Accumulation of Non-Pathological Liver Fat Is Associated with the Loss of Glyoxalase I Activity in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Determination of Liver Triglyceride
2.3. Clinical Chemistry Parameters
2.4. Measurement of Dicarbonyls
2.5. Measurement of Protein-Bound Glycation and Oxidation Biomarkers
2.6. Real-Time PCR
2.7. Western Blotting
2.8. Measurement of Glo1 Activity
2.9. Measurement of Glo2 Activity
2.10. Statistical Analyses and Calculations
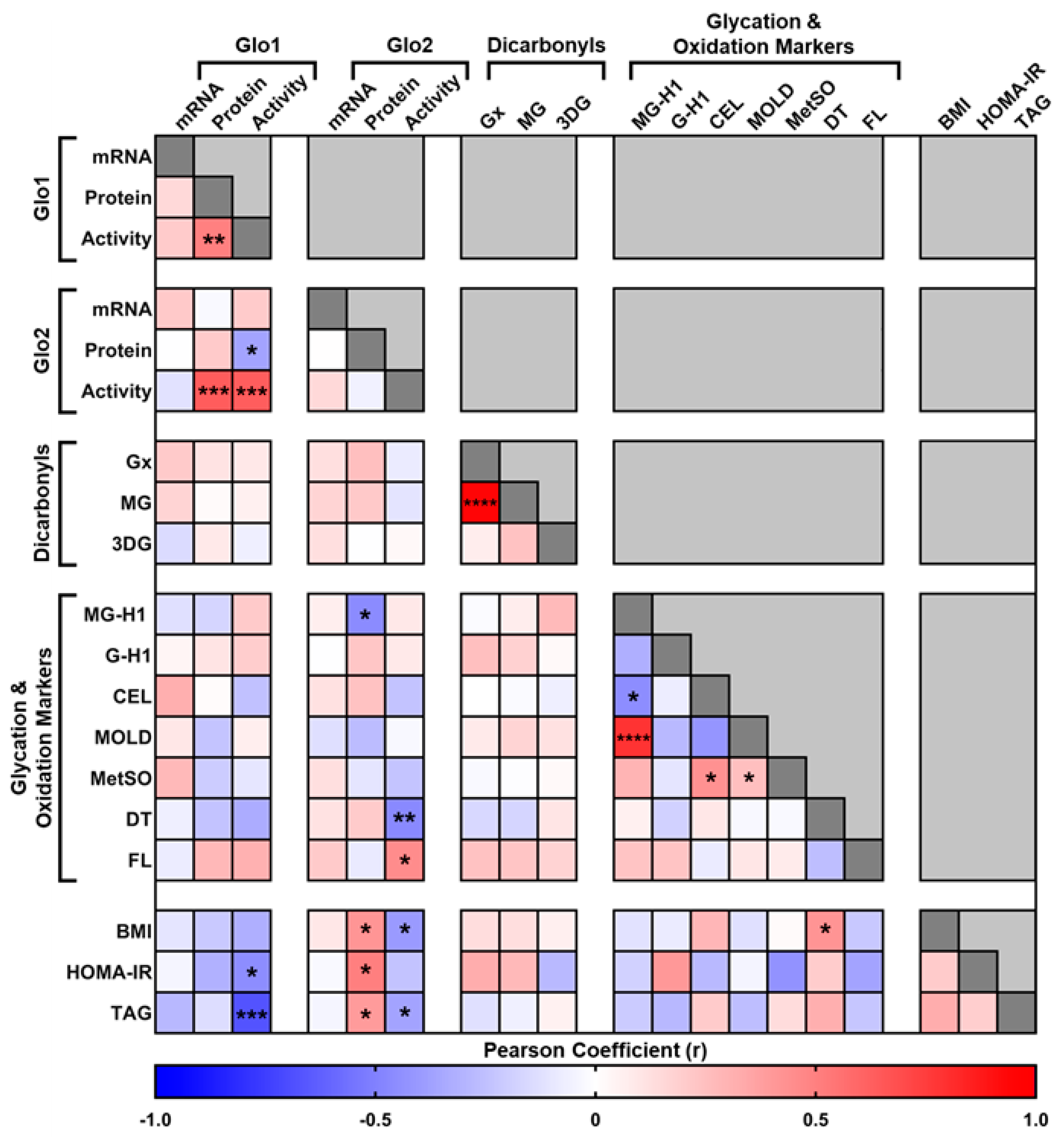
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefan, N.; Häring, H.-U.; Cusi, K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and Diabetes Mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-Alcoholic Fatty Liver Disease and Its Relationship with Cardiovascular Disease and Other Extrahepatic Diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide Burden of Cancer Attributable to Diabetes and High Body-Mass Index: A Comparative Risk Assessment. Lancet Diabetes Endocrinol. 2018, 6, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding Lipotoxicity in NAFLD Pathogenesis: Is CD36 a Key Driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Bence, K.K.; Birnbaum, M.J. Metabolic Drivers of Non-Alcoholic Fatty Liver Disease. Mol. Metab. 2021, 50, 101143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, B.; Lu, M.; Wang, Y.; Ding, N.; Liu, C.; Gao, M.; Yao, Z.; Zhang, S.; Zhao, Y.; et al. The Methyltransferase METTL3 Negatively Regulates Nonalcoholic Steatohepatitis (NASH) Progression. Nat. Commun. 2021, 12, 7213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhang, X.-J.; Zhang, P.; Cai, J.; She, Z.-G.; Li, H. Recent Updates on Targeting the Molecular Mediators of NAFLD. J. Mol. Med. 2023, 101, 101–124. [Google Scholar] [CrossRef]
- Horn, C.L.; Morales, A.L.; Savard, C.; Farrell, G.C.; Ioannou, G.N. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol. Commun. 2022, 6, 12–35. [Google Scholar] [CrossRef]
- Dashti, Z.; Yousefi, Z.; Kiani, P.; Taghizadeh, M.; Maleki, M.H.; Borji, M.; Vakili, O.; Shafiee, S.M. Autophagy and the Unfolded Protein Response Shape the Non-Alcoholic Fatty Liver Landscape: Decoding the Labyrinth. Metabolism 2024, 154, 155811. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Methylglyoxal, Glyoxalase 1 and the Dicarbonyl Proteome. Amino Acids 2012, 42, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.; Herzig, S.; Nawroth, P. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide Mapping Identifies Hotspot Site of Modification in Human Serum Albumin by Methylglyoxal Involved in Ligand Binding and Esterase Activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased Dicarbonyl Metabolism in Endothelial Cells in Hyperglycemia Induces Anoikis and Impairs Angiogenesis by RGD and GFOGER Motif Modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Godfrey, L.; Xue, M.; Shaheen, F.; Geoffrion, M.; Milne, R.; Thornalley, P.J. Glycation of LDL by Methylglyoxal Increases Arterial Atherogenicity. Diabetes 2011, 60, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I—Structure, Function and a Critical Role in the Enzymatic Defence against Glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Campos Campos, M.; Nawroth, P.; Fleming, T. The Glyoxalase System—New Insights into an Ancient Metabolism. Antioxidants 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Traber, M.G.; Bobe, G.; Kensicki, E.; Bohren, K.M.; Milne, G.; Jump, D.B. A Metabolomic Analysis of Omega-3 Fatty Acid-Mediated Attenuation of Western Diet-Induced Nonalcoholic Steatohepatitis in LDLR-/- Mice. PLoS ONE 2013, 8, e83756. [Google Scholar] [CrossRef]
- Sanchez, J.-C.; Converset, V.; Nolan, A.; Schmid, G.; Wang, S.; Heller, M.; Sennitt, M.V.; Hochstrasser, D.F.; Cawthorne, M.A. Effect of Rosiglitazone on the Differential Expression of Diabetes-Associated Proteins in Pancreatic Islets of C57Bl/6 Lep/Lep Mice. Mol. Cell. Proteom. 2002, 1, 509–516. [Google Scholar] [CrossRef]
- Spanos, C.; Maldonado, E.M.; Fisher, C.P.; Leenutaphong, P.; Oviedo-Orta, E.; Windridge, D.; Salguero, F.J.; Bermúdez-Fajardo, A.; Weeks, M.E.; Evans, C.; et al. Proteomic Identification and Characterization of Hepatic Glyoxalase 1 Dysregulation in Non-Alcoholic Fatty Liver Disease. Proteome Sci. 2018, 16, 4. [Google Scholar] [CrossRef]
- Maessen, D.; Brouwers, O.; Miyata, T.; Stehouwer, C.; Schalkwijk, C. Glyoxalase-1 Overexpression Reduces Body Weight and Adipokine Expression, and Improves Insulin Sensitivity in High-Fat Diet-Induced Obese Mice. Diabetologia 2014, 57, S290–S291. [Google Scholar]
- Gaens, K.H.J.; Niessen, P.M.G.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Driessen, A.; Wolfs, M.G.M.; Hofker, M.H.; Bloemen, J.G.; Dejong, C.H.; et al. Endogenous Formation of Nε-(Carboxymethyl)Lysine Is Increased in Fatty Livers and Induces Inflammatory Markers in an in Vitro Model of Hepatic Steatosis. J. Hepatol. 2012, 56, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of Insulin Resistance in Overweight and Obese Subjects by Trans-Resveratrol and Hesperetin Combination—Link to Dysglycemia, Blood Pressure, Dyslipidemia, and Low-Grade Inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef] [PubMed]
- Mey, J.T.; Blackburn, B.K.; Miranda, E.R.; Chaves, A.B.; Briller, J.; Bonini, M.G.; Haus, J.M. Dicarbonyl Stress and Glyoxalase Enzyme System Regulation in Human Skeletal Muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R181–R190. [Google Scholar] [CrossRef]
- Gancheva, S.; Kahl, S.; Pesta, D.; Mastrototaro, L.; Dewidar, B.; Strassburger, K.; Sabah, E.; Esposito, I.; Weiß, J.; Sarabhai, T.; et al. Impaired Hepatic Mitochondrial Capacity in Nonalcoholic Steatohepatitis Associated With Type 2 Diabetes. Diabetes Care 2022, 45, 928–937. [Google Scholar] [CrossRef]
- Masania, J.; Malczewska-Malec, M.; Razny, U.; Goralska, J.; Zdzienicka, A.; Kiec-Wilk, B.; Gruca, A.; Stancel-Mozwillo, J.; Dembinska-Kiec, A.; Rabbani, N.; et al. Dicarbonyl Stress in Clinical Obesity. Glycoconj. J. 2016, 33, 581–589. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glyoxalase 1 Modulation in Obesity and Diabetes. Antioxid. Redox Signal 2019, 30, 354–374. [Google Scholar] [CrossRef]
- Peter, A.; Kovarova, M.; Staiger, H.; Machann, J.; Schick, F.; Königsrainer, A.; Königsrainer, I.; Schleicher, E.; Fritsche, A.; Häring, H.-U.; et al. The Hepatokines Fetuin-A and Fetuin-B Are Upregulated in the State of Hepatic Steatosis and May Differently Impact on Glucose Homeostasis in Humans. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E266–E273. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Measurement of Methylglyoxal by Stable Isotopic Dilution Analysis LC-MS/MS with Corroborative Prediction in Physiological Samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef]
- Rabbani, N.; Shaheen, F.; Anwar, A.; Masania, J.; Thornalley, P.J. Assay of Methylglyoxal-Derived Protein and Nucleotide AGEs. Biochem. Soc. Trans. 2014, 42, 511–517. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.C.; Thornalley, P.J. Glyoxalase Activity in Human Red Blood Cells Fractioned by Age. Mech. Ageing Dev. 1989, 48, 63–71. [Google Scholar] [CrossRef]
- Allen, R.E.; Lo, T.W.C.; Thornalley, P.J. Purification and Characterisation of Glyoxalase II from Human Red Blood Cells. Eur. J. Biochem. 1993, 213, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the Regulation of Protein Abundance from Proteomic and Transcriptomic Analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Buccitelli, C.; Selbach, M. MRNAs, Proteins and the Emerging Principles of Gene Expression Control. Nat. Rev. Genet. 2020, 21, 630–644. [Google Scholar] [CrossRef]
- Mitsumoto, A.; Kim, K.-R.; Oshima, G.; Kunimoto, M.; Okawa, K.; Iwamatsu, A.; Nakagawa, Y. Nitric Oxide Inactivates Glyoxalase I in Cooperation with Glutathione. J. Biochem. 2000, 128, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Birkenmeier, G.; Stegemann, C.; Hoffmann, R.; Günther, R.; Huse, K.; Birkemeyer, C. Posttranslational Modification of Human Glyoxalase 1 Indicates Redox-Dependent Regulation. PLoS ONE 2010, 5, e10399. [Google Scholar] [CrossRef]
- de Hemptinne, V.; Rondas, D.; Toepoel, M.; Vancompernolle, K. Phosphorylation on Thr-106 and NO-Modification of Glyoxalase I Suppress the TNF-Induced Transcriptional Activity of NF-ΚB. Mol. Cell Biochem. 2009, 325, 169–178. [Google Scholar] [CrossRef]
- Morgenstern, J.; Katz, S.; Krebs-Haupenthal, J.; Chen, J.; Saadatmand, A.; Cortizo, F.G.; Moraru, A.; Zemva, J.; Campos, M.C.; Teleman, A.; et al. Phosphorylation of T107 by CamKIIδ Regulates the Detoxification Efficiency and Proteomic Integrity of Glyoxalase 1. Cell Rep. 2020, 32, 108160. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, F.G.; Pfaff, D.; Wirth, A.; Schlotterer, A.; Medert, R.; Morgenstern, J.; Weber, T.; Hammes, H.-P.; Fleming, T.; Nawroth, P.P.; et al. The Activity of Glyoxylase 1 Is Regulated by Glucose-Responsive Phosphorylation on Tyr136. Mol. Metab. 2022, 55, 101406. [Google Scholar] [CrossRef]
- de Hemptinne, V.; Rondas, D.; Vandekerckhove, J.; Vancompernolle, K. Tumour Necrosis Factor Induces Phosphorylation Primarily of the Nitric-Oxide-Responsive Form of Glyoxalase I. Biochem. J. 2007, 407, 121–128. [Google Scholar] [CrossRef]
- Sreekumar, P.G. Methionine Sulfoxide Reductase A: Structure, Function and Role in Ocular Pathology. World J. Biol. Chem. 2011, 2, 184. [Google Scholar] [CrossRef]
- Fromenty, B.; Roden, M. Mitochondrial Alterations in Fatty Liver Diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Xi, H.S.; Li, S. HIF1α Is Required for Survival Maintenance of Chronic Myeloid Leukemia Stem Cells. Blood 2012, 119, 2595–2607. [Google Scholar] [CrossRef]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Romaldi, B.; Laudadio, E.; Galeazzi, R.; Antognelli, C.; Armeni, T. Glyoxalase 2: Towards a Broader View of the Second Player of the Glyoxalase System. Antioxidants 2022, 11, 2131. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, J.; Fleming, T.; Schumacher, D.; Eckstein, V.; Freichel, M.; Herzig, S.; Nawroth, P. Loss of Glyoxalase 1 Induces Compensatory Mechanism to Achieve Dicarbonyl Detoxification in Mammalian Schwann Cells. J. Biol. Chem. 2017, 292, 3224–3238. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory Mechanisms for Methylglyoxal Detoxification in Experimental & Clinical Diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.-F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef]
- Rando, G.; Wahli, W. Sex Differences in Nuclear Receptor-Regulated Liver Metabolic Pathways. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.S.; Hakimi, M.; Vittas, S.; Fleming, T.H.; Nawroth, P.P.; Böckler, D.; Dihlmann, S. Gender Difference in Glyoxalase 1 Activity of Atherosclerotic Carotid Artery Lesions. J. Vasc. Surg. 2015, 62, 471–476. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Median (25–75 Percentile) |
|---|---|
| Gender | 30 (25 M/5 F) |
| Age (years) | 64.5 (56–71.25) |
| Weight (kg) | 81.5 (76.5–91) |
| Height (m) | 175 (169–180) |
| BMI (kg/m2) | 26.6 (24.6–29.8) |
| Liver fat (%) | 1.9 (1.1–5.4) |
| HOMA-IR (μU/mL × mmol/L; n = 21 (18 M/3 F)) | 1.78 (1.11–4.39) |
| Liver Glyoxalase System | |
| Glo1 mRNA (Rps13 mRNA Normalized) | 0.412 (0.319–0.490) |
| Glo1 Protein Expression (Actin Normalized) | 0.889 (0.736–1.163) |
| Glo1 Activity (mU/mg) | 3.46 (2.77–4.15) |
| Glo2 mRNA (Rps13 mRNA Normalized) | 0.047 (0.040–0.058) |
| Glo2 Protein Expression (Actin Normalized) | 1.08 (0.98–1.30) |
| Glo2 Activity (mU/mg) | 8.49 (7.84–9.32) |
| Liver Dicarbonyls (pmol/mg) | |
| Glyoxal | 7.79 (4.81–9.85) |
| Methylglyoxal | 4.50 (3.58–6.18) |
| 3DG | 0.299 (0.269–0.418) |
| Liver Glycation Biomarkers | |
| MG-H1 (mmol/mol Arg) | 1.22 (0.93–1.50) |
| G-H1 (mmol/mol Arg) | 0.245 (0.131–0.308) |
| CEL (mmol/mol Lys) | 1.004 (0.253–1.810) |
| MOLD (mmol/mol Lys) | 0.222 (0.183–0.302) |
| Fructosyl-lysine (mmol/mol Lys) | 9.995 (8.583–12.841) |
| Liver Oxidation Biomarkers | |
| Methionine Sulphoxide (mmol/mol Met) | 32.17 (20.88–42.87) |
| Dityrosine (mmol/mol Tyr) | 0.07 (0.040–0.105) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, A.; Schleicher, E.; Kliemank, E.; Szendroedi, J.; Königsrainer, A.; Häring, H.-U.; Nawroth, P.P.; Fleming, T. Accumulation of Non-Pathological Liver Fat Is Associated with the Loss of Glyoxalase I Activity in Humans. Metabolites 2024, 14, 209. https://doi.org/10.3390/metabo14040209
Peter A, Schleicher E, Kliemank E, Szendroedi J, Königsrainer A, Häring H-U, Nawroth PP, Fleming T. Accumulation of Non-Pathological Liver Fat Is Associated with the Loss of Glyoxalase I Activity in Humans. Metabolites. 2024; 14(4):209. https://doi.org/10.3390/metabo14040209
Chicago/Turabian StylePeter, Andreas, Erwin Schleicher, Elisabeth Kliemank, Julia Szendroedi, Alfred Königsrainer, Hans-Ulrich Häring, Peter P. Nawroth, and Thomas Fleming. 2024. "Accumulation of Non-Pathological Liver Fat Is Associated with the Loss of Glyoxalase I Activity in Humans" Metabolites 14, no. 4: 209. https://doi.org/10.3390/metabo14040209
APA StylePeter, A., Schleicher, E., Kliemank, E., Szendroedi, J., Königsrainer, A., Häring, H.-U., Nawroth, P. P., & Fleming, T. (2024). Accumulation of Non-Pathological Liver Fat Is Associated with the Loss of Glyoxalase I Activity in Humans. Metabolites, 14(4), 209. https://doi.org/10.3390/metabo14040209







