Chemodiversity of Dissolved Soil Organic Matter from Amazon Rainforest as Influenced by Deforestation
Abstract
:1. Introduction
2. Materials and Methods
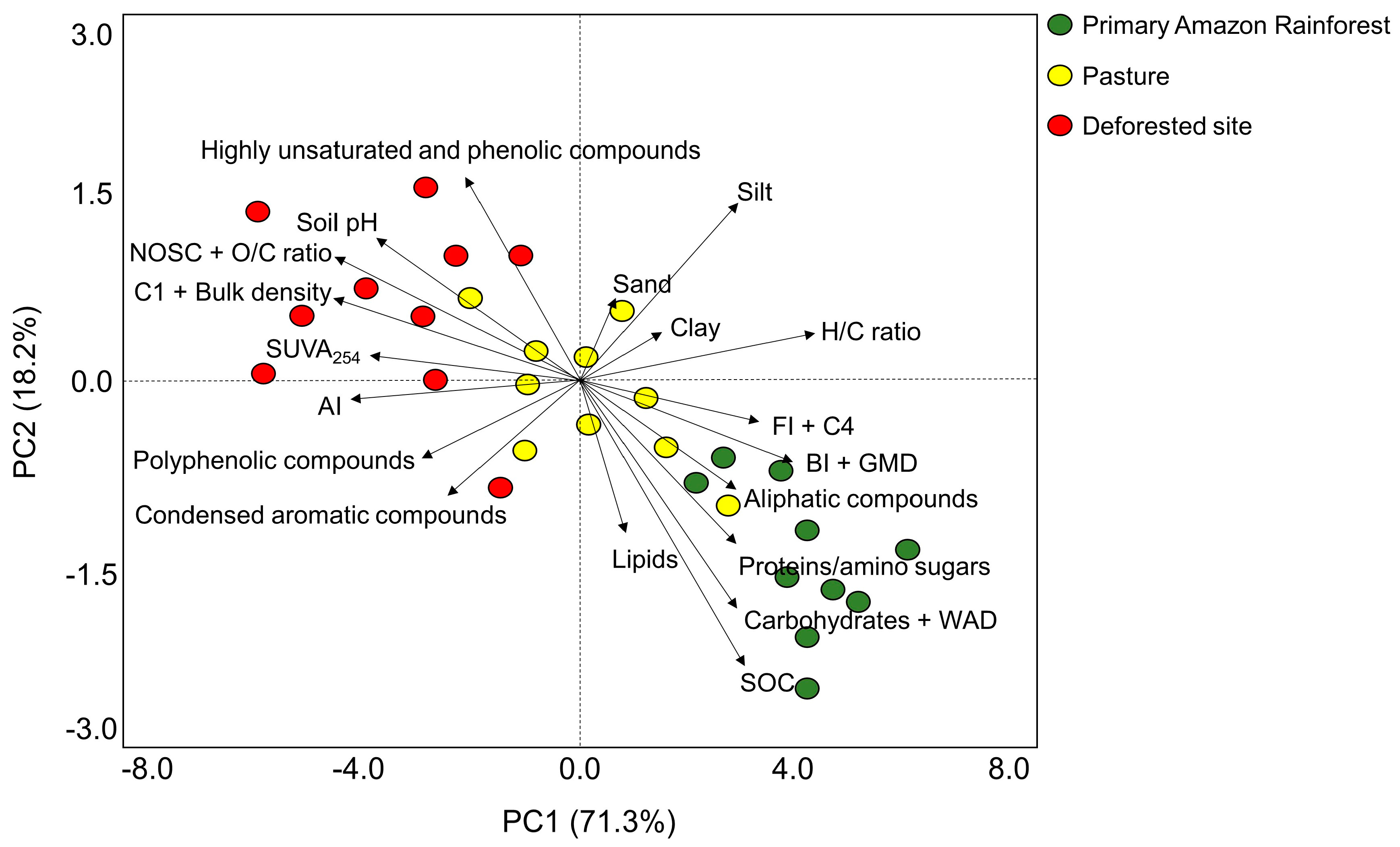
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enck, F.B.; Campos, M.C.C.; Pereira, M.G.; Souza, F.G.; Santos, O.A.Q.; Diniz, Y.V.F.G.; Martins, T.S.; Cunha, J.M.; Lima, A.F.L.; Souza, T.A.F. Forest–Fruticulture Conversion Alters Soil Traits and Soil Organic Matter Compartments. Plants 2022, 11, 2917. [Google Scholar] [CrossRef]
- Ji, H.; Wang, H.; Wu, Z.; Wang, D.; Wang, X.; Fu, P.; Li, C.; Deng, W. Source, composition, and molecular diversity of dissolved and particulate organic matter varied with riparian land use in tropical coastal headstreams. Sci. Total Environ. 2024, 908, 168577. [Google Scholar] [CrossRef]
- Vaughn, D.R.; Kellerman, A.M.; Wickland, K.P.; Striegl, R.G.; Podgorski, D.C.; Hawkings, J.R.; Nienhuis, J.H.; Dornblaser, M.M.; Stets, E.G.; Spencer, R.G.M. Bioavailability of dissolved organic matter varies with anthropogenic landcover in the Upper Mississippi River Basin. Water Res. 2023, 229, 119357. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.A.F.; da Silva, L.J.R.; Nascimento, G.S. Amazonian deforestation and its influence on soil biotic factors and abiotic properties. Pedobiologia 2023, 97–98, 150865. [Google Scholar] [CrossRef]
- Silva, S.F.; Spaccini, R.; Rezende, C.E.; Canellas, L.P. Influence of land use and different plant residues on isotopic carbon distribution of total and water extractable organic matter in an incubation experiment with weathered tropical soil. Land Degrad. Dev. 2022, 34, 1363–1374. [Google Scholar] [CrossRef]
- Minor, E.C.; Oyler, A.R. Dissolved organic matter in large lakes: A key but understudied component of the carbon cycle. Biogeochemistry 2023, 164, 295–318. [Google Scholar] [CrossRef]
- Gu, J.; Bol, R.; Wang, Y.; Zhang, H. Controls on soil dissolved organic carbon along the 4000 km North-South forest transect in Eastern China. Catena 2023, 220, 106691. [Google Scholar] [CrossRef]
- Das, A.; Mishra, G.; Lakra, P.C.; Kumar, S.; Mishra, S.N. Impact of Land Uses on Soil Organic Carbon Dynamics in the Indian Himalayan Region. In Soil Carbon Dynamics in Indian Himalayan Region; Mishra, G., Giri, K., Nath, A.J., Francaviglia, R., Eds.; Springer: Singapore, 2023; pp. 55–75. [Google Scholar] [CrossRef]
- Tu, S.; Li, Q.; Jing, Z.; Gao, H.; Liu, D.; Shao, M.; Yu, H. Characterizing dissolved organic matter and bacterial community interactions in a river network under anthropogenic landcover. Environ. Res. 2023, 238, 117129. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, S.; Liu, C.-Q.; Fu, Q.; Zhang, D.; Hu, W.; Deng, J.; Wu, L.; Li, P.; Yan, Z.; et al. Spatial and molecular variations in forest topsoil dissolved organic matter as revealed by FT-ICR mass spectrometry. Sci. Total Environ. 2023, 895, 165099. [Google Scholar] [CrossRef]
- Yates, C.A.; Johnes, P.J.; Brailsford, F.L.; Evans, C.D.; Evershed, R.P.; Glanville, H.C.; Jones, D.L.; Lloyd, C.E.; Marshall, M.R.; Owen, A.T. Determining patterns in the composition of dissolved organic matter in fresh waters according to land use and management. Biogeochemistry 2023, 164, 143–162. [Google Scholar] [CrossRef]
- Black, C.A. Methods of Soil Analysis, Part 2. In Agronomy Monograph; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; Volume 9, pp. 771–1572. [Google Scholar]
- IITA. Selected Methods for Soil and Plant Analysis; IITA Manual Services: Ibadan, Nigeria, 1979. [Google Scholar]
- Teixeira, P.C.; Donagema, G.K.; Ademir, F.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Plant and Soil Analysis: A Working Manual; Technical Bulletin n.1.; Tropical Soil Biology and Fertility Programme: Nairobi, Kenya, 1993. [Google Scholar]
- Zsolnay, Á. Dissolved organic matter: Artefacts, definitions, and functions. Geoderma 2003, 113, 187–209. [Google Scholar] [CrossRef]
- Kothawala Dolly, N.; Stedmon Colin, A.; Müller Roger, A.; Weyhenmeyer Gesa, A.; Köhler Stephan, J.; Tranvik Lars, J. Controls of dissolved organic matter quality: Evidence from a largeced quinones in dissolved oGlob. Chang. Biol. 2014, 20, 1101–1114. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Mcknight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of DOM for indication of precursor material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Luo, L.; Cao, D. Solid-phase extraction-stepwise elution (SPE-SE) procedure for isolation of dissolved organic matter prior to ESI-FT-ICR-MS analysis. Anal. Chim. Acta 2016, 948, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Stenson, A.C.; Marshall, A.G.; Cooper, W.T. Exact masses and chemical formulas of individual suwannee river fulvic acids from ultrahigh resolution electrospray ionization fourier transform ion cyclotron resonance mass spectra. Anal. Chem. 2003, 75, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- LaRowe, D.E.; Van Cappellen, P. Degradation of natural organic matter: A thermodynamic analysis. Geochim. Cosmochim. Acta 2011, 75, 2030–2042. [Google Scholar] [CrossRef]
- Sleighter, R.L.; Liu, Z.; Xue, J.; Hatcher, P.G. Multivariate statistical approaches for the characterization of dissolved organic matter analyzed by ultrahigh resolution mass spectrometry. Environ. Sci. Technol. 2010, 44, 7576–7582. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Guillemette, F.; Podgorski, D.C.; Aiken, G.R.; Butler, K.D.; Spencer, R.G.M. Unifying concepts linking dissolved organic matter composition to persistence in aquatic ecosystems. Environ. Sci. Technol. 2018, 52, 2538–2548. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. 2018. Available online: http://www.r-project.org/ (accessed on 17 October 2021).
- Vasile, C.; Baican, M. Lignins as Promising Renewable Biopolymers and Bioactive Compounds for High-Performance Materials. Polymers 2023, 15, 3177. [Google Scholar] [CrossRef]
- Shi, S.; Xu, H.; Shui, Y.; Liu, D.; Xie, Q.; Zhou, K.; Zhang, J.; Song, Y.; Wang, J.; Hu, C.; et al. Sedimentary organic molecular compositions reveal the influence of glacier retreat on ecology on the Tibetan Plateau. Sci. Total Environ. 2023, 882, 163629. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Y.; Zhou, Y.; Zhang, Y.; Xu, H.; Jang, K.-S.; Dolfing, J.; Spencer, R.G.M.; Jeppesen, E. Terrestrial dissolved organic matter inputs drive the temporal dynamics of riverine bacterial ecological networks and assembly processes. Water Res. 2024, 249, 120955. [Google Scholar] [CrossRef]
- Li, S.; Harir, M.; Schmitt-Kopplin, P.; Machado-Silva, F.; Gonsior, M.; Bastviken, D.; Enrich-Prast, A.; Valle, J.; Hertkorn, N. Distinct Non-conservative Behavior of Dissolved Organic Matter after Mixing Solimões/Negro and Amazon/Tapajós River Waters. Water 2023, 3, 2083–2095. [Google Scholar] [CrossRef]
- Begum, M.S.; Park, J.-H.; Yang, L.; Shin, K.H.; Hur, J. Optical and molecular indices of dissolved organic matter for estimating biodegradability and resulting carbon dioxide production in inland waters: A review. Water Res. 2023, 228, 119362. [Google Scholar] [CrossRef]
- Vasilchenko, A.V.; Galaktionova, L.V.; Tretyakov, N.Y.; Dyachkov, T.S.M.; Vasilchenko, A.S. Impact of agricultural land use on distribution of microbial biomass and activity within soil aggregates. Soil. Use Manag. 2022, 39, 618–633. [Google Scholar] [CrossRef]
- Wani, O.A.; Kumar, S.S.; Hussain, N.; Wani, A.I.A.; Babu, S.; Alam, P.; Rashid, M.; Popescu, S.M.; Mansoor, S. Multi-scale processes influencing global carbon storage and land-carbon-climate nexus: A critical review. Pedosphere 2023, 33, 250–267. [Google Scholar] [CrossRef]
- Miah, O.; Roy, A.; Sakib, A.A.; Niloy, N.M.; Haque, M.M.; Shammi, M.; Tareq, S.M. Diurnal and seasonal variations of pCO2 and fluorescent dissolved organic matter (FDOM) in different polluted lakes. Environ. Sci. Pollut. Res. 2023, 30, 92720–92735. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Jiang, L.; Yang, J.; Guo, Z.; Li, K.; Peng, Y.; Ibrahim, N.; Liu, H.; Liang, Y.; Yin, H.; et al. Transport Behavior of Cd2+ in Highly Weathered Acidic Soils and Shaping in Soil Microbial Community Structure. Arch. Environ. Contam. Toxicol. 2023, 86, 73–89. [Google Scholar] [CrossRef]
- Smith, J.; Johnson, A.; Davis, R.; Ji, W.; Wang, H.; Wu, Z.; Wang, X.; Fu, P.; Li, C.; Deng, W. Influence of Riparian Land Use on the Source and Molecular Composition of Dissolved and Particulate Organic Matter in Tropical Coastal Headstreams. Environ. Sci. Technol. 2024, 58, 1234–1245. [Google Scholar]
- Zhou, M.; Xiao, Y.; Zhang, X.; Xiao, L.; Ding, G.; Cruse, R.M.; Liu, X. Fifteen years of conservation tillage increases soil aggregate stability by altering the contents and chemical composition of organic carbon fractions in Mollisols. Land Deg. Develop. 2022, 33, 2932–2944. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Cheng, Y.; Yao, H.; Li, H.; You, X.; Zhang, C.; Li, Y. Wheat straw hydrochar induced negative priming effect on carbon decomposition in a coastal soil. iMeta 2023, 2, e134. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, Q.; Liu, M.; Shi, Z.; Liang, Y. Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid. Sci. Total Environ. 2021, 763, 142937. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.; Tang, X.; Zhang, Y.; Jang, K.-S.; Székely, A.J.; Jeppesen, E. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res. 2021, 190, 116776. [Google Scholar] [CrossRef]
- Castañeda-Gómez, L.; Lajtha, K.; Bowden, R.; Jauhar, F.N.M.; Jia, J.; Feng, X.; Simpson, M.J. Soil organic matter molecular composition with long-term detrital alterations is controlled by site-specific forest properties. Global Chang. Biol. 2022, 29, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, S.; Li, Y.; Wang, Q.; Zhong, X.; Yang, Z.; Lin, C.; Yang, Y. Conversion of Natural Evergreen Broadleaved Forests Decreases Soil Organic Carbon but Increases the Relative Contribution of Microbial Residue in Subtropical China. Forests 2019, 10, 468. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.-Z.; Widyastuti, R.; Scheu, S.; Potapov, A.; Krashevska, V. Plant roots are more strongly linked to microorganisms in leaf litter rather than in soil across tropical land-use systems. Soil. Biol. Biochem. 2024, 190, 109329. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Z.; Ye, Q.; Liang, Y.; Liu, M.; Dang, Z.; Wang, Y.; Liu, C. Chemodiversity of Soil Dissolved Organic Matter. Environ. Sci. Technol. 2020, 54, 6174–6184. [Google Scholar] [CrossRef]
- Luo, H.; Du, P.; Wang, P.; Chen, J.; Li, Y.; Wang, H.; Teng, Y.; Li, F. Chemodiversity of dissolved organic matter in cadmium-contaminated paddy soil amended with different materials. Sci. Total Environ. 2022, 825, 153985. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, H.; Wang, H.T.; Xin, P.Y.; Xu, X.H.; Ma, Y.; Liu, W.P.; Teng, C.Y.; Jiang, C.L.; Lou, L.P.; et al. The chemodiversity of paddy soil dissolved organic matter correlates with microbial community at continental scales. Microbiome 2018, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, P.; Li, G.; Petropoulos, E.; Feng, Y.; Li, Z. The chemodiversity of paddy soil dissolved organic matter is shaped and homogenized by bacterial communities that are orchestrated by geographic distance and fertilizations. Soil. Biol. Biochem. 2021, 161, 108374. [Google Scholar] [CrossRef]
| Studied Site | Location | Geographic Coordinates | Elevation (m.a.s.l) |
|---|---|---|---|
| Site 1 | Cruzeiro do Sul, Acre | 7°37′17″ S 72°42′43″ W | 192 |
| Site 2 | Rio Branco, Acre | 9°57′11″ S 67°52′18″ W | 165 |
| Site 3 | Boca do Acre, Amazonas | 8°45′12″ S 67°23′09″ W | 104 |
| Site 4 | São Sebastião do Uatumã, Amazonas | 2°40′47″ S 58°02′49″ W | 69 |
| Site 5 | Manaus, Amazonas | 2°58′57″ S 59°55′53″ W | 86 |
| Site 6 | Manicoré, Amazonas | 5°47′00″ S 61°15′37″ W | 64 |
| Site 7 | Cerejeiras, Rondônia | 13°10′07″ S 61°14′29″ W | 194 |
| Site 8 | Porto Velho, Rondônia | 8°22′33″ S 63°30′16″ W | 70 |
| Site 9 | Boa Vista, Roraima | 2°49′32″ N 60°38′05″ W | 63 |
| Site 10 | Caracaraí, Roraima | 0°45′35″ N 60°57′05″ W | 60 |
| Variables | Primary Amazon Rainforest | Pasture | Deforested Site |
|---|---|---|---|
| Aliphatic compounds (%) | 20.6 ± 1.3 a | 15.2 ± 0.3 b | 2.1 ± 0.4 c |
| Biological index | 4.6 ± 0.3 a | 1.2 ± 0.4 b | 0.2 ± 0.1 c |
| Carbohydrates (%) | 28.2 ± 2.1 a | 15.2 ± 1.3 b | 2.1 ± 0.9 c |
| Condensed aromatic compounds (%) | 25.2 ± 2.5 b | 30.8 ± 2.8 a | 31.1 ± 3.7 a |
| Fluorescence compounds 1–3 | 4.2 ± 0.5 a | 2.9 ± 0.4 b | 1.5 ± 0.3 c |
| Fluorescence compounds 4 | −4.1 ± 0.2 b | −4.2 ± 0.3 b | −2.1 ± 0.2 a |
| Fluorescence index | 3.1 ± 0.4 a | 1.9 ± 0.2 b | 0.5 ± 0.1 c |
| H/C ratio (%) | 2.4 ± 0.2 a | 1.2 ± 0.1 b | 0.4 ± 0.2 c |
| Highly unsaturated and phenolic compounds (%) | 23.4 ± 3.4 b | 24.8 ± 2.7 b | 60.8 ± 5.4 a |
| Lipids (%) | 19.3 ± 3.2 a | 16.4 ± 1.7 b | 9.3 ± 3.1 c |
| Nominal oxidation stage of carbon (%) | 2.5 ± 0.4 a | 1.3 ± 0.5 b | 0.2 ± 0.1 c |
| O/C ratio (%) | 1.2 ± 0.3 a | 1.1 ± 0.2 a | 1.2 ± 0.3 a |
| Polyphenolic compounds (%) | 24.3 ± 3.7 b | 25.8 ± 2.1 b | 30.7 ± 1.7 a |
| Proteins/amino sugars (%) | 41.3 ± 5.3 a | 19.1 ± 3.1 b | 0.9 ± 0.2 c |
| Specific ultraviolet absorbance at 254 nm | 3.7 ± 1.1 a | 2.1 ± 0.3 b | 1.9 ± 0.4 b |
| Soil Properties | Primary Amazon Rainforest | Pasture | Deforested Site |
|---|---|---|---|
| Bulk density (g cm−3) | 0.92 ± 0.05 c | 1.11 ± 0.16 b | 1.27 ± 0.41 a |
| Geometric mean diameter (mm) | 2.57 ± 0.34 a | 2.38 ± 0.27 a | 1.13 ± 0.17 b |
| Weighted average diameter (mm) | 3.04 ± 0.31 a | 2.96 ± 0.41 a | 1.86 ± 0.19 b |
| Sand (g kg−1) | 238.39 ± 21.23 a | 241.67 ± 19.17 a | 240.19 ± 12.26 a |
| Silt (g kg−1) | 524.70 ± 17.01 a | 520.91 ± 19.16 a | 529.13 ± 21.19 a |
| Clay (g kg−1) | 236.91 ± 21.58 a | 237.42 ± 19.46 a | 230.68 ± 22.93 a |
| Soil pH | 3.74 ± 0.28 c | 5.18 ± 0.31 b | 6.23 ± 0.18 a |
| SOC (g kg−1) | 43.12 ± 3.45 a | 27.93 ± 4.91 b | 6.45 ± 1.29 c |
| Microbial C biomass (g C kg−1) | 543.87 ± 2.98 a | 72.38 ± 0.82 b | 14.98 ± 1.02 c |
| Microbial respiration (mg kg−1 h−1) | 0.065 ± 0.002 b | 0.034 ± 0.004 a | 0.008 ± 0.001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.; Araujo, D.J.; Cassimiro, C.A.L.; Batista, D.S. Chemodiversity of Dissolved Soil Organic Matter from Amazon Rainforest as Influenced by Deforestation. Metabolites 2024, 14, 144. https://doi.org/10.3390/metabo14030144
Souza T, Araujo DJ, Cassimiro CAL, Batista DS. Chemodiversity of Dissolved Soil Organic Matter from Amazon Rainforest as Influenced by Deforestation. Metabolites. 2024; 14(3):144. https://doi.org/10.3390/metabo14030144
Chicago/Turabian StyleSouza, Tancredo, Damiana Justino Araujo, Carlos Alberto Lins Cassimiro, and Diego Silva Batista. 2024. "Chemodiversity of Dissolved Soil Organic Matter from Amazon Rainforest as Influenced by Deforestation" Metabolites 14, no. 3: 144. https://doi.org/10.3390/metabo14030144








