Effects of Summer Heat on Adipose Tissue Activity in Periparturient Simmental Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Blood Sampling and Laboratory Assays
2.3. Statistical Analysis
3. Results
3.1. Temperature–Humidity Index and Airflow
3.2. Animal Vital Parameters (Rectal Temperature, Heart and Respiratory Rates)
3.3. Serum Glucose, Nonesterified Fatty Acid (NEFA) and Betahydrohy-Butyrate (BHB) Concentrations
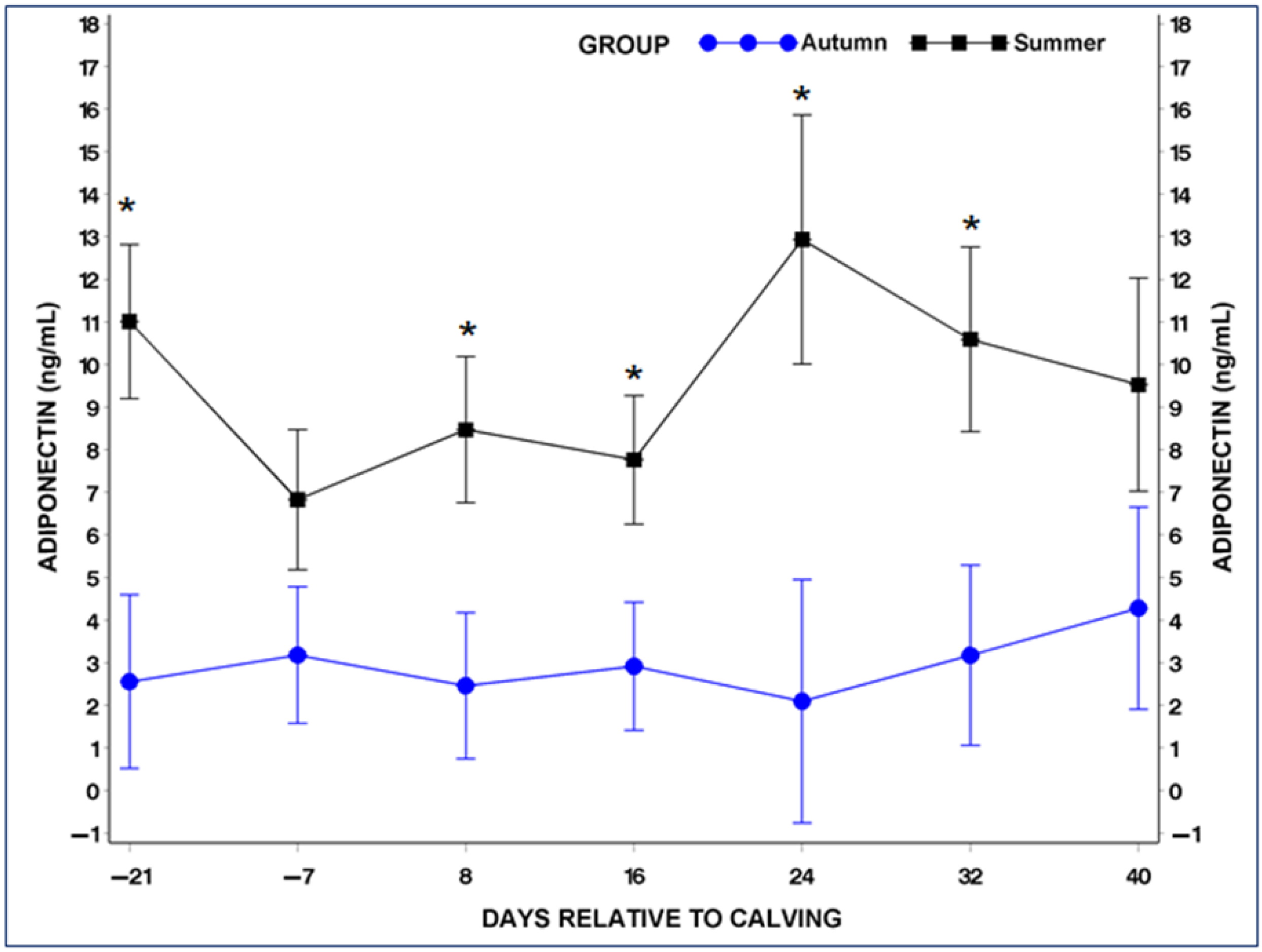
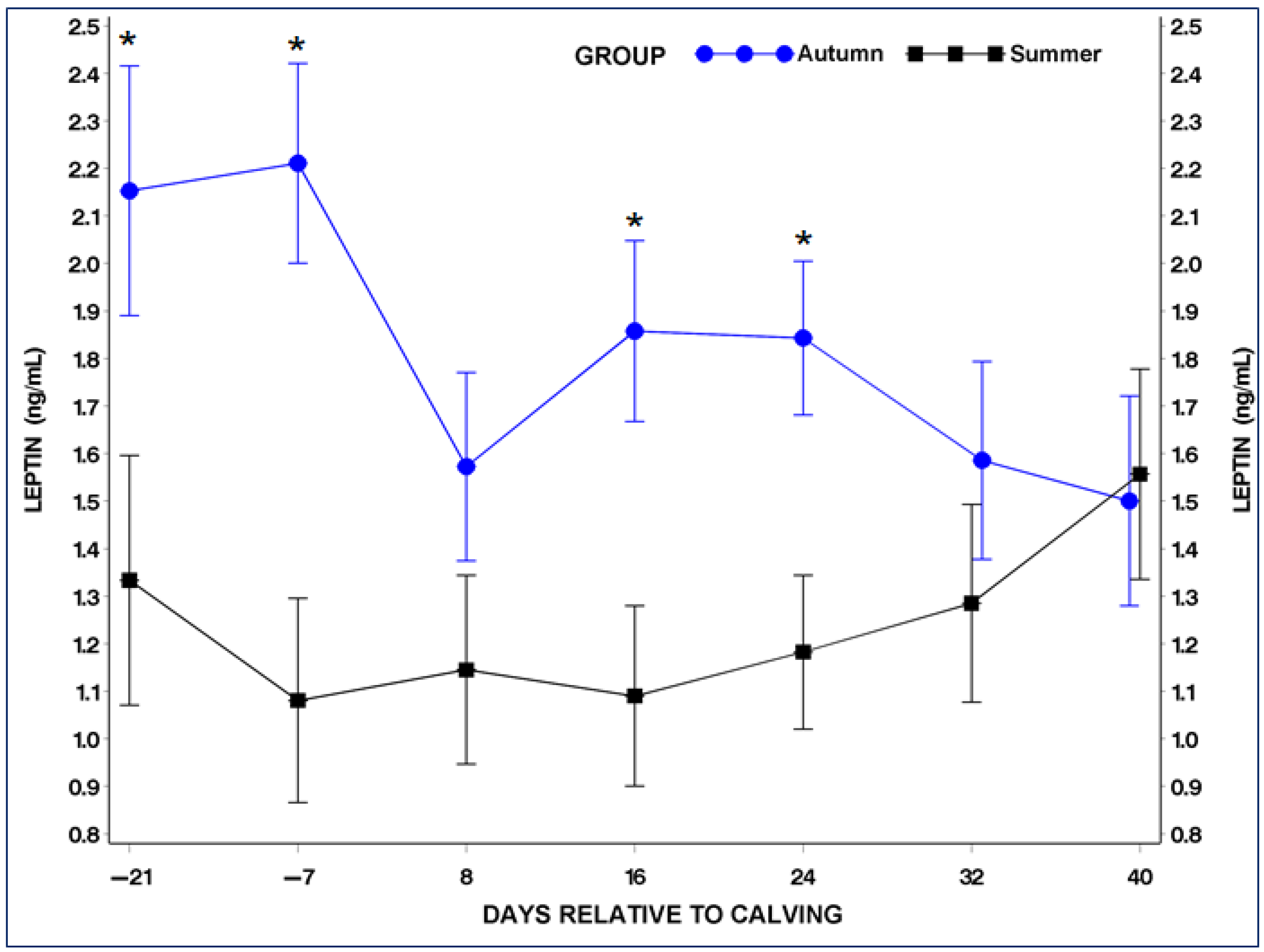
3.4. Serum Adiponectin (ADP) and Leptin (LP) Concentrations
3.5. Correlation between THI, NEFA, BHB, ADP, and LP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, S.; Rivas, R.M.O.; Marins, T.N.; Chen, Y.-C.; Gao, J.; Bernard, J.K. Impact of Heat Stress on Lactational Performance of Dairy Cows. Theriogenology 2020, 150, 437–444. [Google Scholar] [CrossRef]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of Heat Stress on Energetic Metabolism in Lactating Holstein Cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Rhoads, M.L.; Rhoads, R.P.; VanBaale, M.J.; Collier, R.J.; Sanders, S.R.; Weber, W.J.; Crooker, B.A.; Baumgard, L.H. Effects of Heat Stress and Plane of Nutrition on Lactating Holstein Cows: I. Production, Metabolism, and Aspects of Circulating Somatotropin. J. Dairy Sci. 2009, 92, 1986–1997. [Google Scholar] [CrossRef]
- Marins, T.N.; Gao, J.; Yang, Q.; Binda, R.M.; Pessoa, C.M.B.; Orellana Rivas, R.M.; Garrick, M.; Melo, V.H.L.R.; Chen, Y.C.; Bernard, J.K.; et al. Impact of Heat Stress and a Feed Supplement on Hormonal and Inflammatory Responses of Dairy Cows. J. Dairy Sci. 2021, 104, 8276–8289. [Google Scholar] [CrossRef]
- Min, L.; Zheng, N.; Zhao, S.; Cheng, J.; Yang, Y.; Zhang, Y.; Yang, H.; Wang, J. Long-Term Heat Stress Induces the Inflammatory Response in Dairy Cows Revealed by Plasma Proteome Analysis. Biochem. Biophys. Res. Commun. 2016, 471, 296–302. [Google Scholar] [CrossRef]
- Turk, R.; Podpečan, O.; Mrkun, J.; Kosec, M.; Flegar-Meštrić, Z.; Perkov, S.; Starič, J.; Robić, M.; Belić, M.; Zrimšek, P. Lipid Mobilisation and Oxidative Stress as Metabolic Adaptation Processes in Dairy Heifers during Transition Period. Anim. Reprod. Sci. 2013, 141, 109–115. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The Nexus between Nutrient Metabolism, Oxidative Stress and Inflammation in Transition Cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- West, J.W. Effects of Heat-Stress on Production in Dairy Cattle. J. Dairy Sci. 2003, 86, 2131–2144. [Google Scholar] [CrossRef]
- Lamp, O.; Derno, M.; Otten, W.; Mielenz, M.; Nürnberg, G.; Kuhla, B. Metabolic Heat Stress Adaption in Transition Cows: Differences in Macronutrient Oxidation between Late-Gestating and Early-Lactating German Holstein Dairy Cows. PLoS ONE 2015, 10, e0125264. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, B.C.; Connor, E.E.; Tao, S.; Hayen, J.; Bubolz, J.; Dahl, G.E. Heat-Stress Abatement during the Dry Period: Does Cooling Improve Transition into Lactation? J. Dairy Sci. 2009, 92, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Mann, S.; Urh, C.; Sauerwein, H.; Wakshlag, J.J.; Yepes, F.A.L.; Overton, T.R.; Nydam, D.V. The Association of Adiponectin and Leptin Concentrations with Prepartum Dietary Energy Supply, Parity, Body Condition, and Postpartum Hyperketonemia in Transition Dairy Cows. J. Dairy Sci. 2018, 101, 806–811. [Google Scholar] [CrossRef]
- Kabara, E.; Sordillo, L.M.; Holcombe, S.; Contreras, G.A. Adiponectin Links Adipose Tissue Function and Monocyte Inflammatory Responses during Bovine Metabolic Stress. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 49–58. [Google Scholar] [CrossRef]
- Häussler, S.; Sadri, H.; Ghaffari, M.H.; Sauerwein, H. Symposium Review: Adipose Tissue Endocrinology in the Periparturient Period of Dairy Cows. J. Dairy Sci. 2022, 105, 3648–3669. [Google Scholar] [CrossRef]
- Singh, S.P.; Häussler, S.; Gross, J.J.; Schwarz, F.J.; Bruckmaier, R.M.; Sauerwein, H. Circulating and Milk Adiponectin Change Differently during Energy Deficiency at Different Stages of Lactation in Dairy Cows. J. Dairy Sci. 2014, 97, 1535–1542. [Google Scholar] [CrossRef]
- Block, S.S.; Butler, W.R.; Ehrhardt, R.A.; Bell, A.W.; Van Amburgh, M.E.; Boisclair, Y.R. Decreased Concentration of Plasma Leptin in Periparturient Dairy Cows Is Caused by Negative Energy Balance. J. Endocrinol. 2001, 171, 339–348. [Google Scholar] [CrossRef]
- Armstrong, D. Heat Stress Interaction with Shade and Cooling. J. Dairy Sci. 1994, 77, 2044–2050. [Google Scholar] [CrossRef]
- Ekine-Dzivenu, C.C.; Mrode, R.; Oyieng, E.; Komwihangilo, D.; Lyatuu, E.; Msuta, G.; Ojango, J.M.K.; Okeyo, A.M. Evaluating the Impact of Heat Stress as Measured by Temperature-Humidity Index (THI) on Test-Day Milk Yield of Small Holder Dairy Cattle in a Sub-Sahara African Climate. Livest. Sci. 2020, 242, 104314. [Google Scholar] [CrossRef]
- Summer, A.; Lora, I.; Formaggioni, P.; Gottardo, F. Impact of Heat Stress on Milk and Meat Production. Anim. Front. 2019, 9, 39–46. [Google Scholar] [CrossRef]
- Min, L.; Cheng, J.; Shi, B.; Yang, H.; Zheng, N.; Wang, J. Effects of Heat Stress on Serum Insulin, Adipokines, AMP-Activated Protein Kinase, and Heat Shock Signal Molecules in Dairy Cows. J. Zhejiang Univ. Sci. B 2015, 16, 541–548. [Google Scholar] [CrossRef]
- Garcia, A.B.; Angeli, N.; Machado, L.; de Cardoso, F.C.; Gonzalez, F. Relationships between Heat Stress and Metabolic and Milk Parameters in Dairy Cows in Southern Brazil. Trop. Anim. Health Prod. 2015, 47, 889–894. [Google Scholar] [CrossRef]
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183. [Google Scholar] [CrossRef]
- Fathoni, A.; Boonkum, W.; Chankitisakul, V.; Duangjinda, M. An Appropriate Genetic Approach for Improving Reproductive Traits in Crossbred Thai–Holstein Cattle under Heat Stress Conditions. Vet. Sci. 2022, 9, 163. [Google Scholar] [CrossRef]
- Turk, R.; Podpečan, O.; Mrkun, J.; Flegar-Meštrić, Z.; Perkov, S.; Zrimšek, P. The Effect of Seasonal Thermal Stress on Lipid Mobilisation, Antioxidant Status and Reproductive Performance in Dairy Cows. Reprod. Domest. Anim. 2015, 50, 595–603. [Google Scholar] [CrossRef]
- Shwartz, G.; Rhoads, M.L.; VanBaale, M.J.; Rhoads, R.P.; Baumgard, L.H. Effects of a Supplemental Yeast Culture on Heat-Stressed Lactating Holstein Cows. J. Dairy Sci. 2009, 92, 935–942. [Google Scholar] [CrossRef]
- Ouellet, V.; Cabrera, V.E.; Fadul-Pacheco, L.; Charbonneau, É. The Relationship between the Number of Consecutive Days with Heat Stress and Milk Production of Holstein Dairy Cows Raised in a Humid Continental Climate. J. Dairy Sci. 2019, 102, 8537–8545. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Stefanska, B.; Sobolewska, P.; Fievez, V.; Pruszynska-Oszmałek, E.; Purwin, C.; Nowak, W. The Impact of Heat Stress on Performance, Fertility, and Adipokines Involved in Regulating Systemic Immune Response during Lipolysis of Early Lactating Dairy Cows. J. Dairy Sci. 2023, in press. [Google Scholar] [CrossRef]
- Ehrhardt, R.A.; Foskolos, A.; Giesy, S.L.; Wesolowski, S.R.; Krumm, C.S.; Butler, W.R.; Quirk, S.M.; Waldron, M.R.; Boisclair, Y.R. Increased Plasma Leptin Attenuates Ptive Metabolism in Early Lactating Dairy Cows. J. Endocrinol. 2016, 229, 145–157. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Imai, Y.; Shimozawa, N.; Hioki, K.; Uchida, S.; Ito, Y.; Takakuwa, K.; Matsui, J. Globular Adiponectin Protected Ob/Ob Mice from Diabetes and ApoE-Deficient Mice from Atherosclerosis. J. Biol. Chem. 2003, 278, 2461–2468. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 9. [Google Scholar]
| Ingredients | % |
|---|---|
| Crude protein | 32 |
| Crude fats | 2 |
| Crude fibers | 9 |
| Calcium | 2.5 |
| Phosphorus | 1.2 |
| Potassium | 1 |
| Magnesium | 0.25 |
| Days Relative to Calving | THI | Airflow (m/s) | ||
|---|---|---|---|---|
| Autumn | Summer | Autumn | Summer | |
| −21 | 60 | 78 * | 0.45 | 0.18 * |
| −7 | 59 | 79 * | 0.45 | 0.17 * |
| 8 | 58 | 80 * | 0.45 | 0.18 * |
| 16 | 62 | 82 * | 0.45 | 0.18 * |
| 24 | 60 | 81 * | 0.48 | 0.18 * |
| 32 | 57 | 77 * | 0.48 | 0.19 * |
| 40 | 57 | 77 * | 0.48 | 0.20 * |
| Days Relative to Calving | Rectal Temp. (°C) | Heart Rate (Beats/Minute) | Respiratory Rate (Breaths/Minute) | |||
|---|---|---|---|---|---|---|
| Autumn | Summer | Autumn | Summer | Autumn | Summer | |
| −21 | 38.7 | 39.1 * | 90.4 | 88.6 | 31.4 | 37.1 |
| −7 | 38.8 | 39.2 * | 85.9 | 93.8 | 29.6 | 47.4 ** |
| 8 | 38.7 | 39.2 * | 83.0 | 86.7 | 25.8 | 54.3 *** |
| 16 | 38.8 | 39.5 * | 84.3 | 91.8 * | 27.3 | 63.9 *** |
| 24 | 38.7 | 39.3 * | 86.0 | 91.8 | 27.3 | 66.2 *** |
| 32 | 38.6 | 39.0 * | 83.9 | 84.2 | 26.1 | 52.0 *** |
| 40 | 38.7 | 38.8 | 85.3 | 87.0 | 26.3 | 42.6 *** |
| Days Relative to Calving | Glucose (mmol/L) | NEFA (mmol/L) | BHB (mmol/L) | |||
|---|---|---|---|---|---|---|
| Autumn | Summer | Autumn | Summer | Autumn | Summer | |
| −21 | 3.3 a | 3.0 | 0.13 ac | 0.14 a | 0.54 a | 0.51 |
| −7 | 3.2 ac | 2.9 | 0.11 bc | 0.39 b* | 0.57 a | 0.44 |
| 8 | 2.8 bc | 2.9 | 0.67 b | 0.51 b | 0.96 b | 0.58 * |
| 16 | 2.8 b | 2.7 | 0.57 b | 0.31 a* | 1.21 b | 0.55 *** |
| 24 | 2.7 b | 2.8 | 0.46 b | 0.28 a* | 1.34 b | 0.70 ** |
| 32 | 2.7 b | 2.8 | 0.47 b | 0.24 a* | 1.13 b | 0.63 |
| 40 | 2.9 a | 3.2 | 0.28 ac | 0.10 a* | 1.17 b | 0.53 |
| NEFA | BHB | LP | ADP | |
|---|---|---|---|---|
| THI | n.s. | −0.34 <0.0001 | −0.42 <0.0001 | 0.37 <0.0001 |
| NEFA | 0.44 <0.0001 | n.s. | −0.29 <0.001 | |
| BHB | 0.31 <0.0001 | −0.44 <0.0001 | ||
| LP | −0.67 <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turk, R.; Rošić, N.; Beer Ljubić, B.; Vince, S. Effects of Summer Heat on Adipose Tissue Activity in Periparturient Simmental Cows. Metabolites 2024, 14, 207. https://doi.org/10.3390/metabo14040207
Turk R, Rošić N, Beer Ljubić B, Vince S. Effects of Summer Heat on Adipose Tissue Activity in Periparturient Simmental Cows. Metabolites. 2024; 14(4):207. https://doi.org/10.3390/metabo14040207
Chicago/Turabian StyleTurk, Romana, Nikola Rošić, Blanka Beer Ljubić, and Silvijo Vince. 2024. "Effects of Summer Heat on Adipose Tissue Activity in Periparturient Simmental Cows" Metabolites 14, no. 4: 207. https://doi.org/10.3390/metabo14040207
APA StyleTurk, R., Rošić, N., Beer Ljubić, B., & Vince, S. (2024). Effects of Summer Heat on Adipose Tissue Activity in Periparturient Simmental Cows. Metabolites, 14(4), 207. https://doi.org/10.3390/metabo14040207






