Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Irradiation Procedure
2.2. Analysis of Morphology
2.3. Analyses of Lysosomal Enzymes Activity
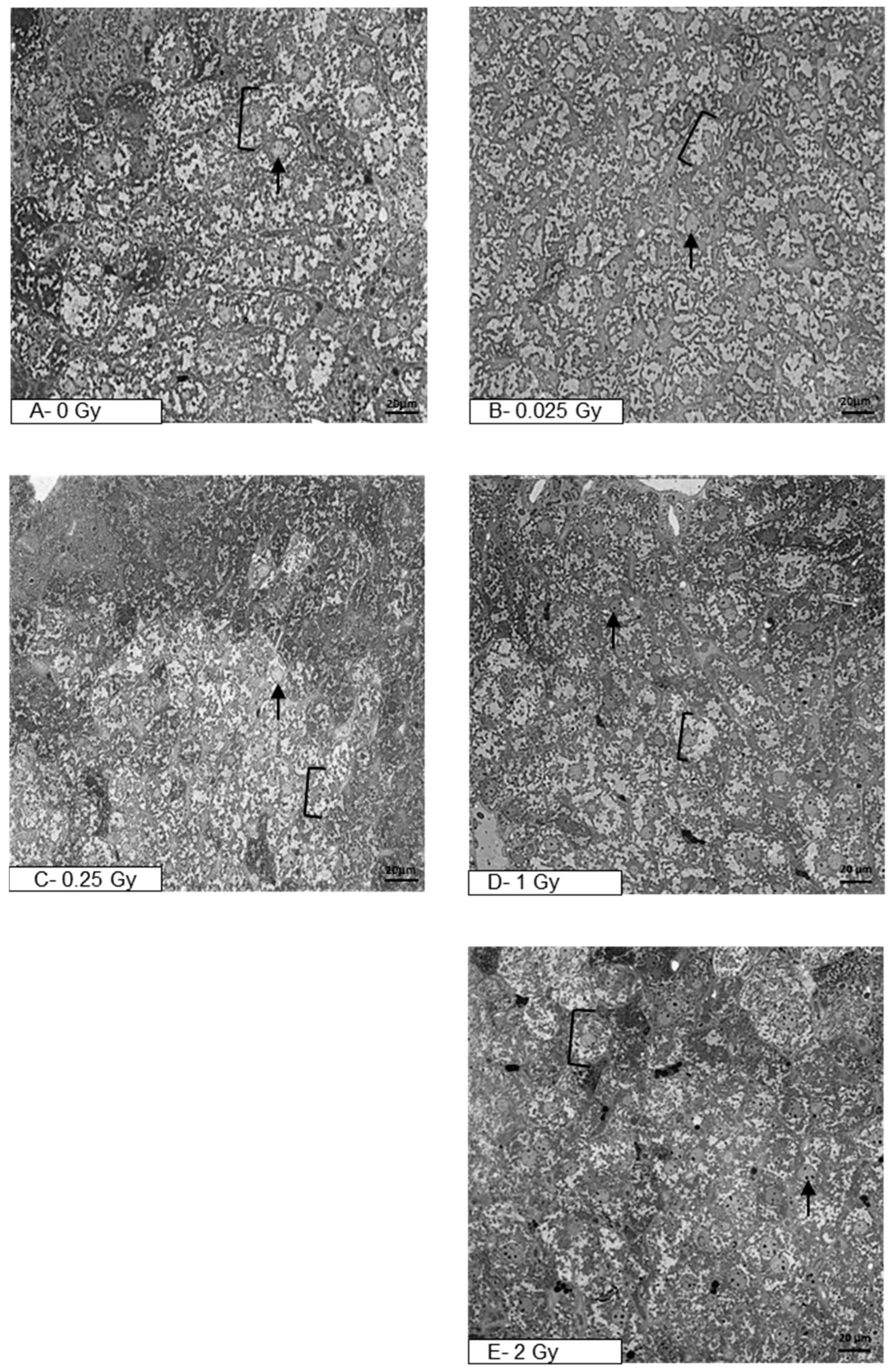
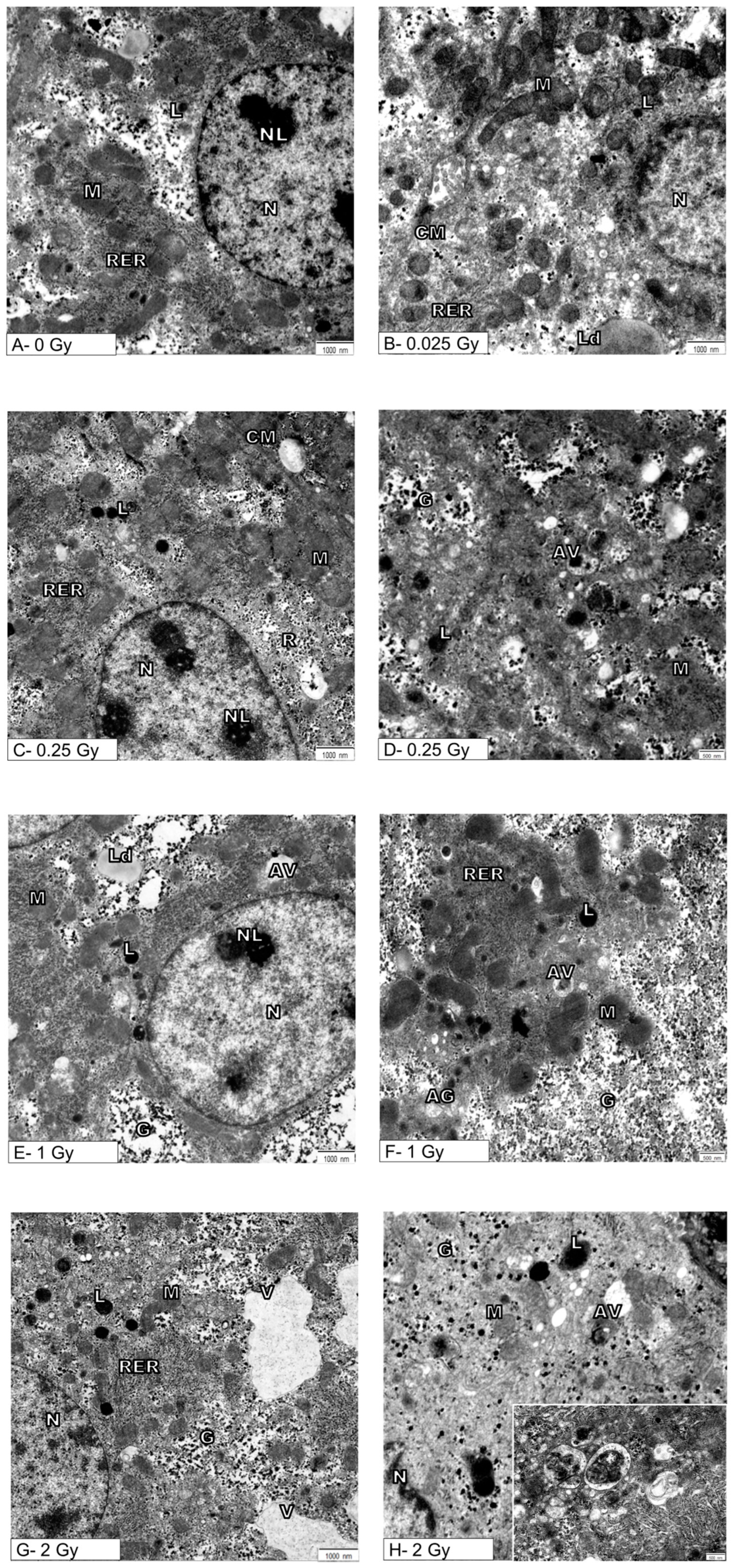
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marcos, R.; Correia-Gomes, C. Long Live the Liver: Immunohistochemical and Stereological Study of Hepatocytes, Liver Sinusoidal Endothelial Cells, Kupffer Cells and Hepatic Stellate Cells of Male and Female Rats throughout Ageing. Cell Tissue Res. 2016, 366, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tian, X.; Wu, H.; Huang, J.; Li, M.; Mei, Z.; Zhou, L.; Xie, H.; Zheng, S. Metabolic Changes of Hepatocytes in NAFLD. Front. Physiol. 2021, 12, 710420. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.R.; Abdul-Hamid, M.; Galaly, S.R.; Hamdalla, H.M. Monosodium Glutamate-Induced Liver Microscopic and Biochemical Changes in Male Rats, and the Possible Amendment of Quercetin. Egypt. J. Zool. 2019, 71, 44–55. [Google Scholar] [CrossRef]
- Parlakgül, G.; Arruda, A.P.; Pang, S.; Cagampan, E.; Min, N.; Güney, E.; Lee, G.Y.; Inouye, K.; Hess, H.F.; Xu, C.S.; et al. Regulation of Liver Subcellular Architecture Controls Metabolic Homeostasis. Nature 2022, 603, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Zoncu, R. The Lysosome as a Regulatory Hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yue, P.; Lu, T.; Wang, Y.; Wei, Y.; Wei, X. Role of Lysosomes in Physiological Activities, Diseases, and Therapy. J. Hematol. Oncol. 2021, 14, 79. [Google Scholar] [CrossRef]
- Johnson, D.E.; Ostrowski, P.; Jaumouillé, V.; Grinstein, S. The Position of Lysosomes within the Cell Determines Their Luminal pH. J. Cell Biol. 2016, 212, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Wang, F.; Muller, S. Lysosomes as a Therapeutic Target. Nat. Rev. Drug Discov. 2019, 18, 923–948. [Google Scholar] [CrossRef] [PubMed]
- Somosy, Z. Radiation Response of Cell Organelles. Micron 2000, 31, 165–181. [Google Scholar] [CrossRef]
- Persson, H.L.; Kurz, T.; Eaton, J.W.; Brunk, U.T. Radiation-Induced Cell Death: Importance of Lysosomal Destabilization. Biochem. J. 2005, 389, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal Membrane Permeabilization and Cell Death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chakraborty, A. Radiation-Induced Bystander Phenomenon: Insight and Implications in Radiotherapy. Int. J. Radiat. Biol. 2019, 95, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.; van der Kogel, A. Basic Clinical Radiobiology, 5th ed.; CRC Press: Boca Raton, FL, US, 2018; ISBN 978-1-4441-7963-7. [Google Scholar]
- Maciejewski, B. Tumor and Normal Tissue Radiation Side Effects. Nowotw. J. Oncol. 2022, 72, 242–246. [Google Scholar] [CrossRef]
- Dörr, W.; Herrmann, T.; Trott, K.-R. Normal Tissue Tolerance. Transl. Cancer Res. 2017, 6, S840–S851. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhong, R.; Sun, L.; Jia, J.; Ma, S.; Liu, X. Ionizing Radiation-Induced Adaptive Response in Fibroblasts under Both Monolayer and 3-Dimensional Conditions. PLoS ONE 2015, 10, e0121289. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic Effects of Ionizing Radiation at the Proteome and Metabolome Levels in the Blood of Cancer Patients Treated with Radiotherapy: The Influence of Inflammation and Radiation Toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, D.; Kulik, R.; Trela, K.; Ślosarek, K. Dosimetric Comparison of Liver Tumour Radiotherapy in All Respiratory Phases and in One Phase Using 4DCT. Radiother. Oncol. 2011, 100, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.G.; Diehn, M.; Kesarwala, A.H.; Maity, A.; Morgan, M.A.; Schwarz, J.K.; Bristow, R.; Demaria, S.; Eke, I.; Griffin, R.J.; et al. The Future of Radiobiology. J. Natl. Cancer Inst. 2018, 110, 329–340. [Google Scholar] [CrossRef]
- Atiq, A.; Atiq, M.; Naeem, H.; Saeed, N.; Abbas, M. Modern Radiation Therapy Techniques and Their Toxicities for Breast Cancer. In Breast Cancer: From Bench to Personalized Medicine; Shakil Malik, S., Masood, N., Eds.; Springer Nature: Singapore, 2022; pp. 429–451. ISBN 978-981-19019-7-3. [Google Scholar]
- Ellahham, S.; Khalouf, A.; Elkhazendar, M.; Dababo, N.; Manla, Y. An Overview of Radiation-Induced Heart Disease. Radiat. Oncol. J. 2022, 40, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Mrotzek, S.M.; Rassaf, T.; Totzeck, M. Cardiovascular Damage Associated With Chest Irradiation. Front. Cardiovasc. Med. 2020, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Güzelöz, Z.; Ayrancıoğlu, O.; Aktürk, N.; Güneş, M.; Alıcıkuş, Z.A. Dose Volume and Liver Function Test Relationship Following Radiotheraphy for Right Breast Cancer: A Multicenter Study. Curr. Oncol. 2023, 30, 8763–8773. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, A.; Szołtysek, K.; Jelonek, K.; Polańska, J.; Dörr, W.; Haagen, J.; Widłak, P.; Gabryś, D. Heart Irradiation Reduces Microvascular Density and Accumulation of HSPA1 in Mice. Strahlenther. Onkol. 2018, 194, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Boguszewicz, ᴌ.; Ciszek, M.; Gabryś, D.; Kulik, R.; Sokół, M. Metabolic Changes in Mice Cardiac Tissue after Low-Dose Irradiation Revealed by 1H NMR Spectroscopy. J. Radiat. Res. 2020, 61, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Marzella, L.; Glaumann, H. Increased Degradation in Rat Liver Induced by Vinblastine. II. Morphologic Characterization. Lab. Investig. 1980, 42, 18–27. [Google Scholar] [PubMed]
- Marzella, L.; Glaumann, H. Increased Degradation in Rat Liver Induced by Vinblastine. I. Biochemical Characterization. Lab. Investig. 1980, 42, 8–17. [Google Scholar] [PubMed]
- Hollander, V.P. 19 Acid Phosphatases. In The Enzymes; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1971; Volume 4, pp. 449–498. [Google Scholar]
- Barrett, A.J.; Heath, M.F. Lysosomal Enzymes. In Lysosomes: A Laboratory Handbook; Dingle, J.T., Ed.; North Holland Publishing Company: Amsterdam, The Netherlands, 1977; pp. 19–145. [Google Scholar]
- McDonald, J.K.; Barrett, A.J. Mammalian Proteases: A Glossary and Bibliography, 2nd ed.; Exopeptidases; Academic Press: London, UK, 1986; p. 357. [Google Scholar]
- Langner, J.; Wakil, A.; Zimmermann, M.; Ansorge, S.; Bohley, P.; Kirschke, H.; Wiederanders, B. [Activity determination of proteolytic enzymes using azocasein as a substrate]. Acta Biol. Med. Ger. 1973, 31, 1–18. [Google Scholar] [PubMed]
- Kirschke, H.; Wiederanders, B. Methoden Zur Aktivitätsbestimmung von Proteinases; Martin-Luther. Universität Halle-Wittenberg, Wissenschaftliche Beitrage: Halle/Salle, Germany, 1984; pp. 11–17. [Google Scholar]
- Nakajima, T.; Ninomiya, Y.; Nenoi, M. Radiation-Induced Reactions in the Liver—Modulation of Radiation Effects by Lifestyle-Related Factors. Int. J. Mol. Sci. 2018, 19, 3855. [Google Scholar] [CrossRef] [PubMed]
- Melin, N.; Yarahmadov, T.; Sanchez-Taltavull, D.; Birrer, F.E.; Brodie, T.M.; Petit, B.; Felser, A.; Nuoffer, J.-M.; Montani, M.; Vozenin, M.-C.; et al. A New Mouse Model of Radiation-Induced Liver Disease Reveals Mitochondrial Dysfunction as an Underlying Fibrotic Stimulus. JHEP Rep. 2022, 4, 100508. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Nakamura, K.; Nishihara, T.; Ichikawa, K.; Nakayama, R.; Takaya, Y.; Toh, N.; Akagi, S.; Miyoshi, T.; Akagi, T.; et al. Association between Cardiovascular Disease and Liver Disease, from a Clinically Pragmatic Perspective as a Cardiologist. Nutrients 2023, 15, 748. [Google Scholar] [CrossRef] [PubMed]
- Gramatyka, M.; Skorupa, A.; Sokół, M. Nuclear Magnetic Resonance Spectroscopy Reveals Metabolic Changes in Living Cardiomyocytes after Low Doses of Ionizing Radiation. Acta Biochim. Pol. 2018, 65, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, J.; Zheng, Q.; Meng, L.; Xin, Y.; Yin, X.; Jiang, X. Radiation-Induced Heart Disease: A Review of Classification, Mechanism and Prevention. Int. J. Biol. Sci. 2019, 15, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.d.A.; Villar, R.C. Radiotherapy and Immune Response: The Systemic Effects of a Local Treatment. Clinics 2018, 73, e557s. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.M.; Mukherjee, D. Liver Abnormalities in Cardiac Diseases and Heart Failure. Int. J. Angiol. 2011, 20, 135–142. [Google Scholar] [CrossRef]
- Shah Shailja, C.; Sass, D.A. “Cardiac Hepatopathy”: A Review of Liver Dysfunction in Heart Failure. Liver Res. Open J. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Telbisz, Á.; Kovács, A.L.; Somosy, Z. Influence of X-Ray on the Autophagic-Lysosomal System in Rat Pancreatic Acini. Micron 2002, 33, 143–151. [Google Scholar] [CrossRef]
- Bright, S.; Fletcher, A.G.; Fell, D.; Kadhim, M.A. Evidence of a Role for Radiation-Induced Lysosomal Damage in Non-Targeted Effects: An Experimental and Theoretical Analysis. bioRxiv 2015. [Google Scholar] [CrossRef]
- Murdica, V.; Mancini, G.; Loberto, N.; Bassi, R.; Giussani, P.; Di Muzio, N.; Deantoni, C.; Prinetti, A.; Aureli, M.; Sonnino, S. Abiraterone and Ionizing Radiation Alter the Sphingolipid Homeostasis in Prostate Cancer Cells. Adv. Exp. Med. Biol. 2018, 1112, 293–307. [Google Scholar] [CrossRef]
- Roy, A.; Bera, S.; Saso, L.; Dwarakanath, B.S. Role of Autophagy in Tumor Response to Radiation: Implications for Improving Radiotherapy. Front. Oncol. 2022, 12, 957373. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.G. Damage Pattern as Function of Various Types of Radiations. Med. J. Islam. World Acad. Sci. 2005, 15, 135–147. [Google Scholar] [CrossRef][Green Version]
- Boyer, M.J.; Tannock, I.F. Lysosomes, Lysosomal Enzymes, and Cancer. Adv. Cancer Res. 1993, 60, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Lysek-Gladysinska, M.; Wieczorek, A.; Walaszczyk, A.; Jelonek, K.; Jozwik, A.; Pietrowska, M.; Dörr, W.; Gabrys, D.; Widlak, P. Long-Term Effects of Low-Dose Mouse Liver Irradiation Involve Ultrastructural and Biochemical Changes in Hepatocytes That Depend on Lipid Metabolism. Radiat. Environ. Biophys. 2018, 57, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Loke, W.K. Molecular Mechanisms of Low Dose Ionizing Radiation-Induced Hormesis, Adaptive Responses, Radioresistance, Bystander Effects, and Genomic Instability. Int. J. Radiat. Biol. 2015, 91, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, G.; Pissakas, G.; Ganos, C.G.; Sivolapenko, G.; Kardamakis, D. Effectiveness and Tolerability of Natural Herbal Formulations in the Prevention of Radiation-Induced Skin Toxicity in Patients Undergoing Radiotherapy. Int. J. Low. Extrem. Wounds 2022, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose Response 2018, 16, 1559325818796331. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Doll, R.; Goodhead, D.T.; Hall, E.J.; Land, C.E.; Little, J.B.; Lubin, J.H.; Preston, D.L.; Preston, R.J.; Puskin, J.S.; et al. Cancer Risks Attributable to Low Doses of Ionizing Radiation: Assessing What We Really Know. Proc. Natl. Acad. Sci. USA 2003, 100, 13761–13766. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Chew, M.T.; Alqahtani, A.; Jones, B.; Hill, M.A.; Nisbet, A.; Bradley, D.A. Low Dose Ionising Radiation-Induced Hormesis: Therapeutic Implications to Human Health. Appl. Sci. 2021, 11, 8909. [Google Scholar] [CrossRef]
- Khan, M.G.M.; Wang, Y. Advances in the Current Understanding of How Low-Dose Radiation Affects the Cell Cycle. Cells 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A.; Lysek-Gladysinska, M.; Walaszczyk, A.; Jelonek, K.; Smolarz, M.; Pietrowska, M.; Gabrys, D.; Kulik, R.; Widlak, P. Changes in Activity and Structure of Lysosomes from Liver of Mouse Irradiated in Vivo. Int. J. Radiat. Biol. 2018, 94, 443–453. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łysek-Gładysińska, M.; Wieczorek, A.; Walaszczyk, A.; Jelonek, K.; Pietrowska, M.; Widłak, P.; Kulik, R.; Gabryś, D. Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo. Metabolites 2024, 14, 212. https://doi.org/10.3390/metabo14040212
Łysek-Gładysińska M, Wieczorek A, Walaszczyk A, Jelonek K, Pietrowska M, Widłak P, Kulik R, Gabryś D. Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo. Metabolites. 2024; 14(4):212. https://doi.org/10.3390/metabo14040212
Chicago/Turabian StyleŁysek-Gładysińska, Małgorzata, Anna Wieczorek, Anna Walaszczyk, Karol Jelonek, Monika Pietrowska, Piotr Widłak, Roland Kulik, and Dorota Gabryś. 2024. "Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo" Metabolites 14, no. 4: 212. https://doi.org/10.3390/metabo14040212
APA StyleŁysek-Gładysińska, M., Wieczorek, A., Walaszczyk, A., Jelonek, K., Pietrowska, M., Widłak, P., Kulik, R., & Gabryś, D. (2024). Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo. Metabolites, 14(4), 212. https://doi.org/10.3390/metabo14040212






