Environ. Health Perspect. 2023, 131, 11309. [Google Scholar] [CrossRef]
- Tian, T.; Hao, Y.; Wang, Y.; Xu, X.; Long, X.; Yan, L.; Zhao, Y.; Qiao, J. Mixed and single effects of endocrine disrupting chemicals in follicular fluid on likelihood of diminished ovarian reserve: A case-control study. Chemosphere 2023, 330, 138727. [Google Scholar] [CrossRef]
- Boucret, L.; Tramon, L.; Riou, J.; Ferré-L’Hôtellier, V.; Bouet, P.-E.; May-Panloup, P. Influence of diminished ovarian reserve on early embryo morphokinetics during in vitro fertilization: A time-lapse study. J. Clin. Med. 2022, 11, 7173. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xia, Z. Diminished ovarian reserve is associated with metabolic disturbances and hyperhomocysteinemia in women with infertility. J. Obstet. Gynaecol. 2023, 43, 2282722. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—A plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef]
- Xiao, J.; Song, J.; Sa, Y.; Yuan, L.; Guo, J.; Sun, Z. The mechanisms of improving ivf outcomes of liu-wei-di-huang pill acting on DOR patients. Evid. Based Complement. Altern. Med. 2020, 2020, 5183017. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Jin, H.; Huang, X.; Lin, J.; Wang, P. Comparison of modified agonist, mild-stimulation and antagonist protocols for in vitro fertilization in patients with diminished ovarian reserve. J. Int. Med. Res. 2018, 46, 2327–2337. [Google Scholar] [CrossRef]
- Buergel, T.; Steinfeldt, J.; Ruyoga, G.; Pietzner, M.; Bizzarri, D.; Vojinovic, D.; Upmeier Zu Belzen, J.; Loock, L.; Kittner, P.; Christmann, L.; et al. Metabolomic profiles predict individual multidisease outcomes. Nat. Med. 2022, 28, 2309–2320. [Google Scholar] [CrossRef]
- Jansson, J.K.; Baker, E.S. A multi-omic future for microbiome studies. Nat. Microbiol. 2016, 1, 16049. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Keshari, K.R. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell 2022, 185, 2678–2689. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L.; Gao, M.; Wei, L.; Liu, A.; Wang, B.; Wang, L.; Zhang, L.; Jia, T.; Wang, Y.; et al. The follicular fluid metabolome in infertile individuals between polycystic ovary syndrome and diminished ovarian reserve. Arch. Biochem. Biophys. 2022, 732, 109453. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Qi, C.; Hu, H.; Zhang, Q.; Zhu, X.; Fu, Y. UHPLC-MS-MS analysis of oxylipins metabolomics components of follicular fluid in infertile individuals with diminished ovarian reserve. Reprod. Biol. Endocrinol. 2021, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- de la Barca, J.M.C.; Boueilh, T.; Simard, G.; Boucret, L.; Ferre-L’Hotellier, V.; Tessier, L.; Gadras, C.; Bouet, P.E.; Descamps, P.; Procaccio, V.; et al. Targeted metabolomics reveals reduced levels of polyunsaturated choline plasmalogens and a smaller dimethylarginine/arginine ratio in the follicular fluid of patients with a diminished ovarian reserve. Hum. Reprod. 2017, 32, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhao, Z.; Yang, Y.; Liang, X. Using bioinformatics and metabolomics to identify altered granulosa cells in patients with diminished ovarian reserve. PeerJ 2020, 8, e9812. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Fan, Q.; Li, H.; Zhao, L.; Liu, C.; Liang, X. Cholesterol metabolism is decreased in patients with diminished ovarian reserve. Reprod. Biomed. Online 2022, 44, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Al Rashid, K.; Taylor, A.; Lumsden, M.A.; Goulding, N.; Lawlor, D.A.; Nelson, S.M. Association of the functional ovarian reserve with serum metabolomic profiling by nuclear magnetic resonance spectroscopy: A cross-sectional study of ~400 women. BMC Med. 2020, 18, 247. [Google Scholar] [CrossRef]
- Shen, H.; Gao, M.; Li, Q.; Sun, H.; Jiang, Y.; Liu, L.; Wu, J.; Yu, X.; Jia, T.; Xin, Y.; et al. Effect of PFOA exposure on diminished ovarian reserve and its metabolism. Reprod. Biol. Endocrinol. 2023, 21, 16. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, C.; Yuan, X.Q.; Yao, W.; Yao, Q.Y.; Huang, Y.; Li, N.J.; Deng, Y.L.; Chen, P.P.; Miao, Y.; et al. Urinary phthalate metabolites and the risk of endometrial polyp: A pilot study from the TREE cohort. Environ. Pollut. 2023, 317, 120711. [Google Scholar] [CrossRef]
- Liu, C.; Deng, Y.L.; Yuan, X.Q.; Chen, P.P.; Miao, Y.; Luo, Q.; Zhang, M.; Cui, F.P.; Yao, W.; Zeng, J.Y.; et al. Exposure to disinfection by-products and reproductive hormones among women: Results from the Tongji Reproductive and Environmental (TREE) study. Environ. Res. 2022, 209, 112863. [Google Scholar] [CrossRef]
- Deng, Y.L.; Luo, Q.; Liu, C.; Zeng, J.Y.; Lu, T.T.; Shi, T.; Cui, F.P.; Yuan, X.Q.; Miao, Y.; Zhang, M.; et al. Urinary biomarkers of exposure to drinking water disinfection byproducts and ovarian reserve: A cross-sectional study in China. J. Hazard. Mater. 2022, 421, 126683. [Google Scholar] [CrossRef]
- Chehab, R.F.; Ferrara, A.; Zheng, S.; Barupal, D.K.; Ngo, A.L.; Chen, L.; Fiehn, O.; Zhu, Y. In utero metabolomic signatures of refined grain intake and risk of gestational diabetes: A metabolome-wide association study. Am. J. Clin. Nutr. 2023, 117, 731–740. [Google Scholar] [CrossRef]
- DeFelice, B.C.; Mehta, S.S.; Samra, S.; Cajka, T.; Wancewicz, B.; Fahrmann, J.F.; Fiehn, O. Mass spectral feature list optimizer (MS-FLO): A tool to minimize false positive peak reports in untargeted liquid chromatography-mass spectroscopy (LC-MS) data processing. Anal. Chem. 2017, 89, 3250–3255. [Google Scholar] [CrossRef]
- Bijlsma, S.; Bobeldijk, I.; Verheij, E.R.; Ramaker, R.; Kochhar, S.; Macdonald, I.A.; Van Ommen, B.; Smilde, A.K. Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Anal. Chem. 2006, 78, 567–574. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Tsugawa, H.; Rai, A.; Saito, K.; Nakabayashi, R. Metabolomics and complementary techniques to investigate the plant phytochemical cosmos. Nat. Prod. Rep. 2021, 38, 1729–1759. [Google Scholar] [CrossRef]
- Deng, Y.-L.; Liu, C.; Yuan, X.-Q.; Luo, Q.; Miao, Y.; Chen, P.-P.; Cui, F.-P.; Zhang, M.; Zeng, J.-Y.; Shi, T. Associations between urinary concentrations of disinfection byproducts and in vitro fertilization outcomes: A prospective cohort study in China. Environ. Health Perspect. 2023, 131, 097003. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Macones, G.A.; Odem, R.R.; Patterson, B.W.; Lanzendorf, S.E.; Ratts, V.S.; Moley, K.H. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011, 95, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Clower, C.; Nelsen, D.; Butler, W.; Carvalho, A.; Hok, E.; Garelnabi, M. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J. Assist. Reprod. Genet. 2012, 29, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- McKeegan, P.J.; Sturmey, R.G. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 2011, 24, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Mirabi, P.; Chaichi, M.J.; Esmaeilzadeh, S.; Ali Jorsaraei, S.G.; Bijani, A.; Ehsani, M.; Hashemi Karooee, S.F. The role of fatty acids on ICSI outcomes: A prospective cohort study. Lipids Health Dis. 2017, 16, 18. [Google Scholar] [CrossRef]
- Bender, K.; Walsh, S.; Evans, A.C.; Fair, T.; Brennan, L. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 2010, 139, 1047–1055. [Google Scholar] [CrossRef]
- Stamenkovic, A. Oxidized Phosphatidylcholines (OxPCs) as Mediators of Myocardial Ischemia/Reperfusion Injury. 2020. Available online: http://hdl.handle.net/1993/35116 (accessed on 1 February 2020).
- Prates, E.G.; Nunes, J.T.; Pereira, R.M. A role of lipid metabolism during cumulus-oocyte complex maturation: Impact of lipid modulators to improve embryo production. Mediat. Inflamm. 2014, 2014, 692067. [Google Scholar] [CrossRef]
- Stoffel, W.; Schmidt-Soltau, I.; Binczek, E.; Thomas, A.; Thevis, M.; Wegner, I. Dietary omega3-and omega6-Polyunsaturated fatty acids reconstitute fertility of Juvenile and adult Fads2-Deficient mice. Mol. Metab. 2020, 36, 100974. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ning, N.; Wei, J.A.; Huang, Q.L.; Lu, Y.; Pang, X.F.; Wu, J.J.; Zhou, J.B.; Zhou, J.W.; Luo, G.A.; et al. Metabonomics study on the infertility treated with zishen yutai pills combined with in vitro fertilization-embryo transfer. Front. Pharmacol. 2021, 12, 686133. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to know endometriosis-related infertility better: A review on how endometriosis affects oocyte quality and embryo development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, H.; Gu, X.; Boots, C.; Moley, K.H.; Wang, Q. Metabolic control of oocyte development: Linking maternal nutrition and reproductive outcomes. Cell. Mol. Life Sci. 2015, 72, 251–271. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y. The sphingosine 1-phosphate axis: An emerging therapeutic opportunity for endometriosis. Reprod. Sci. 2023, 30, 2040–2059. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, L.M.; Song, J.L.; Yau, L.F.; Mi, J.N.; Zhang, C.R.; Wu, W.T.; Lai, M.H.; Jiang, Z.H.; Wang, J.R.; et al. Alterations of sphingolipid metabolism in different types of polycystic ovary syndrome. Sci. Rep. 2019, 9, 3204. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Li, S.; Zhang, Y.; Feng, R.; Huang, R.; Chen, M.; Qian, Y. Metabolic signatures in human follicular fluid identify lysophosphatidylcholine as a predictor of follicular development. Commun. Biol. 2022, 5, 763. [Google Scholar] [CrossRef]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Guzel, Y.; Bildik, G.; Oktem, O. Sphingosine-1-phosphate protects human ovarian follicles from apoptosis in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 222, 19–24. [Google Scholar] [CrossRef]
- Guo, L.; Ou, X.; Li, H.; Han, Z. Roles of sphingosine-1-phosphate in reproduction. Reprod. Sci. 2014, 21, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Li, X.; Zhang, J.; Li, Y.; Wu, X.M.; Yang, Y.Z.; Zhang, X.F.; Ma, L.Z.; Liu, Y.D.; Wang, Z.; et al. Plasma metabolomic characterization of premature ovarian insufficiency. J. Ovarian Res. 2023, 16, 2. [Google Scholar] [CrossRef]
- Liu, L.; Yin, T.L.; Chen, Y.; Li, Y.; Yin, L.; Ding, J.; Yang, J.; Feng, H.L. Follicular dynamics of glycerophospholipid and sphingolipid metabolisms in polycystic ovary syndrome patients. J. Steroid Biochem. Mol. Biol. 2019, 185, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, H.M.; Wang, A.; Song, J.; Zhang, X.; Guo, J.; Leung, P.C.K.; Lian, F. Identification of potential metabolic biomarkers of polycystic ovary syndrome in follicular fluid by SWATH mass spectrometry. Reprod. Biol. Endocrinol. 2019, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Wei, Y.; Zhu, P.; Yin, T.; Wan, Q. Metabonomic analysis of follicular fluid in patients with diminished ovarian reserve. Front. Endocrinol. 2023, 14, 1132621. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, W.; Liu, J.; Sun, Y.; Li, Y.; Li, H.; Xiao, S.; Shen, X. Metabolomic changes in follicular fluid induced by soy isoflavones administered to rats from weaning until sexual maturity. Toxicol. Appl. Pharmacol. 2013, 269, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Sun, L.; Cheng, J.; Lu, Y.; Wei, Y.; Qin, G.; Liang, J.; Lan, G. Non-targeted metabolomics reveals metabolic characteristics of porcine atretic follicles. Front. Vet. Sci. 2021, 8, 679947. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Discovery Set | Validation Set | p-Value a | p-Value b | ||
|---|---|---|---|---|---|---|
| Non-DOR (N = 96) | DOR (N = 67) | Non-DOR (N = 22) | DOR (N = 22) | |||
| Age (years old) | 31.9 ± 2.7 | 34.0 ± 6.9 | 32.5 ± 3.0 | 32.5 ± 3.0 | 0.233 | 0.999 |
| Age at menarche (years old) | 13.4 ± 1.1 | 13.4 ± 1.3 | 13.2 ± 0.8 | 12.9 ± 1.2 | 0.555 | 0.603 |
| Infertility duration (years) | 3.8 ± 2.6 (3 missing) | 3.3 ± 3.2 (1 missing) | 4.4 ± 3.6 (1 missing) | 4.3 ± 2.5 | 0.041 | 0.816 |
| BMI (kg/m2) | 21.8 ± 3.2 | 22.9 ± 3.8 | 22.4 ± 2.6 | 22.4 ± 2.8 | 0.078 | 0.916 |
| <25.0 | 80 (83.3%) | 48 (71.6%) | 18 (81.8%) | 18 (81.8%) | 0.074 | 0.999 |
| ≥25.0 | 16 (16.7%) | 19 (28.4%) | 4 (18.2%) | 4 (18.2%) | ||
| Race | ||||||
| Han | 93 (96.9%) | 65 (97.0%) | 21 (95.5%) | 22 (100.0%) | 0.999 | 0.999 |
| Others | 3 (3.1%) | 2 (3.0%) | 1 (4.5%) | 0 (0%) | ||
| Marital status | ||||||
| First marriage | 83 (86.5%) | 54 (80.6%) | 18 (81.8%) | 20 (90.9%) | 0.315 | 0.664 |
| Remarriage | 13 (13.5%) | 13 (19.4%) | 4 (18.2%) | 2 (9.1%) | ||
| Gravidity | ||||||
| Yes | 55 (57.3%) | 20 (29.9%) | 8 (36.4%) | 11 (50.0%) | 0.001 | 0.361 |
| No | 41 (42.7%) | 47 (70.1%) | 14 (63.6%) | 11 (50.0%) | ||
| Parity | ||||||
| Nulliparous | 78 (81.3%) | 46 (68.7%) | 12 (54.5%) | 19 (86.4%) | 0.064 | 0.021 |
| Parous | 18 (18.8%) | 21 (31.3%) | 10 (45.5%) | 3 (13.6%) | ||
| Household income (yuan/month) | ||||||
| ≤5000 | 50 (52.1%) | 38 (56.7%) | 12 (54.5%) | 11 (50.0%) | 0.559 | 0.763 |
| >5000 | 46 (47.9%) | 29 (43.3%) | 10 (45.5%) | 11 (50.0%) | ||
| Passive smoking status | ||||||
| Yes | 18 (18.8%) | 21 (31.3%) | 7 (31.8%) | 7 (31.8%) | 0.064 | 0.999 |
| No | 78 (81.3%) | 46 (68.7%) | 15 (68.2%) | 15 (68.2%) | ||
| Alcohol status | ||||||
| Never | 78 (81.3%) | 62 (92.5%) | 21 (95.5%) | 21 (95.5%) | 0.042 | 0.999 |
| Ever/Current | 18 (18.8%) | 5 (7.5%) | 1 (4.5%) | 1 (4.5%) | ||
| Educational level | ||||||
| Less than high school | 35 (36.5%) | 27 (40.3%) | 11 (50.0%) | 9 (40.9%) | 0.619 | 0.545 |
| High school and above | 61 (63.5%) | 40 (59.7%) | 11 (50.0%) | 13 (59.1%) | ||
| Exercise frequency | ||||||
| Never | 31 (32.3%) | 27 (40.3%) | 11 (50.0%) | 12 (52.3%) | 0.575 | 0.029 |
| Occasionally | 49 (51.0%) | 30 (44.8%) | 11 (50.0%) | 6 (27.3%) | ||
| Frequently | 16 (16.7%) | 10 (14.9%) | 0 (0.0%) | 4 (18.2%) | ||
| Total AFC (n) | 13.6 ± 4.7 | 4.6 ± 2.2 | 12.5 ± 3.4 | 4.8 ± 1.7 | <0.001 | <0.001 |
| FSH (IU/L) | 6.9 ± 1.4 (1 missing) | 9.1 ± 3.1 (1 missing) | 7.8 ± 1.4 | 9.9 ± 5.6 | <0.001 | 0.222 |
| E2 (pg/mL) | 42.9 ± 17.2 (1 missing) | 49.1 ± 28.2 (2 missing) | 35.4 ± 12.1 | 45.6 ± 31.2 (2 missing) | 0.195 | 0.208 |
| AMH (ng/mL) | 4.0 ± 2.2 (1 missing) | 1.1 ± 0.6 (1 missing) | 3.7 ± 2.3 | 1.1 ± 0.7 | <0.001 | <0.001 |
| Infertility diagnosis of couples | ||||||
| Female factor | 35 (36.4%) | 50 (74.6%) | 9 (40.9%) | 18 (81.8%) | <0.001 | 0.011 |
| Male factor | 24 (25.0%) | 0 (0.0%) | 5 (22.7%) | 0 (0.0%) | ||
| Mixed factor | 21 (21.8%) | 17 (25.4%) | 5 (22.7%) | 4 (18.2%) | ||
| Unexplained factor | 16 (16.6%) | 0 (0.0%) | 3 (13.6%) | 0 (0.0%) | ||
| Treatment protocol | ||||||
| Long GnRH agonist | 55 (71.4%) | 3 (4.6%) | 15 (75.0%) | 2 (9.5%) | <0.001 | <0.001 |
| GnRH antagonist | 20 (25.9%) | 40 (61.5%) | 5 (25.0%) | 16 (76.1%) | ||
| Others | 2 (2.4%) | 22 (33.7%) | 0 (0.0%) | 3 (14.2%) | ||
| IVF outcomes | ||||||
| Total number of oocytes retrieved | 13.8 ± 6.4 | 5.9 ± 3.8 | 12.9 ± 6.5 | 6.2 ± 3.8 | <0.001 | 0.001 |
| Mature (MII) oocytes retrieved | 11.3 ± 5.6 | 5.1 ± 3.2 | 10.9 ± 5.7 | 5.1 ± 3.6 | <0.001 | 0.001 |
| Normal fertilized (2PN) oocytes | 8.3 ± 4.7 | 3.6 ± 2.9 | 8.1 ± 4.8 | 3.0 ± 2.5 | <0.001 | <0.001 |
| 2PN cleavage zygotes | 8.1 ± 4.6 | 3.5 ± 3.0 | 8.0 ± 4.7 | 3.0 ± 2.4 | <0.001 | <0.001 |
| High-quality embryos | 4.3 ± 3.2 | 1.7 ± 1.7 | 4.5 ± 2.9 | 1.5 ± 1.4 | <0.001 | <0.001 |
| Fertilization rate (%) c | 72.2 ± 18.5 | 66.9 ± 31.4 | 73.7 ± 17.2 | 63.7 ± 28.0 | 0.807 | 0.237 |
| 2PN cleavage rate (%) d | 97.3 ± 7.4 | 95.3 ± 19.2 | 98.6 ± 5.1 | 98.6 ± 6.4 | 0.173 | 0.594 |
| High-quality embryo rate (%) e | 52.4 ± 24.3 | 50.0 ± 33.9 | 63.2 ± 25.4 | 45.9 ± 36.0 | 0.635 | 0.086 |
| Implantation success f | 57 (77.0%) | 25 (49.0%) | 16 (84.2%) | 8 (44.4%) | 0.001 | 0.011 |
| Clinical pregnancy g | 53 (71.6%) | 19 (37.3%) | 15 (78.9%) | 7 (38.9%) | <0.001 | 0.013 |
| Live birth h | 46 (62.2%) | 17 (33.3%) | 13 (68.4%) | 6 (33.3%) | 0.002 | 0.033 |
| Characteristic | NOR | DOR | p-Value |
|---|---|---|---|
| β (95% CI) | |||
| Total number of oocytes | ref | −0.77 (−0.88, −0.67) | <0.001 |
| MII oocytes | ref | −0.76 (−0.88, −0.65) | <0.001 |
| 2PN oocytes | ref | −0.84 (−0.98, −0.70) | <0.001 |
| 2PN cleavage zygotes | ref | −0.85 (−0.99, −0.71) | <0.001 |
| High-quality embryos | ref | −0.99 (−1.19, −0.78) | <0.001 |
| Fertilization rate | ref | −0.25 (−0.51, 0.01) | 0.06 |
| 2PN cleavage rate | ref | −0.22 (−1.17, 0.74) | 0.66 |
| High-quality embryo rate | ref | −0.28 (−0.58, 0.01) | 0.06 |
| RR (95% CI) | |||
| Implantation success | ref | 0.23 (0.11, 0.49) | <0.001 |
| Clinical pregnancy | ref | 0.22 (0.10, 0.46) | <0.001 |
| Live birth | ref | 0.29 (0.14, 0.60) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, N.; Zhang, M.; Zhu, Q.-F.; Chen, Y.-Y.; Deng, Y.-L.; Liu, X.-Y.; Zeng, Q.; Feng, Y.-Q. Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes. Metabolites 2024, 14, 143. https://doi.org/10.3390/metabo14030143
An N, Zhang M, Zhu Q-F, Chen Y-Y, Deng Y-L, Liu X-Y, Zeng Q, Feng Y-Q. Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes. Metabolites. 2024; 14(3):143. https://doi.org/10.3390/metabo14030143
Chicago/Turabian StyleAn, Na, Min Zhang, Quan-Fei Zhu, Yao-Yu Chen, Yan-Ling Deng, Xiao-Ying Liu, Qiang Zeng, and Yu-Qi Feng. 2024. "Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes" Metabolites 14, no. 3: 143. https://doi.org/10.3390/metabo14030143
APA StyleAn, N., Zhang, M., Zhu, Q.-F., Chen, Y.-Y., Deng, Y.-L., Liu, X.-Y., Zeng, Q., & Feng, Y.-Q. (2024). Metabolomic Analysis Reveals Association between Decreased Ovarian Reserve and In Vitro Fertilization Outcomes. Metabolites, 14(3), 143. https://doi.org/10.3390/metabo14030143






