Heat-Induced Cross-Tolerance to Salinity Due to Thermopriming in Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental and Priming Conditions
- first two weeks: 0.122 g N m−2 d−1 (FertyBasis1/Ca(NO3)2/NH4NO3) and 0.2 g K2O m−2 d−1,
- next two weeks: 0.244 g N m−2 d−1 (FertyBasis1/Ca(NO3)2/NH4NO3) and 0.4 g K2O m−2 d−1,
- consecutive weeks: 0.366 g N m−2 d−1 (FertyBasis1/Ca(NO3)2/NH4NO3) and 0.6 g K2O m−2 d−1.
2.2. Growth and Yield Parameters
2.3. Leaf Compound Analysis
2.4. Fruit Compound Analysis
- 1.
- For colorimetric analysis, mixed samples consisting of one-eighth of each fruit were immediately frozen in liquid nitrogen and stored at −80 °C;
- 2.
- For the analysis of ascorbic acid, approximately 100 g of one eighth of each fruit were mixed, weighed in a vessel, filled up with 200 g of 1% (w/v) aqueous oxalic acid dihydrate (≥99.5%; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and frozen at −20 °C;
- 3.
- For the determination of titratable acidity, the remaining fruit parts were frozen in liquid nitrogen and stored at −80 °C.
- 4.
- Sugar Extraction:
- 5.
- Preparation of Standard Solutions:
- 6.
- Preparation of Buffers and Enzymes:
- Citrate buffer. For 250 mL buffer, 8.625 g of citric acid (≥99.5%; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and 11.375 g tri-sodium citrate dihydrate (≥99%; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) were dissolved in a 250 mL volumetric flask with DI water. The pH was adjusted to 4.6 with 2 mol L−1 sodium hydroxide solution. The buffer was stored at 4 °C.
- Tris buffer. For making 250 mL tris(2-hydroxyethyl)ammonium chloride (tris buffer), 43.75 g triethanolamine hydrochloride (≥99%; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and 0.78 g magnesium sulphate heptahydrate (100%; Carl Roth GmbH + Co. KG, Karlsruhe, Germany) were dissolved in a volume of 250 mL DI water. The pH was adjusted to 7.6 with 5 mol L−1 sodium hydroxide solution. This buffer solution was also stored at 4 °C.
- Tris-combi buffer. In a 15 mL test tube with 10 mL tris buffer, 100 mg sodium hydrogen carbonate (≥99.5%), 100 mg adenosin-5′-triphosphate disodium salt (≥98%), and 20 mg NADP disodium salt (≥85%; all obtained from Carl Roth GmbH + Co. KG, Karlsruhe, Germany) were dissolved.
- Hexokinase for glucose/glucose 6-phosphate dehydrogenase (HK/G6P-DH). For this buffer, 500 µL HK/G6P-DH (Roche Pharma AG, Basel, Suisse) was diluted with 500 µL DI water.
- Phosphoglucose isomerase (PGI) fructose. For this buffer, 50 µL PGI (Roche Pharma AG, Basel, Suisse) was diluted with 950 µL DI water.
- Amyloglucosidase (AMG). In 2 mL citrate buffer 5.6 mg AMG was dissolved.
- 7.
- Measurements of Glucose and Fructose:
- 8.
- Starch Measurements:
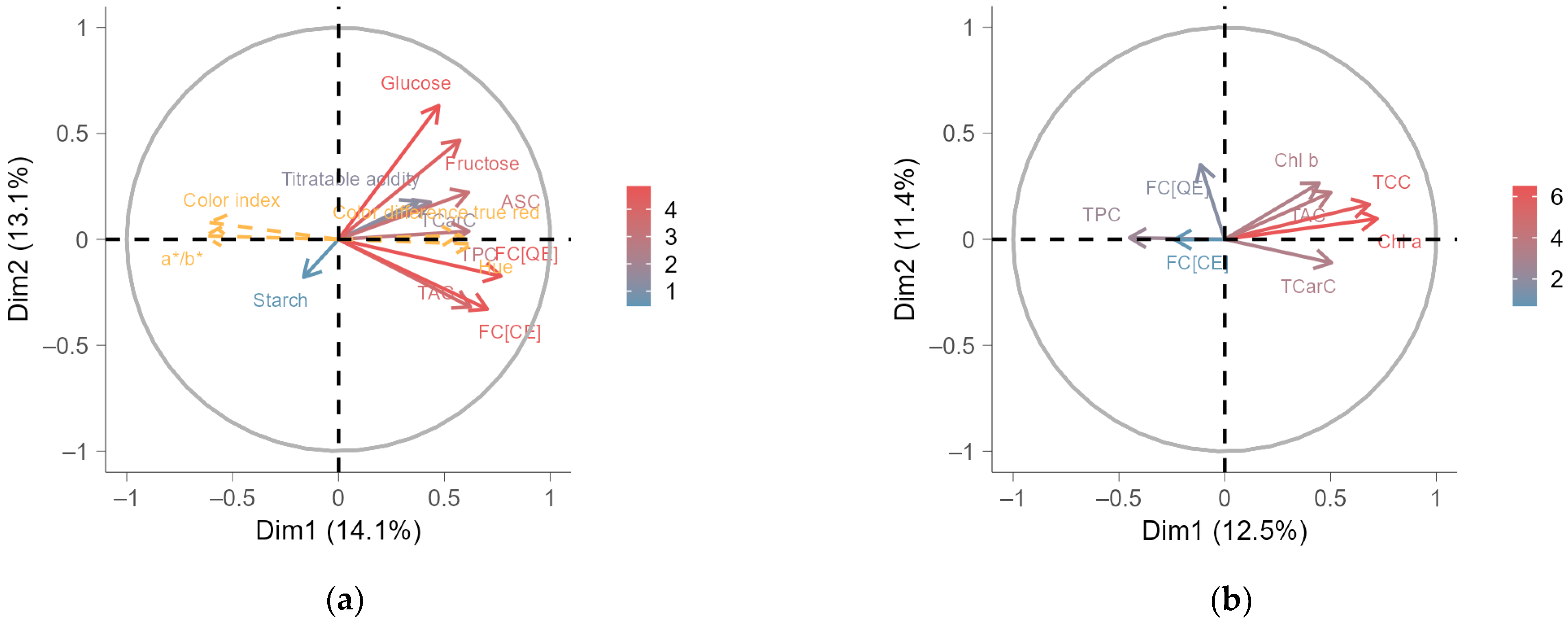
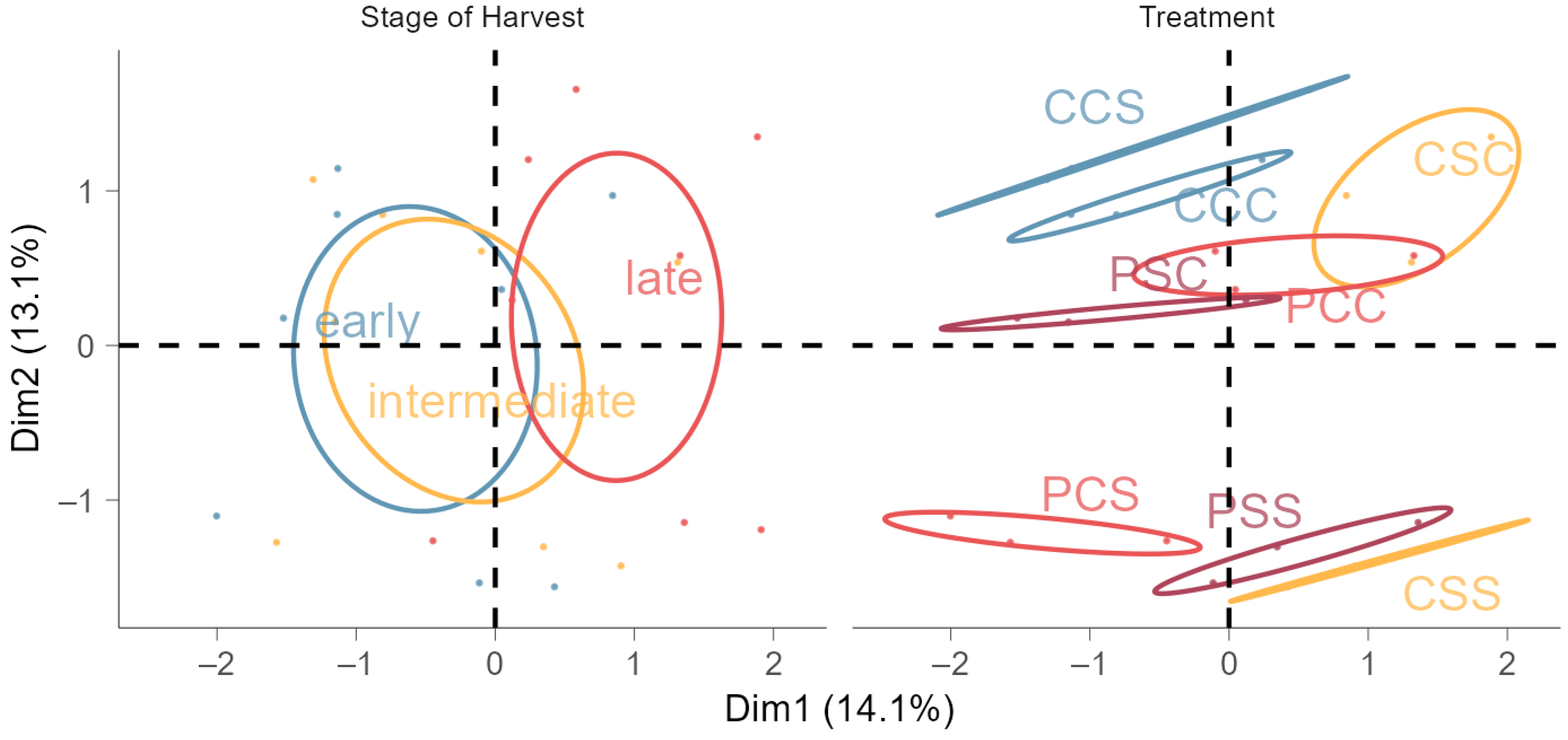
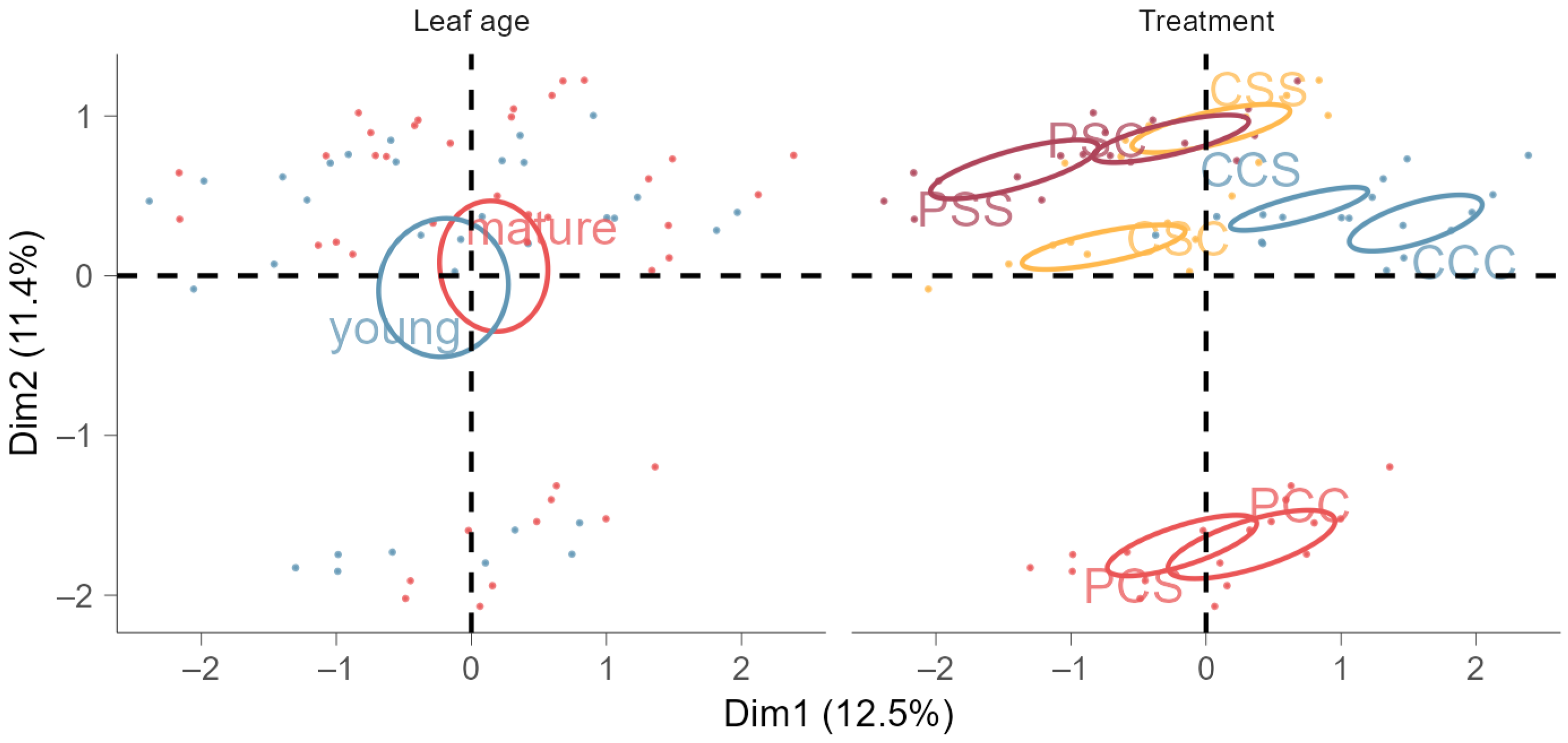
2.5. Multiple Factor Analysis
2.6. Data Analysis
3. Results
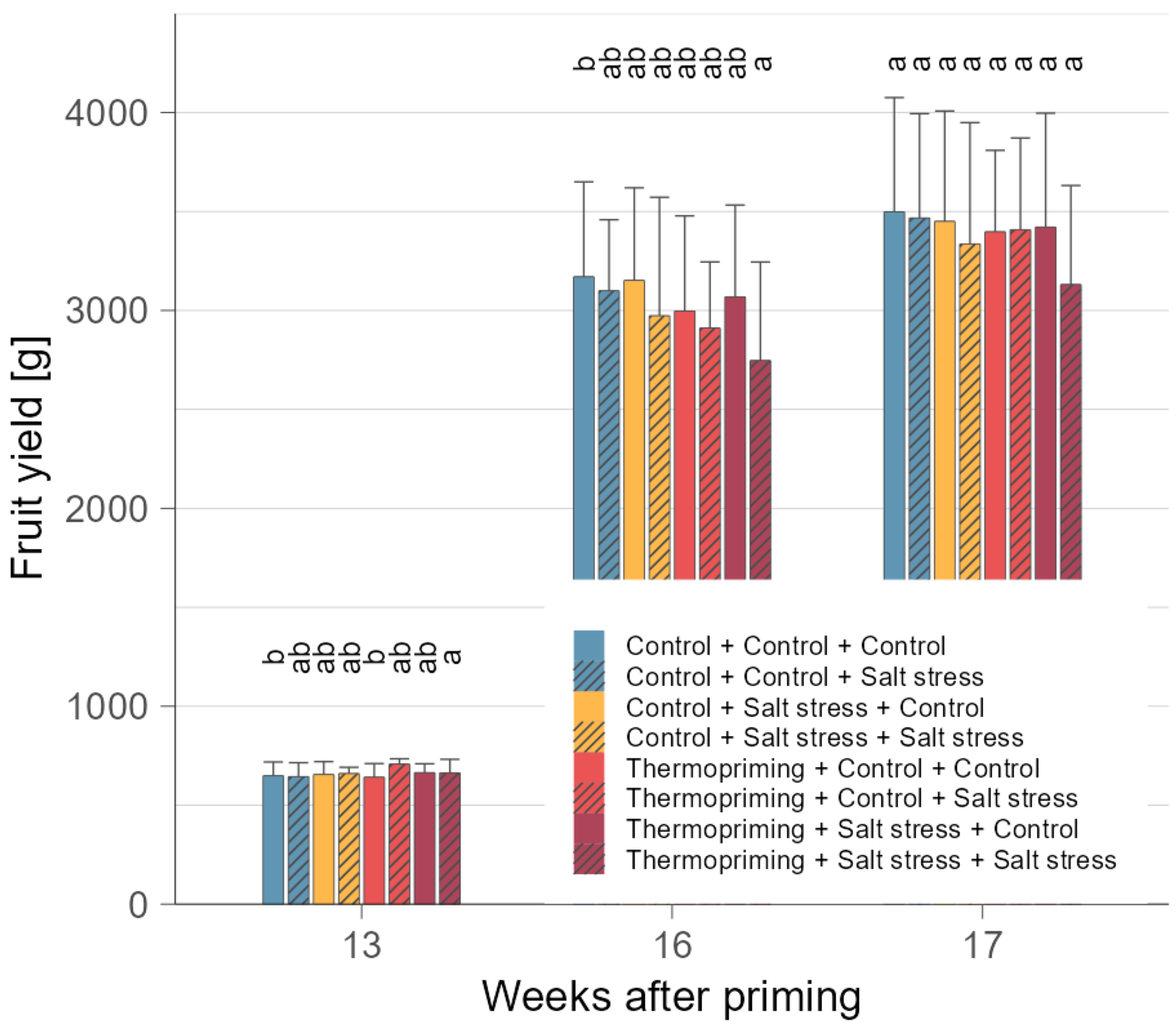
3.1. Thermopriming Did Not Impair Plant Growth and Yield Performance after Subsequent Stress
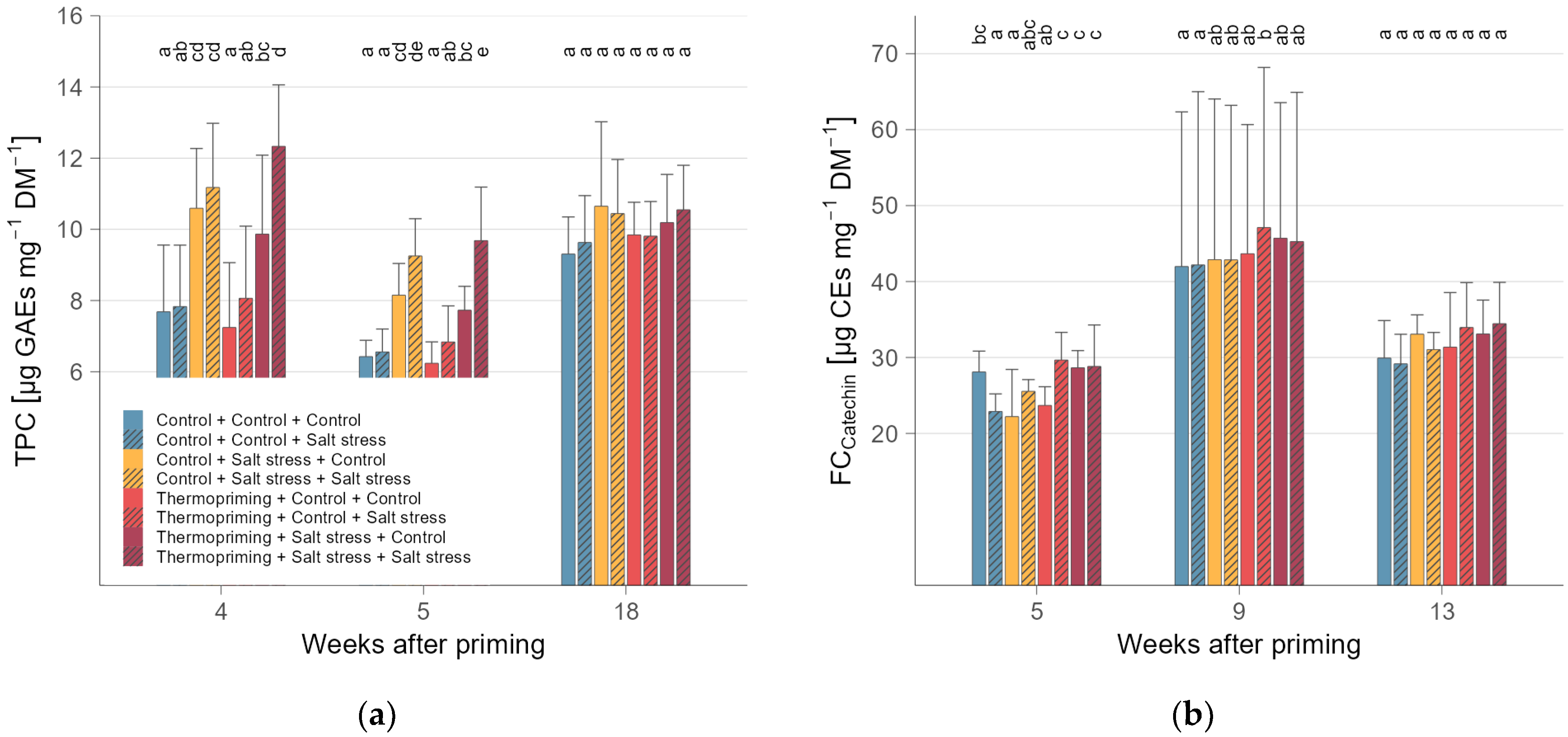
3.2. Thermopriming Decreased Acidity and Sugars, but Increased Starch and Carotenoids in Fruits
3.3. Accumulated Phenols and Flavonoids in Leaves Indicate Cross-Tolerance to Salinity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | WAP | Number of Leaves | n | Number of Inflorescences | n | Number of Infructescences | n |
|---|---|---|---|---|---|---|---|
| C–C–C | 0 | 0.5 ± 0.5 b | 154 | ||||
| 2 | 4.0 ± 0.4 b | 63 | |||||
| 3 | 6.5 ± 0.6 b | 63 | 1.0 ± 0.0 a | 54 | |||
| 4 | 9.4 ± 0.5 c | 24 | 1.9 ± 0.3 c | 24 | |||
| 6 | 15.9 ± 0.5 c | 24 | 4.0 ± 0.0 c | 24 | |||
| 7 | 19.5 ± 0.8 c | 24 | 3.6 ± 0.5 a | 24 | 1.3 ± 0.5 ab | 24 | |
| 8 | 22.1 ± 1.1 b | 24 | 3.0 ± 0.5 a | 24 | 3.0 ± 0.3 b | 24 | |
| 10 | 27.8 ± 1.4 abc | 24 | 3.0 ± 0.8 a | 24 | 4.9 ± 0.4 b | 24 | |
| 12 | 33.8 ± 2.5 a | 24 | 2.1 ± 0.8 a | 24 | 6.9 ± 2.2 a | 24 | |
| C–C–S | 4 | 8.9 ± 0.6 bc | 24 | 1.8 ± 0.4 bc | 24 | ||
| 6 | 15.2 ± 0.7 bc | 24 | 3.8 ± 0.4 bc | 24 | |||
| 7 | 19.2 ± 1.0 bc | 24 | 3.4 ± 0.5 a | 24 | 1.4 ± 0.5 ab | 23 | |
| 8 | 22.2 ± 1.6 b | 24 | 3.1 ± 0.4 a | 24 | 2.8 ± 0.4 ab | 24 | |
| 10 | 28.0 ± 1.5 abc | 23 | 3.1 ± 0.3 a | 23 | 4.8 ± 0.4 b | 23 | |
| 12 | 34.1 ± 1.6 a | 23 | 2.3 ± 0.5 a | 23 | 7.4 ± 0.8 a | 24 | |
| C–S–C | 2 | 3.8 ± 0.4 a | 62 | ||||
| 3 | 6.3 ± 0.7 ab | 62 | 1.0 ± 0.0 a | 46 | |||
| 4 | 9.0 ± 0.7 bc | 24 | 1.8 ± 0.4 bc | 24 | |||
| 6 | 15.5 ± 0.7 bc | 24 | 3.8 ± 0.4 bc | 24 | |||
| 7 | 19.2 ± 0.9 bc | 24 | 3.3 ± 0.6 a | 24 | 1.5 ± 0.6 b | 24 | |
| 8 | 22.2 ± 1.0 b | 24 | 3.0 ± 0.3 a | 24 | 2.8 ± 0.4 ab | 24 | |
| 10 | 28.1 ± 1.0 bc | 24 | 3.2 ± 0.4 ab | 24 | 4.9 ± 0.4 b | 24 | |
| 12 | 34.2 ± 1.0 a | 24 | 2.2 ± 0.5 a | 24 | 7.4 ± 0.6 a | 24 | |
| C–S–S | 4 | 8.6 ± 0.6 ab | 24 | 1.6 ± 0.5 abc | 24 | ||
| 6 | 14.9 ± 0.9 ab | 24 | 3.8 ± 0.4 bc | 24 | |||
| 7 | 18.5 ± 1.0 ab | 24 | 3.4 ± 0.5 a | 24 | 1.3 ± 0.5 ab | 23 | |
| 8 | 21.5 ± 1.1 b | 24 | 2.9 ± 0.5 a | 24 | 2.8 ± 0.4 ab | 24 | |
| 10 | 27.2 ± 1.3 ab | 24 | 3.0 ± 0.2 a | 24 | 4.7 ± 0.5 ab | 24 | |
| 12 | 33.5 ± 1.5 a | 24 | 2.2 ± 0.6 a | 24 | 7.1 ± 0.4 a | 24 | |
| P–C–C | 0 | 0.0 ± 0.2 a | 154 | ||||
| 2 | 4.0 ± 0.4 ab | 63 | |||||
| 3 | 6.5 ± 0.6 b | 63 | 1.0 ± 0.0 a | 51 | |||
| 4 | 9.1 ± 0.5 bc | 24 | 1.7 ± 0.5 bc | 24 | |||
| 6 | 15.8 ± 0.8 c | 24 | 3.7 ± 0.6 bc | 24 | |||
| 7 | 19.3 ± 0.8 bc | 24 | 3.7 ± 0.5 a | 24 | 1.2 ± 0.4 ab | 22 | |
| 8 | 22.3 ± 0.8 b | 24 | 3.2 ± 0.6 a | 24 | 2.9 ± 0.7 ab | 24 | |
| 10 | 28.4 ± 0.8 c | 24 | 3.2 ± 0.4 ab | 24 | 4.7 ± 0.7 ab | 24 | |
| 12 | 34.1 ± 1.1 a | 24 | 2.2 ± 0.4 a | 24 | 7.2 ± 0.7 a | 24 | |
| P–C–S | 4 | 9.0 ± 0.6 bc | 24 | 1.5 ± 0.5 abc | 24 | ||
| 6 | 15.2 ± 0.8 bc | 24 | 3.6 ± 0.5 ab | 24 | |||
| 7 | 18.8 ± 1.0 bc | 24 | 3.5 ± 0.5 a | 24 | 1.2 ± 0.4 ab | 24 | |
| 8 | 21.5 ± 1.3 b | 24 | 3.0 ± 0.5 a | 24 | 2.7 ± 0.5 ab | 24 | |
| 10 | 27.4 ± 1.3 abc | 24 | 3.2 ± 0.4 ab | 24 | 4.7 ± 0.5 ab | 24 | |
| 12 | 33.3 ± 1.8 a | 24 | 2.4 ± 0.5 a | 24 | 7.3 ± 0.6 a | 24 | |
| P–S–C | 2 | 3.8 ± 0.5 a | 63 | ||||
| 3 | 6.2 ± 0.7 a | 63 | 1.0 ± 0.0 a | 36 | |||
| 4 | 8.9 ± 0.6 ab | 24 | 1.5 ± 0.5 ab | 24 | |||
| 6 | 15.4 ± 0.7 bc | 24 | 3.7 ± 0.5 abc | 24 | |||
| 7 | 19.0 ± 1.0 bc | 24 | 3.5 ± 0.6 a | 24 | 1.3 ± 0.5 ab | 24 | |
| 8 | 21.6 ± 1.1 b | 24 | 2.9 ± 0.4 a | 24 | 2.9 ± 0.3 ab | 24 | |
| 10 | 27.6 ± 1.2 abc | 23 | 3.3 ± 0.5 ab | 23 | 4.6 ± 0.5 ab | 23 | |
| 12 | 33.3 ± 1.1 a | 23 | 2.2 ± 0.7 a | 24 | 7.0 ± 1.5 a | 24 | |
| P–S–S | 4 | 8.4 ± 0.7 a | 24 | 1.2 ± 0.4 a | 24 | ||
| 6 | 14.4 ± 1.2 a | 24 | 3.3 ± 0.5 a | 24 | |||
| 7 | 18.0 ± 1.1 a | 24 | 3.4 ± 0.5 a | 24 | 1.1 ± 0.3 a | 22 | |
| 8 | 20.4 ± 1.0 a | 24 | 3.1 ± 0.4 a | 24 | 2.6 ± 0.5 a | 24 | |
| 10 | 26.9 ± 1.4 a | 24 | 3.5 ± 0.7 b | 24 | 4.3 ± 0.7 a | 24 | |
| 12 | 33.0 ± 1.6 a | 24 | 2.4 ± 0.5 a | 24 | 7.0 ± 0.5 a | 24 |
| Treatment | FM | DM | n | Fruit Yield | n |
|---|---|---|---|---|---|
| [g] | [g] | ||||
| C–C–C | 2321 ± 293 a | 316 ± 61 a | 15 | 4682 ± 836 a | 24 |
| C–C–S | 2421 ± 434 a | 316 ± 58 a | 15 | 4572 ± 581 a | 24 |
| C–S–C | 2485 ± 235 a | 338 ± 37 a | 15 | 4722 ± 596 a | 24 |
| C–S–S | 2191 ± 289 a | 299 ± 46 a | 15 | 4881 ± 693 a | 24 |
| P–C–C | 2513 ± 273 a | 341 ± 51 a | 15 | 4786 ± 497 a | 24 |
| P–C–S | 2207 ± 306 a | 294 ± 42 a | 15 | 4772 ± 548 a | 24 |
| P–S–C | 2284 ± 348 a | 305 ± 51 a | 15 | 4809 ± 658 a | 23 |
| P–S–S | 2220 ± 274 a | 302 ± 49 a | 15 | 4699 ± 587 a | 24 |
| Treatment | WAP | gsw | ETR | n |
|---|---|---|---|---|
| [mol m−2 s−1] | [μmol e− m−2 s−1] | |||
| C–C–C | 3 1 | 1.01 ± 0.14 c | 52.21 ± 11.06 b | 63 |
| 3 2 | 0.94 ± 0.12 e | 23.17 ± 5.88 a | 32 | |
| 4 | 0.80 ± 0.20 cd | 54.15 ± 20.41 a | 24 | |
| 7 | 0.75 ± 0.16 a | 35.84 ± 12.96 ab | 24 | |
| 9 | 1.23 ± 0.43 a | 125.02 ± 33.21 a | 21 | |
| 11 | 1.00 ± 0.21 ab | 72.79 ± 28.55 ab | 24 | |
| 13 | 0.36 ± 0.19 ab | 49.67 ± 62.32 a | 24 | |
| C–C–S | 3 | 0.81 ± 0.18 cd | 27.05 ± 5.71 abc | 30 |
| 4 | 0.60 ± 0.22 b | 68.98 ± 43.09 ab | 24 | |
| 7 | 0.72 ± 0.09 a | 31.53 ± 7.29 a | 24 | |
| 9 | 1.29 ± 0.33 a | 118.42 ± 56.78 a | 21 | |
| 11 | 1.10 ± 0.26 b | 76.24 ± 36.98 ab | 24 | |
| 13 | 0.41 ± 0.14 ab | 44.29 ± 46.15 a | 24 | |
| C–S–C | 3 | 0.88 ± 0.17 ab | 51.77 ± 12.46 b | 62 |
| 3 | 0.71 ± 0.16 bc | 28.52 ± 8.94 bc | 32 | |
| 4 | 0.69 ± 0.21 bc | 75.70 ± 41.46 ab | 24 | |
| 7 | 0.80 ± 0.10 a | 37.52 ± 17.14 ab | 24 | |
| 9 | 1.23 ± 0.30 a | 163.30 ± 67.37 a | 21 | |
| 11 | 1.04 ± 0.28 ab | 54.88 ± 18.46 a | 24 | |
| 13 | 0.32 ± 0.13 ab | 53.30 ± 59.30 a | 24 | |
| C–S–S | 3 | 0.41 ± 0.22 a | 30.57 ± 10.78 c | 32 |
| 4 | 0.34 ± 0.17 a | 83.65 ± 46.83 b | 24 | |
| 7 | 0.78 ± 0.14 a | 43.55 ± 25.10 b | 24 | |
| 9 | 1.25 ± 0.20 a | 158.40 ± 59.59 a | 19 | |
| 11 | 1.07 ± 0.17 ab | 81.95 ± 36.76 b | 24 | |
| 13 | 0.36 ± 0.10 ab | 62.58 ± 74.03 a | 24 | |
| P–C–C | 3 | 0.94 ± 0.14 bc | 46.67 ± 6.82 a | 63 |
| 3 | 0.84 ± 0.09 de | 31.03 ± 13.90 c | 30 | |
| 4 | 0.92 ± 0.24 d | 53.05 ± 22.56 a | 24 | |
| 7 | 0.70 ± 0.12 a | 32.53 ± 3.74 a | 24 | |
| 9 | 1.18 ± 0.26 a | 137.14 ± 59.63 a | 22 | |
| 11 | 0.89 ± 0.32 a | 68.52 ± 34.48 ab | 24 | |
| 13 | 0.31 ± 0.15 a | 54.28 ± 49.49 a | 24 | |
| P–C–S | 3 | 0.68 ± 0.16 b | 25.57 ± 15.11 ab | 32 |
| 4 | 0.65 ± 0.15 bc | 55.11 ± 20.52 a | 24 | |
| 7 | 0.76 ± 0.20 a | 38.48 ± 11.20 ab | 24 | |
| 9 | 1.05 ± 0.21 a | 141.52 ± 63.80 a | 20 | |
| 11 | 1.04 ± 0.19 ab | 72.64 ± 30.84 ab | 24 | |
| 13 | 0.43 ± 0.10 b | 63.47 ± 67.12 a | 24 | |
| P–S–C | 3 | 0.83 ± 0.14 a | 51.34 ± 11.56 b | 63 |
| 3 | 0.70 ± 0.13 bc | 25.35 ± 5.25 ab | 32 | |
| 4 | 0.55 ± 0.17 b | 70.69 ± 44.22 ab | 24 | |
| 7 | 0.77 ± 0.12 a | 38.94 ± 12.00 ab | 24 | |
| 9 | 1.20 ± 0.22 a | 120.58 ± 53.35 a | 18 | |
| 11 | 0.93 ± 0.29 ab | 75.64 ± 28.40 ab | 23 | |
| 13 | 0.36 ± 0.16 ab | 48.83 ± 55.93 a | 24 | |
| P–S–S | 3 | 0.41 ± 0.23 a | 22.98 ± 10.53 a | 31 |
| 4 | 0.36 ± 0.20 a | 79.40 ± 41.42 ab | 24 | |
| 7 | 0.79 ± 0.17 a | 39.30 ± 8.60 ab | 24 | |
| 9 | 1.11 ± 0.18 a | 131.10 ± 58.95 a | 22 | |
| 11 | 1.07 ± 0.16 ab | 70.06 ± 18.76 ab | 24 | |
| 13 | 0.40 ± 0.13 ab | 61.94 ± 61.02 a | 24 |
| Treatment | Stage of Harvest | TCarC 1 | TAC 2 | TPC 3 | FCCatechin 4 | FCQuercetin 4 | n |
|---|---|---|---|---|---|---|---|
| [µg mg−1 DM−1] | [µg CyEs mg−1 DM−1] | [µg GAEs mg−1 DM−1] | [µg CEs mg−1 DM−1] | [µg QEs mg−1 DM−1] | |||
| C–C–C | early 5 | 1.0 ± 0.2 ab | 0.7 ± 0.3 a | 3.3 ± 0.2 a | 4.2 ± 0.8 a | 3.1 ± 0.4 a | 9 |
| C–C–S | 1.0 ± 0.2 ab | 0.9 ± 0.4 a | 3.7 ± 0.5 a | 4.6 ± 1.0 a | 3.1 ± 0.7 a | 9 | |
| C–S–C | 1.0 ± 0.3 ab | 0.9 ± 0.3 a | 3.6 ± 0.8 a | 4.5 ± 1.0 a | 3.3 ± 0.4 a | 10 | |
| C–S–S | 0.8 ± 0.2 a | 1.0 ± 0.3 a | 3.5 ± 0.2 a | 4.9 ± 1.0 a | 3.3 ± 0.3 a | 7 | |
| P–C–C | 1.0 ± 0.2 ab | 0.9 ± 0.5 a | 3.6 ± 0.3 a | 4.7 ± 1.4 a | 3.3 ± 0.5 a | 10 | |
| P–C–S | 1.0 ± 0.3 ab | 0.7 ± 0.1 a | 3.5 ± 0.1 a | 4.0 ± 0.6 a | 3.2 ± 0.4 a | 10 | |
| P–S–C | 1.0 ± 0.1 ab | 0.7 ± 0.4 a | 3.4 ± 0.2 a | 3.9 ± 1.1 a | 3.1 ± 0.5 a | 9 | |
| P–S–S | 1.1 ± 0.1 b | 0.9 ± 0.3 a | 3.5 ± 0.3 a | 4.5 ± 0.8 a | 3.4 ± 0.6 a | 11 | |
| C–C–C | inter- mediate 6 | 1.0 ± 0.3 a | 0.9 ± 0.3 ab | 3.9 ± 0.2 a | 4.0 ± 0.8 ab | 3.3 ± 0.4 a | 10 |
| C–C–S | 1.0 ± 0.3 a | 0.8 ± 0.3 a | 3.8 ± 0.3 a | 3.7 ± 0.6 a | 3.1 ± 0.5 a | 12 | |
| C–S–C | 0.9 ± 0.2 a | 1.2 ± 0.2 b | 3.9 ± 0.3 a | 5.0 ± 1.0 b | 3.6 ± 0.3 a | 10 | |
| C–S–S | 1.0 ± 0.3 a | 1.1 ± 0.3 ab | 3.9 ± 0.4 a | 4.7 ± 0.9 ab | 3.5 ± 0.3 a | 12 | |
| P–C–C | 1.0 ± 0.3 a | 0.7 ± 0.4 a | 3.9 ± 0.4 a | 4.1 ± 1.5 ab | 3.3 ± 0.6 a | 9 | |
| P–C–S | 0.8 ± 0.2 a | 0.9 ± 0.3 ab | 3.8 ± 0.4 a | 4.3 ± 0.9 ab | 3.4 ± 0.4 a | 10 | |
| P–S–C | 0.9 ± 0.2 a | 0.8 ± 0.3 ab | 3.8 ± 0.3 a | 4.2 ± 1.1 ab | 3.1 ± 0.5 a | 10 | |
| P–S–S | 0.9 ± 0.2 a | 1.0 ± 0.5ab | 4.1 ± 0.3 a | 4.8 ± 1.3 ab | 3.4 ± 0.4 a | 11 | |
| C–C–C | late 7 | 1.1 ± 0.2 a | 0.9 ± 0.4 a | 4.5 ± 0.3 a | 4.4 ± 0.8 a | 3.6 ± 0.5 a | 10 |
| C–C–S | 1.2 ± 0.1 a | 0.9 ± 0.4 a | 4.9 ± 0.3 a | 5.0 ± 1.8 a | 3.6 ± 0.5 a | 10 | |
| C–S–C | 1.1 ± 0.2 a | 0.8 ± 0.3 a | 4.8 ± 0.2 a | 4.4 ± 1.0 a | 3.6 ± 0.6 a | 11 | |
| C–S–S | 1.2 ± 0.2 a | 1.0 ± 0.3 a | 5.0 ± 0.6 a | 5.3 ± 1.3 a | 3.8 ± 0.5 a | 11 | |
| P–C–C | 1.1 ± 0.2 a | 1.0 ± 0.5 a | 4.9 ± 0.4 a | 5.2 ± 2.3 a | 3.7 ± 0.4 a | 10 | |
| P–C–S | 1.2 ± 0.2 a | 1.0 ± 0.2 a | 5.0 ± 0.4 a | 5.2 ± 1.0 a | 3.6 ± 0.3 a | 12 | |
| P–S–C | 1.1 ± 0.2 a | 1.1 ± 0.5 a | 4.8 ± 0.4 a | 5.2 ± 2.0 a | 3.6 ± 0.7 a | 11 | |
| P–S–S | 1.1 ± 0.2 a | 1.0 ± 0.5 a | 4.9 ± 0.4 a | 5.9 ± 1.9 a | 3.7 ± 0.7 a | 11 |
| Treatment | Stage of Harvest | ASC 1 | n | Glucose | Fructose | Starch | n | TA 2 | n |
|---|---|---|---|---|---|---|---|---|---|
| [mg kg−1 fruit FM−1] | [µg mg−1 fruit FM−1] | [µg mg−1 fruit FM−1] | [µg mg−1 fruit FM−1] | [µg CAEs mg−1 DM−1] | |||||
| C–C–C | early 3 | 231.5 ± 19.7 ab | 12 | 220.8 ± 42.4 bc | 219.3 ± 34.6 ab | 4.4 ± 1.0 a | 9 | 2.9 ± 0.4 a | 11 |
| C–C–S | 226.0 ± 15.3 ab | 12 | 217.6 ± 32.4 bc | 211.7 ± 22.0 ab | 4.7 ± 1.8 a | 10 | 2.8 ± 0.4 a | 9 | |
| C–S–C | 236.7 ± 37.1 b | 12 | 239.0 ± 34.8 c | 238.0 ± 31.0 b | 4.7 ± 1.7 a | 11 | 2.9 ± 0.3 a | 10 | |
| C–S–S | 214.8 ± 15.6 ab | 12 | 192.2 ± 33.0 ab | 199.4 ± 30.9 ab | 4.4 ± 1.2 a | 12 | 2.8 ± 0.3 a | 12 | |
| P–C–C | 236.8 ± 20.2 b | 11 | 213.0 ± 28.1 bc | 214.6 ± 21.7 ab | 4.4 ± 1.6 a | 12 | 2.9 ± 0.4 a | 12 | |
| P–C–S | 207.8 ± 12.0 a | 11 | 194.3 ± 40.3 ab | 203.6 ± 35.4 ab | 3.9 ± 1.7 a | 12 | 2.6 ± 0.5 a | 12 | |
| P–S–C | 212.5 ± 14.5 ab | 12 | 205.5 ± 28.2 abc | 215.3 ± 35.4 ab | 4.5 ± 1.8 a | 10 | 2.8 ± 0.4 a | 11 | |
| P–S–S | 218.1 ± 11.9 ab | 11 | 165.7 ± 38.0 a | 178.8 ± 29.6 a | 4.8 ± 1.5 a | 12 | 3.0 ± 0.5 a | 11 | |
| C–C–C | inter- mediate 4 | 261.7 ± 34.9 a | 12 | 205.4 ± 27.4 ab | 211.9 ± 28.0 ab | 2.4 ± 2.8 a | 12 | 2.9 ± 0.3 a | 12 |
| C–C–S | 255.3 ± 23.1 a | 12 | 187.3 ± 24.6 ab | 189.9 ± 25.0 ab | 2.4 ± 1.3 a | 12 | 2.7 ± 0.2 a | 12 | |
| C–S–C | 269.2 ± 19.6 a | 12 | 200.2 ± 25.4 ab | 206.2 ± 25.5 ab | 2.3 ± 1.0 a | 11 | 2.8 ± 0.5 a | 12 | |
| C–S–S | 262.5 ± 34.4 a | 12 | 198.1 ± 32.4 ab | 204.7 ± 34.0 ab | 2.5 ± 1.2 a | 12 | 2.8 ± 0.2 a | 12 | |
| P–C–C | 284.1 ± 38.7 a | 12 | 229.8 ± 42.7 b | 236.7 ± 48.4 b | 4.0 ± 2.5 a | 12 | 2.7 ± 0.5 a | 12 | |
| P–C–S | 258.3 ± 27.9 a | 12 | 181.6 ± 23.0 a | 188.0 ± 20.4 a | 2.6 ± 2.4 a | 11 | 2.9 ± 0.2 a | 12 | |
| P–S–C | 264.5 ± 29.1 a | 12 | 191.4 ± 28.2 a | 199.0 ± 26.9 a | 2.4 ± 1.5 a | 12 | 3.0 ± 0.4 a | 12 | |
| P–S–S | 257.0 ± 19.2 a | 12 | 207.1 ± 43.6 ab | 217.2 ± 46.1 ab | 3.2 ± 2.5 a | 12 | 2.8 ± 0.4 a | 12 | |
| C–C–C | late 5 | 323.5 ± 28.5 a | 11 | 241.8 ± 60.7 a | 256.4 ± 55.0 a | 4.1 ± 3.0 ab | 11 | 3.0 ± 0.3 b | 11 |
| C–C–S | 331.9 ± 26.1 a | 12 | 249.6 ± 65.2 a | 273.0 ± 58.9 a | 2.7 ± 1.0 a | 12 | 3.0 ± 0.4 ab | 12 | |
| C–S–C | 333.4 ± 21.2 a | 12 | 246.8 ± 55.1 a | 265.4 ± 54.0 a | 2.3 ± 1.0 a | 11 | 3.0 ± 0.3 ab | 12 | |
| C–S–S | 324.8 ± 32.8 a | 12 | 214.0 ± 60.0 a | 240.6 ± 58.2 a | 3.4 ± 2.3 ab | 11 | 2.9 ± 0.2 ab | 12 | |
| P–C–C | 335.9 ± 30.2 a | 12 | 228.0 ± 56.3 a | 244.1 ± 49.5 a | 4.4 ± 3.0 ab | 12 | 2.9 ± 0.4 ab | 12 | |
| P–C–S | 310.2 ± 25.3 a | 12 | 199.6 ± 55.2 a | 223.0 ± 52.2 a | 6.3 ± 4.1 b | 12 | 2.8 ± 0.4 ab | 12 | |
| P–S–C | 305.1 ± 32.1 a | 12 | 223.7 ± 59.3 a | 237.5 ± 55.6 a | 3.5 ± 2.3 ab | 12 | 3.0 ± 0.3 ab | 11 | |
| P–S–S | 314.6 ± 30.4 a | 12 | 217.6 ± 71.3 a | 246.5 ± 64.4 a | 3.2 ± 1.7 ab | 12 | 2.7 ± 0.3 a | 12 |
| Treatment | WAP | TCC 1 | TCarC 2 | TAC 3 | TPC 4 | FCCatechin 5 | FCQuercetin 5 | n |
|---|---|---|---|---|---|---|---|---|
| [µg mg−1 DM−1] | [µg CyEs mg−1 DM−1] | [µg GAEs mg−1 DM−1] | [µg CEs mg−1 DM−1] | [µg QEs mg−1 DM−1] | ||||
| C–C–C | 1 | 4.2 ± 0.2 b | 3.4 ± 0.3 b | 3.0 ± 0.4 a | 6.7 ± 0.6 a | 13.3 ± 2.8 a | 12.8 ± 0.4 a | 16 |
| 3 | 4.7 ± 0.4 a | 4.2 ± 0.3 ab | 2.3 ± 0.5 a | 10.9 ± 2.5 b | 21.3 ± 4.6 a | 15.4 ± 1.1 ab | 8 | |
| 4 | 5.1 ± 0.5 b | 3.4 ± 0.6 c | 3.1 ± 0.8 ab | 11.0 ± 1.4 e | 24.7 ± 2.5 a | 15.0 ± 0.6 d | 12 | |
| 5 | 5.1 ± 0.3 a | 3.8 ± 0.6 a | 5.5 ± 0.7 bc | 10.5 ± 1.0 a | 28.0 ± 2.8 bc | 16.2 ± 1.5 a | 12 | |
| 9 | 3.8 ± 0.5 b | 2.6 ± 0.4 a | 4.5 ± 0.9 a | 17.1 ± 1.5 a | 41.9 ± 20.4 a | 18.0 ± 0.9 a | 12 | |
| 13 | 3.1 ± 0.3 c | 1.7 ± 0.2 a | 3.6 ± 0.5 b | 15.7 ± 1.2 a | 29.9 ± 5.0 a | 17.6 ± 2.2 a | 12 | |
| C–C–S | 4 | 5.1 ± 0.4 b | 2.8 ± 0.7 bc | 3.2 ± 0.5 b | 10.0 ± 1.2 de | 24.2 ± 6.1 a | 14.4 ± 0.9 cd | 12 |
| 5 | 5.2 ± 0.5 a | 3.8 ± 0.7 a | 4.0 ± 1.3 a | 10.5 ± 0.8 a | 22.8 ± 2.4 a | 16.8 ± 1.0 a | 12 | |
| 9 | 3.6 ± 0.4 ab | 2.6 ± 0.2 a | 4.7 ± 0.8 a | 16.6 ± 1.4 a | 42.1 ± 22.9 a | 17.5 ± 0.8 a | 12 | |
| 13 | 3.0 ± 0.4 bc | 1.7 ± 0.2 a | 3.5 ± 0.6 b | 16.0 ± 2.0 a | 29.1 ± 3.9 a | 17.3 ± 1.3 a | 12 | |
| C–S–C | 3 | 4.6 ± 0.5 a | 3.4 ± 1.3 a | 2.3 ± 0.6 a | 9.5 ± 2.3 ab | 21.2 ± 2.9 a | 14.7 ± 0.8 ab | 8 |
| 4 | 5.0 ± 0.5 ab | 2.8 ± 1.0 bc | 3.3 ± 0.4 b | 8.4 ± 1.4 abc | 19.9 ± 4.0 a | 12.6 ± 1.0 ab | 12 | |
| 5 | 4.7 ± 1.3 a | 3.8 ± 0.7 a | 4.1 ± 1.9 a | 9.5 ± 2.9 a | 22.2 ± 6.3 a | 15.1 ± 4.4 a | 12 | |
| 9 | 3.4 ± 0.4 ab | 2.5 ± 0.2 a | 4.1 ± 0.5 a | 17.2 ± 1.1 a | 42.8 ± 21.2 ab | 17.7 ± 0.7 a | 12 | |
| 13 | 2.5 ± 0.3 a | 1.4 ± 0.2 a | 2.7 ± 0.6 a | 17.4 ± 1.3 a | 33.0 ± 2.6 a | 16.9 ± 1.6 a | 12 | |
| C–S–S | 4 | 4.7 ± 0.4 ab | 2.8 ± 0.7 bc | 3.2 ± 0.5 b | 8.3 ± 0.8 ab | 20.7 ± 6.0 a | 13.2 ± 1.8 ab | 12 |
| 5 | 5.3 ± 0.3 a | 3.9 ± 0.7 a | 6.2 ± 0.7 c | 9.9 ± 1.2 a | 25.5 ± 1.6 abc | 15.8 ± 0.5 a | 12 | |
| 9 | 3.6 ± 0.5 ab | 2.5 ± 0.3 a | 4.0 ± 0.7 a | 17.5 ± 1.9 a | 42.8 ± 20.4 ab | 17.7 ± 1.0 a | 12 | |
| 13 | 2.9 ± 0.3 abc | 1.6 ± 0.2 a | 3.2 ± 0.7 ab | 15.9 ± 1.7 a | 31.0 ± 2.3 a | 17.1 ± 1.7 a | 12 | |
| P–C–C | 1 | 4.0 ± 0.3 a | 3.1 ± 0.4 a | 3.1 ± 0.5 a | 7.0 ± 0.9 a | 12.6 ± 2.6 a | 13.0 ± 1.4 a | 16 |
| 3 | 4.8 ± 0.3 a | 4.4 ± 0.2 b | 2.2 ± 0.5 a | 10.5 ± 1.0 ab | 23.8 ± 3.2 a | 15.7 ± 0.7 b | 8 | |
| 4 | 5.0 ± 0.4 b | 3.0 ± 0.4 bc | 2.7 ± 0.5 ab | 9.5 ± 1.0 bcd | 22.7 ± 4.2 a | 13.5 ± 0.7 abc | 12 | |
| 5 | 5.0 ± 0.5 a | 3.8 ± 0.7 a | 4.1 ± 1.7 a | 11.0 ± 0.9 a | 23.6 ± 2.5 ab | 17.0 ± 0.9 a | 12 | |
| 9 | 3.2 ± 0.4 a | 2.4 ± 0.2 a | 4.0 ± 0.7 a | 17.7 ± 1.3 a | 43.6 ± 17.1 ab | 17.3 ± 1.2 a | 12 | |
| 13 | 2.6 ± 0.3 ab | 1.6 ± 0.3 a | 3.0 ± 0.6 ab | 16.5 ± 1.7 a | 31.3 ± 7.2 a | 17.0 ± 1.7 a | 12 | |
| P–C–S | 4 | 4.8 ± 0.3 ab | 2.4 ± 0.5 b | 2.7 ± 0.3 ab | 9.7 ± 1.3 cde | 23.9 ± 7.1 a | 12.9 ± 0.9 ab | 12 |
| 5 | 5.0 ± 0.4 a | 3.8 ± 0.7 a | 4.2 ± 1.7 ab | 10.6 ± 1.3 a | 29.6 ± 3.7 c | 16.2 ± 1.2 a | 12 | |
| 9 | 3.5 ± 0.4 ab | 2.6 ± 0.3 a | 4.2 ± 0.6 a | 17.6 ± 0.9 a | 47.1 ± 21.1 b | 17.3 ± 1.0 a | 11 | |
| 13 | 2.9 ± 0.4 abc | 1.6 ± 0.3 a | 3.1 ± 0.7 ab | 16.2 ± 2.1 a | 33.9 ± 6.0 a | 16.8 ± 1.5 a | 12 | |
| P–S–C | 3 | 4.6 ± 0.5 a | 3.6 ± 0.5 ab | 2.2 ± 0.6 a | 8.2 ± 1.1 a | 20.3 ± 3.5 a | 14.4 ± 0.7 a | 8 |
| 4 | 4.9 ± 0.4 ab | 2.3 ± 0.4 b | 3.1 ± 0.5 ab | 7.7 ± 1.0 a | 19.9 ± 3.4 a | 13.5 ± 0.4 bc | 12 | |
| 5 | 5.0 ± 0.3 a | 3.9 ± 0.6 a | 5.6 ± 0.6 c | 10.7 ± 1.1 a | 28.6 ± 2.3 c | 16.1 ± 1.6 a | 12 | |
| 9 | 3.5 ± 0.3 ab | 2.5 ± 0.3 a | 4.2 ± 0.5 a | 17.9 ± 1.3 a | 45.7 ± 17.9 ab | 17.4 ± 1.0 a | 12 | |
| 13 | 2.8 ± 0.4 abc | 1.6 ± 0.3 a | 3.2 ± 0.5 ab | 16.3 ± 1.7 a | 33.0 ± 4.5 a | 17.0 ± 2.5 a | 12 | |
| P–S–S | 4 | 4.4 ± 0.5 a | 0.8 ± 1.4 a | 2.5 ± 0.6 a | 7.7 ± 0.6 a | 22.8 ± 5.3 a | 12.4 ± 0.6 a | 12 |
| 5 | 5.1 ± 0.4 a | 3.8 ± 0.7 a | 5.6 ± 0.5 c | 10.4 ± 1.0 a | 28.8 ± 5.5 c | 15.8 ± 0.7 a | 12 | |
| 9 | 3.5 ± 0.5 ab | 2.6 ± 0.4 a | 4.0 ± 0.6 a | 17.7 ± 1.7 a | 45.2 ± 19.7 ab | 18.0 ± 0.9 a | 12 | |
| 13 | 2.9 ± 0.5 abc | 1.6 ± 0.4 a | 3.0 ± 0.7 ab | 16.3 ± 1.5 a | 34.4 ± 5.5 a | 17.4 ± 1.7 a | 12 | |
| Treatment | WAP | TCC 1 | TAC 2 | TPC 3 | FCCatechin 4 | FCQuercetin 4 | n |
|---|---|---|---|---|---|---|---|
| [µg mg−1 DM−1] | [µg CyEs mg−1 DM−1] | [µg GAEs mg−1 DM−1] | [µg CEs mg−1 DM−1] | [µg QEs mg−1 DM−1] | |||
| C–C–C | 3 | 4.0 ± 0.4 a | 2.2 ± 0.4 b | 6.2 ± 0.5 a | 18.9 ± 1.5 b | 12.8 ± 0.6 a | 8 |
| 4 | 4.1 ± 0.2 b | 2.3 ± 0.4 bc | 7.7 ± 1.9 a | 19.0 ± 4.3 abc | 12.7 ± 0.3 ab | 12 | |
| 5 | 4.0 ± 0.2 c | 4.8 ± 0.7 c | 6.4 ± 0.5 a | 17.6 ± 2.3 bcd | 12.9 ± 1.0 a | 12 | |
| 9 | 5.3 ± 0.8 a | 7.4 ± 1.2 a | 8.8 ± 1.2 a | 35.5 ± 26.4 a | 16.0 ± 1.1 ab | 12 | |
| 13 | 5.7 ± 0.7 a | 8.1 ± 1.4 a | 9.6 ± 1.1 a | 26.4 ± 5.8 a | 18.1 ± 2.4 a | 12 | |
| 18 | 4.0 ± 0.7 a | 5.7 ± 0.7 a | 9.3 ± 1.1 a | 16.0 ± 3.0 a | 16.5 ± 1.4 a | 12 | |
| C–C–S | 4 | 4.1 ± 0.3 b | 2.4 ± 0.3 bc | 7.8 ± 1.7 ab | 20.2 ± 4.2 bc | 14.0 ± 2.3 bc | 12 |
| 5 | 3.9 ± 0.2 c | 3.2 ± 1.0 b | 6.5 ± 0.7 a | 14.9 ± 1.6 a | 17.3 ± 3.1 c | 12 | |
| 9 | 5.0 ± 0.7 a | 7.5 ± 1.0 a | 8.6 ± 0.6 a | 33.1 ± 24.1 a | 15.7 ± 1.3 ab | 12 | |
| 13 | 5.3 ± 0.8 a | 7.9 ± 1.1 a | 9.4 ± 1.2 a | 23.2 ± 4.9 a | 17.5 ± 2.2 a | 12 | |
| 18 | 3.8 ± 0.8 a | 5.6 ± 1.3 a | 9.6 ± 1.3 a | 16.1 ± 3.2 a | 16.4 ± 2.3 a | 12 | |
| C–S–C | 3 | 3.9 ± 0.3 a | 1.4 ± 0.4 a | 7.5 ± 0.9 c | 15.6 ± 2.9 a | 16.4 ± 1.4 b | 8 |
| 4 | 4.0 ± 0.2 ab | 2.1 ± 0.4 abc | 10.6 ± 1.7 cd | 14.6 ± 1.5 a | 15.6 ± 1.8 cd | 12 | |
| 5 | 3.9 ± 0.4 c | 2.7 ± 0.9 ab | 8.1 ± 0.9 cd | 16.7 ± 0.9 abc | 18.5 ± 1.8 c | 12 | |
| 9 | 4.7 ± 1.0 a | 6.5 ± 1.3 a | 8.7 ± 1.3 a | 32.5 ± 20.0 a | 15.7 ± 2.4 ab | 12 | |
| 13 | 5.3 ± 0.4 a | 7.5 ± 0.9 a | 8.7 ± 1.1 a | 24.3 ± 3.0 a | 17.5 ± 2.0 a | 12 | |
| 18 | 3.5 ± 0.7 a | 5.3 ± 1.2 a | 10.6 ± 2.4 a | 16.2 ± 2.5 a | 17.2 ± 2.5 a | 12 | |
| C–S–S | 4 | 4.1 ± 0.3 b | 2.5 ± 0.4 c | 11.2 ± 1.8 cd | 17.6 ± 1.3 ab | 16.3 ± 1.4 d | 12 |
| 5 | 3.3 ± 0.6 ab | 2.9 ± 0.9 ab | 9.2 ± 1.1 de | 17.2 ± 1.4 abcd | 16.2 ± 1.9 bc | 12 | |
| 9 | 5.0 ± 0.8 a | 7.0 ± 1.4 a | 9.0 ± 1.1 a | 32.8 ± 20.9 a | 16.3 ± 1.7 ab | 12 | |
| 13 | 5.3 ± 0.6 a | 7.7 ± 0.9 a | 10.0 ± 1.8 a | 23.5 ± 2.2 a | 17.7 ± 1.9 a | 12 | |
| 18 | 4.3 ± 0.6 a | 6.2 ± 1.0 a | 10.4 ± 1.5 a | 17.3 ± 2.8 a | 17.7 ± 1.8 a | 12 | |
| P–C–C | 3 | 3.7 ± 0.3 a | 1.7 ± 0.3 a | 6.4 ± 0.7 ab | 17.8 ± 1.6 b | 12.4 ± 0.6 a | 8 |
| 4 | 3.6 ± 0.3 a | 1.9 ± 0.3 ab | 7.2 ± 1.8 a | 15.9 ± 3.2 ab | 10.8 ± 0.7 a | 12 | |
| 5 | 3.6 ± 0.3 bc | 3.0 ± 1.1 ab | 6.2 ± 0.6 a | 16.1 ± 2.5 abc | 12.7 ± 0.5 a | 12 | |
| 9 | 5.0 ± 0.7 a | 7.3 ± 1.2 a | 8.0 ± 1.0 a | 35.8 ± 23.5 a | 14.8 ± 1.6 a | 12 | |
| 13 | 5.7 ± 0.4 a | 8.1 ± 1.3 a | 8.9 ± 0.9 a | 27.8 ± 5.0 a | 17.8 ± 2.1 a | 12 | |
| 18 | 4.1 ± 0.6 a | 6.0 ± 0.8 a | 9.8 ± 0.9 a | 17.4 ± 2.5 a | 17.4 ± 1.3 a | 12 | |
| P–C–S | 4 | 3.7 ± 0.4 ab | 2.0 ± 0.3 ab | 8.1 ± 2.0 ab | 17.1 ± 5.7 ab | 11.2 ± 0.8 a | 12 |
| 5 | 3.6 ± 0.4 bc | 3.0 ± 0.9 ab | 6.8 ± 1.0 ab | 15.7 ± 2.6 ab | 13.3 ± 2.0 a | 12 | |
| 9 | 4.9 ± 1.0 a | 6.7 ± 1.4 a | 8.7 ± 0.9 a | 34.8 ± 18.0 a | 15.2 ± 1.2 a | 12 | |
| 13 | 5.3 ± 0.8 a | 7.3 ± 0.9 a | 9.6 ± 1.8 a | 27.7 ± 6.7 a | 16.9 ± 2.2 a | 12 | |
| 18 | 4.0 ± 0.8 a | 5.9 ± 0.9 a | 9.8 ± 1.0 a | 17.6 ± 4.2 a | 16.9 ± 1.6 a | 12 | |
| P–S–C | 3 | 3.8 ± 0.4 a | 1.6 ± 0.3 a | 7.4 ± 0.9 bc | 15.1 ± 3.1 a | 15.4 ± 1.9 b | 8 |
| 4 | 3.9 ± 0.3 ab | 2.1 ± 0.3 abc | 9.9 ± 2.2 bc | 15.8 ± 2.7 ab | 16.5 ± 1.6 d | 12 | |
| 5 | 3.5 ± 0.4 bc | 3.2 ± 0.8 b | 7.7 ± 0.7 bc | 19.9 ± 2.7 d | 17.3 ± 2.3 c | 12 | |
| 9 | 5.0 ± 0.4 a | 7.2 ± 0.9 a | 8.9 ± 1.1 a | 35.6 ± 22.2 a | 15.7 ± 2.3 ab | 12 | |
| 13 | 5.5 ± 0.7 a | 8.0 ± 1.2 a | 10.1 ± 1.2 a | 26.7 ± 5.0 a | 18.1 ± 2.5 a | 12 | |
| 18 | 3.9 ± 0.9 a | 5.7 ± 1.4 a | 10.2 ± 1.4 a | 18.1 ± 5.8 a | 16.8 ± 1.8 a | 12 | |
| P–S–S | 4 | 3.6 ± 0.5 a | 1.9 ± 0.5 a | 12.3 ± 1.7 d | 23.0 ± 5.3 c | 15.9 ± 2.1 d | 12 |
| 5 | 2.9 ± 0.7 a | 2.1 ± 0.7 a | 9.7 ± 1.5 e | 18.7 ± 2.1 cd | 14.4 ± 2.3 ab | 11 | |
| 9 | 4.9 ± 0.8 a | 6.5 ± 1.0 a | 8.4 ± 0.7 a | 37.9 ± 21.4 a | 18.1 ± 4.5 b | 12 | |
| 13 | 5.4 ± 0.7 a | 7.3 ± 0.8 a | 9.3 ± 1.5 a | 26.6 ± 4.8 a | 17.4 ± 1.5 a | 12 | |
| 18 | 4.1 ± 0.6 a | 5.9 ± 0.7 a | 10.5 ± 1.3 a | 18.3 ± 3.5 a | 18.1 ± 1.7 a | 12 |
References
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1998, 78, 83–125. [Google Scholar] [CrossRef]
- Karaca, C.; Aslan, G.E.; Buyuktas, D.; Kurunc, A.; Bastug, R.; Navarro, A. Effects of Salinity Stress on Drip-Irrigated Tomatoes Grown under Mediterranean-Type Greenhouse Conditions. Agronomy 2023, 13, 36. [Google Scholar] [CrossRef]
- Maas, E.V. Salt Tolerance of Plants. Appl. Agric. Res. 1986, 1, 12–25. [Google Scholar]
- Li, J.; Gao, Y.; Zhang, X.; Tian, P.; Li, J.; Tian, Y. Comprehensive comparison of different saline water irrigation strategies for tomato production: Soil properties, plant growth, fruit yield and fruit quality. Agric. Water Manag. 2019, 213, 521–533. [Google Scholar] [CrossRef]
- Tola, E.; Al-Gaadi, K.A.; Madugundu, R.; Patil, V.C.; Sygrimis, N. Impact of water salinity levels on the spectral behavior and yield of tomatoes in hydroponics. J. King Saud Univ. Sci. 2023, 35, 102515. [Google Scholar] [CrossRef]
- Botella, M.Á.; Hernández, V.; Mestre, T.; Hellín, P.; García-Legaz, M.F.; Rivero, R.M.; Martínez, V.; Fenoll, J.; Flores, P. Bioactive Compounds of Tomato Fruit in Response to Salinity, Heat and Their Combination. Agriculture 2021, 11, 534. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Rodrigues, F.; Sousa, B.; Soares, C.; Moreira, D.; Pereira, C.; Moutinho-Pereira, J.; Cunha, A.; Fidalgo, F. Are tomato plants co-exposed to heat and salinity able to ensure a proper carbon metabolism?—An insight into the photosynthetic hub. Plant Physiol. Biochem. 2023, 206, 108270. [Google Scholar] [CrossRef]
- Körner, T.; Zinkernagel, J.; Röhlen-Schmittgen, S. Induction of Time-Dependent Tolerance through Thermopriming in Tomatoes. Sustainability 2024, 16, 1163. [Google Scholar] [CrossRef]
- Charng, Y.-Y.; Mitra, S.; Yu, S.-J. Maintenance of abiotic stress memory in plants: Lessons learned from heat acclimation. Plant Cell 2023, 35, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple heat priming enhances thermo-tolerance to a later high temperature stress via improving subcellular antioxidant activities in wheat seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Serano, N.L.G. Understanding the Molecular Basis of Thermopriming in Plants. Ph.D. Thesis, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia, 2019. [Google Scholar]
- Frey, C.; Hernández-Barriuso, A.; Encina, A.; Acebes, J.L. Non-invasive monitoring of tomato graft dynamics using thermography and fluorescence quantum yields measurements. Physiol. Plant. 2023, 175, e13935. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Hossain, M.A. Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Elsevier Science & Technology: San Diego, CA, USA, 2020; ISBN 978-0-12-817892-8. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; p. 129. [Google Scholar] [CrossRef]
- Dörr, O.S.; Zimmermann, B.F.; Kögler, S.; Mibus, H. Influence of leaf temperature and blue light on the accumulation of rosmarinic acid and other phenolic compounds in Plectranthus scutellarioides (L.). Environ. Exp. Bot. 2019, 167, 103830. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- López Camelo, A.F.; Gómez, P.A. Comparison of color indexes for tomato ripening. Hortic. Bras. 2004, 22, 534–537. [Google Scholar] [CrossRef]
- Abe-Matsumoto, L.T.; Sampaio, G.R.; Bastos, D.H.M. Is Titration as Accurate as HPLC for Determination of Vitamin C in Supplements?—Titration versus HPLC for Vitamin C Analysis. AJAC 2020, 11, 269–279. [Google Scholar] [CrossRef]
- Tanner, H.; Brunner, H.R. Getränke-Analytik: Untersuchungsmethoden für die Labor- und Betriebspraxis, 2nd ed.; Aufl.; Verl. Heller Chemie- u. Verwaltungsges: Schwäbisch Hall, Germany, 1987; ISBN 978-3-9800498-1-8. [Google Scholar]
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Amtliche Sammlung von Untersuchungsverfahren nach §35 LMBG. Untersuchung von Lebensmitteln. Bestimmung der Titrierbaren Säure von Frucht- und Gemüsesäften. DIN EN 12147. 1997. Available online: https://www.methodensammlung-bvl.de/de (accessed on 20 December 2023).
- Organisation for Economic Cooperation and Development. OECD Fruit and Vegetables Scheme: Guidelines on Objective Tests To Determine Quality of Fruit and Vegetables, Dry And Dried Produce. 1918. Available online: https://www.oecd.org/agriculture/fruit-vegetables/publications/ (accessed on 20 December 2023).
- Gomez, L.; Bancel, D.; Rubio, E.; Vercambre, G. The microplate reader: An efficient tool for the separate enzymatic analysis of sugars in plant tissues—Validation of a micro-method. J. Sci. Food Agric. 2007, 87, 1893–1905. [Google Scholar] [CrossRef]
- Zhao, D.; MacKown, C.T.; Starks, P.J.; Kindiger, B.K. Rapid Analysis of Nonstructural Carbohydrate Components in Grass Forage Using Microplate Enzymatic Assays. Crop Sci. 2010, 50, 1537–1545. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat Priming During Early Reproductive Stages Enhances Thermo-Tolerance to Post-anthesis Heat Stress via Improving Photosynthesis and Plant Productivity in Winter Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Olas, J.J.; Apelt, F.; Annunziata, M.G.; John, S.; Richard, S.I.; Gupta, S.; Kragler, F.; Balazadeh, S.; Mueller-Roeber, B. Primary carbohydrate metabolism genes participate in heat-stress memory at the shoot apical meristem of Arabidopsis thaliana. Mol. Plant 2021, 14, 1508–1524. [Google Scholar] [CrossRef]
- Shaheen, M.R.; Ayyub, C.M.; Amjad, M.; Waraich, E.A. Morpho-physiological evaluation of tomato genotypes under high temperature stress conditions. J. Sci. Food Agric. 2016, 96, 2698–2704. [Google Scholar] [CrossRef]
- Wigge, P.A. Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 2013, 16, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, V.A.; Ayala-Garay, O.J.; Arévalo-Galarza, M.d.L.; Gautier, H. Increased Temperature Affects Tomato Fruit Physicochemical Traits at Harvest Depending on Fruit Developmental Stage and Genotype. Horticulturae 2023, 9, 212. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 2000, 109, 435–442. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; de Pascale, S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Bolarín, M.C.; Pérez-Alfocea, F.; Cano, E.A.; Estañ, M.T.; Caro, M. Growth, Fruit Yield, and Ion Concentration in Tomato Genotypes after Pre- and Post-emergence Salt Treatments. J. Amer. Soc. Hort. Sci. 1993, 118, 655–660. [Google Scholar] [CrossRef]
- Liu, F.-Y.; Li, K.-T.; Yang, W.-J. Differential responses to short-term salinity stress of heat-tolerant cherry tomato cultivars grown at high temperatures. Hortic. Environ. Biotechnol. 2014, 55, 79–90. [Google Scholar] [CrossRef]
- Rosadi, R.A.B.; Senge, M.; Suhandy, D.; Tusi, A. The Effect of EC Levels of Nutrient Solution on the Growth, Yield, and Quality of Tomatoes (Solanum lycopersicum) under the Hydroponic System. JAEB 2014, 2, 7–12. [Google Scholar] [CrossRef]
- Adams, P. Effects of watering on the yield, quality and composition of tomatoes grown in bags of peat. J. Hortic. Sci. 1990, 65, 667–674. [Google Scholar] [CrossRef]
- Parvin, K.; Ahamed, K.U.; Islam, M.M.; Haque, M.N. Response of Tomato Plant Under Salt Stress: Role of Exogenous Calcium. J. Plant Sci. 2015, 10, 222–233. [Google Scholar] [CrossRef]
- Hocking, P. The Distribution and Identity of Assimilates in Tomato with Special Reference to Stem Reserves. Ann. Bot. 1994, 73, 315–325. [Google Scholar] [CrossRef]
- Taffouo, V.; Nouck, A.; Dibong, S.; Amougou, Y.A. Effects of salinity stress on seedlings growth, mineral nutrients and total chlorophyll of some tomato (Lycopersicum esculentum L.) cultivars. Afr. J. Biotechnol. 2010, 9, 5366–5372. [Google Scholar]
- Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi, A. Impact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv. Microtom leaves. S. Afr. J. Bot. 2017, 108, 364–369. [Google Scholar] [CrossRef]
- Al Hassan, M.; Fuertes, M.; Sánchez, F.; Vicente, O.; Boscaiu, M. Effects of Salt and Water Stress on Plant Growth and on Accumulation of Osmolytes and Antioxidant Compounds in Cherry Tomato. Not. Bot. Horti Agrobo. 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef] [PubMed]
- Qaryouti, M.M.; Qawasmi, W.; Hamdan, H.; Edwan, M. Influence of NaCl Salinity Stress on Yield, Plant Water Uptake and Drainage Water of Tomato Grown in Soilless Culture. Acta Hortic. 2007, 747, 539–545. [Google Scholar] [CrossRef]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Lorenzo, P. Effects of salinity on fruit yield and quality of tomato grown in soil-less culture in greenhouses in Mediterranean climatic conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- Mizrahi, Y.; Taleisnik, E.; Kagan-Zur, V.; Zohar, Y.; Offenbach, R.; Matan, E.; Golan, R. A Saline Irrigation Regime for Improving Tomato Fruit Quality without Reducing Yield. J. Amer. Soc. Hort. Sci. 1988, 113, 202–205. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Shennan, C.; Grattan, S.R.; May, D.M. Tomato Fruit Yields and Quality under Water Deficit and Salinity. J. Amer. Soc. Hort. Sci. 1991, 116, 215–221. [Google Scholar] [CrossRef]
- Azarmi, R.; Didar, R.; Gikloo, A. Effects of salinity on morphological and physiological changes and yield of tomato in hydroponics system. J. Food Agric. Environ. 2010, 8, 573–576. [Google Scholar]
- Zhang, P.; Senge, M.; Yoshiyama, K.; Ito, K.; Dai, Y.; Zhang, F. Effects of Low Salinity Stress on Growth, Yield and Water Use Efficiency of Tomato under Soilless Cultivation. J. Irrig. Drain. Rural Eng. 2017, 85, 15–21. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Seasonal and Cultivar Differences in Salt-induced Change in Ascorbic Acid and Dehydroascorbic Acid Contents of Tomato Fruit. Environ. Control Biol. 2007, 45, 165–171. [Google Scholar] [CrossRef][Green Version]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of salt stress on tomato fruit antioxidant systems depends on fruit development stage. Physiol. Mol. Biol. Plants 2014, 20, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernández, J.M.; Bolarin, M.C.; Egea, I. Recovering Tomato Landraces to Simultaneously Improve Fruit Yield and Nutritional Quality Against Salt Stress. Front. Plant Sci. 2018, 9, 1778. [Google Scholar] [CrossRef] [PubMed]
- van Ieperen, W. Effects of different day and night salinity levels on vegetative growth, yield and quality of tomato. J. Hortic. Sci. 1996, 71, 99–111. [Google Scholar] [CrossRef]
- Incerti, A.; Navari-Izzo, F.; Pardossi, A.; Izzo, R. Seasonal variations in polyphenols and lipoic acid in fruits of tomato irrigated with sea water. J. Sci. Food Agric. 2009, 89, 1326–1331. [Google Scholar] [CrossRef]
| Duration: | 20 weeks |
| Period: | February–July 2023 |
| Timing of thermopriming (week after sowing): | 2nd |
| Timing of 1st salt stress (weeks after sowing): | 3 |
| Timing of 2nd salt stress (weeks after sowing): | 5 |
| Number of treatments: | 8 |
| Total number of blocks (incl. border): | 6 |
| Number of blocks with experimental plants: | 4 |
| Total number of plants (incl. border) per block: | 8 |
| Number of experimental plants per block: | 6 |
| Total number of experimental plants per treatment: | 24 |
| Number of harvested trusses per experimental plant: | 3 (3rd/5th/7th truss per plant) |
| Treatment | Weeks after Sowing | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |||
| C–C–C | ||||||||||||||||||||||
| C–C–S | ||||||||||||||||||||||
| C–S–C | ||||||||||||||||||||||
| C–S–S | ||||||||||||||||||||||
| P–C–C | ||||||||||||||||||||||
| P–C–S | ||||||||||||||||||||||
| P–S–C | ||||||||||||||||||||||
| P–S–S | ||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körner, T.; Gierholz, R.; Zinkernagel, J.; Röhlen-Schmittgen, S. Heat-Induced Cross-Tolerance to Salinity Due to Thermopriming in Tomatoes. Metabolites 2024, 14, 213. https://doi.org/10.3390/metabo14040213
Körner T, Gierholz R, Zinkernagel J, Röhlen-Schmittgen S. Heat-Induced Cross-Tolerance to Salinity Due to Thermopriming in Tomatoes. Metabolites. 2024; 14(4):213. https://doi.org/10.3390/metabo14040213
Chicago/Turabian StyleKörner, Tobias, Ruven Gierholz, Jana Zinkernagel, and Simone Röhlen-Schmittgen. 2024. "Heat-Induced Cross-Tolerance to Salinity Due to Thermopriming in Tomatoes" Metabolites 14, no. 4: 213. https://doi.org/10.3390/metabo14040213
APA StyleKörner, T., Gierholz, R., Zinkernagel, J., & Röhlen-Schmittgen, S. (2024). Heat-Induced Cross-Tolerance to Salinity Due to Thermopriming in Tomatoes. Metabolites, 14(4), 213. https://doi.org/10.3390/metabo14040213







