Metabolomic Analysis Reveals the Association of Severe Bronchopulmonary Dysplasia with Gut Microbiota and Oxidative Response in Extremely Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Urine Sample Preparation
2.3. 1H–Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. NMR Data Processing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Urinary Metabolite Sets Categorized by Different GA and BPD Severity
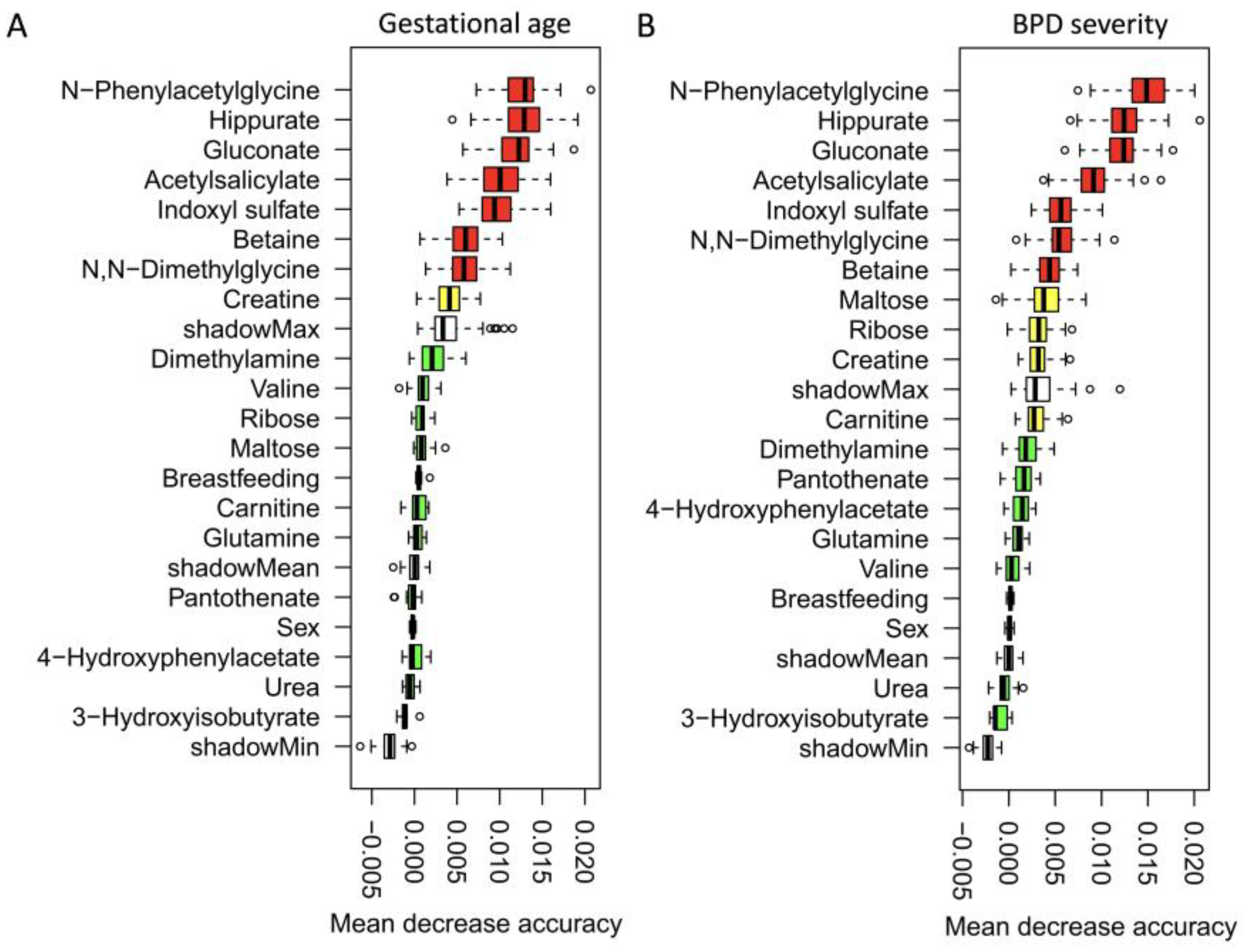
3.3. Dynamic Metabolic Changes across Different GA and BPD Severity
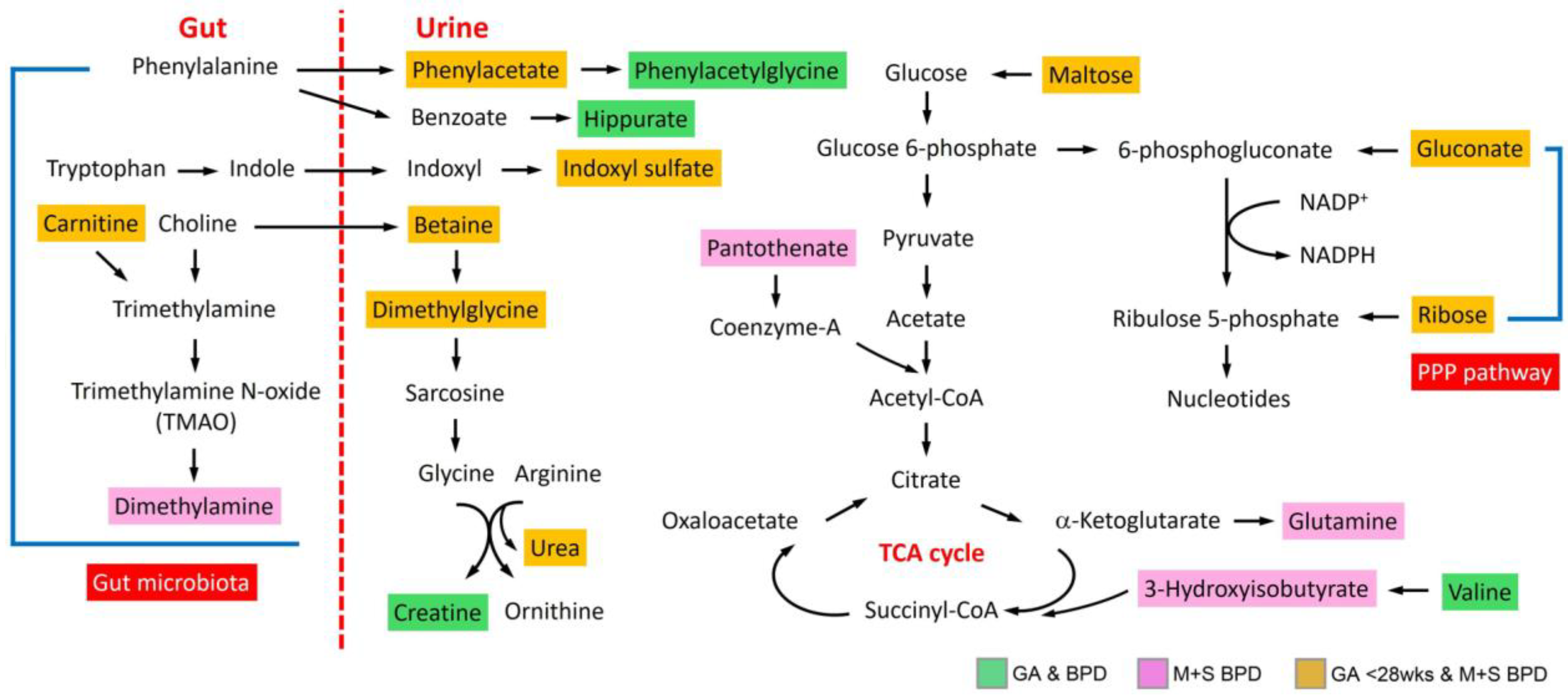
3.4. Metabolic Pathway and Functional Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, I.C.; Yang, Y.C.; Chen, K.T. The trends and associated factors of preterm deliveries from 2001 to 2011 in Taiwan. Medicine 2019, 98, e15060. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Holzfurtner, L.; Shahzad, T.; Dong, Y.; Rekers, L.; Selting, A.; Staude, B.; Lauer, T.; Schmidt, A.; Rivetti, S.; Zimmer, K.P.; et al. When inflammation meets lung development-an update on the pathogenesis of bronchopulmonary dysplasia. Mol. Cell. Pediatr. 2022, 9, 7. [Google Scholar] [CrossRef]
- Davidson, L.M.; Berkelhamer, S.K. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J. Clin. Med. 2017, 6, 4. [Google Scholar] [CrossRef]
- Jeon, G.W.; Oh, M.; Lee, J.; Jun, Y.H.; Chang, Y.S. Comparison of definitions of bronchopulmonary dysplasia to reflect the long-term outcomes of extremely preterm infants. Sci. Rep. 2022, 12, 18095. [Google Scholar] [CrossRef]
- Xu, E.Y.; Schaefer, W.H.; Xu, Q. Metabolomics in pharmaceutical research and development: Metabolites, mechanisms and pathways. Curr. Opin. Drug Discov. Devel. 2009, 12, 40–52. [Google Scholar] [PubMed]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, N.; Ge, W.; Han, P.; Siddiqui, J.; Wei, J.T.; Raghunathan, T.; Chinnaiyan, A.M.; Rajendiran, T.M.; Ramamoorthy, A. NMR-Based Metabolomic Profiling of Urine: Evaluation for Application in Prostate Cancer Detection. Nat. Prod. Commun. 2019, 14, 1934578X19849978. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Yeh, K.W.; Lin, G.; Chiang, M.H.; Yang, S.C.; Chao, W.J.; Yao, T.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; et al. Metabolomics Reveals Dynamic Metabolic Changes Associated with Age in Early Childhood. PLoS ONE 2016, 11, e0149823. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Lin, G.; Cheng, M.L.; Chiang, M.H.; Tsai, M.H.; Lai, S.H.; Wong, K.S.; Hsieh, S.Y. Metabolomic Profiling of Infectious Parapneumonic Effusions Reveals Biomarkers for Guiding Management of Children with Streptococcus pneumoniae Pneumonia. Sci. Rep. 2016, 6, 24930. [Google Scholar] [CrossRef] [PubMed]
- Jacob, D.; Deborde, C.; Lefebvre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Giuliani, F.; Barros, F.; Roggero, P.; Coronado Zarco, I.A.; Rego, M.A.S.; Ochieng, R.; Gianni, M.L.; Rao, S.; Lambert, A.; et al. Monitoring the Postnatal Growth of Preterm Infants: A Paradigm Change. Pediatrics 2018, 141, e20172467. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Volpi, E.; Wolfe, R.R. Whole body and skeletal muscle glutamine metabolism in healthy subjects. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E323–E333. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, W.J.; Shi, J.Y.; Su, Y.L.; Liu, Y.C.; Wang, L.; Xiao, C.X.; Chen, C.; Lu, Q. The gut microbial metabolite phenylacetylglycine protects against cardiac injury caused by ischemia/reperfusion through activating β2AR. Arch. Biochem. Biophys. 2021, 697, 108720. [Google Scholar] [CrossRef] [PubMed]
- Ticinesi, A.; Guerra, A.; Nouvenne, A.; Meschi, T.; Maggi, S. Disentangling the Complexity of Nutrition, Frailty and Gut Microbial Pathways during Aging: A Focus on Hippuric Acid. Nutrients 2023, 15, 1138. [Google Scholar] [CrossRef] [PubMed]
- Pruss, K.M.; Chen, H.; Liu, Y.; Van Treuren, W.; Higginbottom, S.K.; Jarman, J.B.; Fischer, C.R.; Mak, J.; Wong, B.; Cowan, T.M.; et al. Host-microbe co-metabolism via MCAD generates circulating metabolites including hippuric acid. Nat. Commun. 2023, 14, 512. [Google Scholar] [CrossRef]
- Sim, K.; Powell, E.; Cornwell, E.; Simon Kroll, J.; Shaw, A.G. Development of the gut microbiota during early life in premature and term infants. Gut Pathog. 2023, 15, 3. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial community. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, M.; Schulz, C.; Stoeger, T.; Hilgendorff, A. Association of immune cell recruitment and BPD development. Mol. Cell. Pediatr. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Veskovic, M.; Mladenovic, D.; Milenkovic, M.; Tosic, J.; Borozan, S.; Gopcevic, K.; Labudovic-Borovic, M.; Dragutinovic, V.; Vucevic, D.; Jorgacevic, B.; et al. Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 2019, 848, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Sasaki, K.; Somfai, T.; Nagai, T.; Manabe, N.; Edashige, K. N,N-Dimethylglycine decreases oxidative stress and improves in vitro development of bovine embryos. J. Reprod. Dev. 2016, 62, 209–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marino, L.V.; Paulson, S.; Ashton, J.J.; Weeks, C.; Young, A.; Pappachan, J.V.; Swann, J.; Johnson, M.J.; Beattie, R.M. A Scoping Review: Urinary Markers of Metabolic Maturation in Preterm Infants and Future Interventions to Improve Growth. Nutrients 2022, 14, 3957. [Google Scholar] [CrossRef] [PubMed]
- Kimble, A.; Robbins, M.E.; Perez, M. Pathogenesis of Bronchopulmonary Dysplasia: Role of Oxidative Stress from ‘Omics’ Studies. Antioxidants 2022, 11, 2380. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, M.; den Heijer, M.; Semmekrot, B.A.; Sporken, J.M.; Ueland, P.M.; Blom, H.J. Umbilical choline and related methylamines betaine and dimethylglycine in relation to birth weight. Pediatr. Res. 2013, 73, 783–787. [Google Scholar] [CrossRef]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef]
| GA | BPD Severity | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | ≥37 Weeks (n = 50) | 28–32 Weeks (n = 48) | <28 Weeks (n = 41) | p-Value | HC (n = 50) | No + Mild (n = 40) | M + S (n = 49) | p-Value |
| Sex, male | 20 (40.0%) | 22 (45.8%) | 20 (48.8%) | 0.688 | 20 (40.0%) | 19 (47.5%) | 23 (46.9) | 0.714 |
| Gestational age (wk) | 39.1 ± 0.8 | 30.7 ± 0.9 | 26.1 ± 1.4 | <0.001 | 39.1 ± 0.80 | 30.2 ± 1.70 | 27.2 ± 2.40 | <0.001 |
| Birth body weight (g) | 3171.3 ± 365.7 | 1369 ± 273.9 | 775.8 ± 203.7 | <0.001 | 3171.3 ± 365.7 | 1404.9 ± 287.2 | 843.4 ± 241.8 | <0.001 |
| Age, corrected (month) | 6.89 ± 1.25 | 7.17 ± 3.03 | 7.83 ± 2.10 | 0.129 | 6.89 ± 1.25 | 7.62 ± 3.33 | 7.36 ± 1.95 | 0.293 |
| Body weight (g) | 7.87 ± 0.75 | 7.71 ± 1.24 | 7.14 ± 1.42 | 0.009 | 7.87 ± 0.75 | 8.05 ± 1.00 | 6.96 ± 1.40 | <0.001 |
| Body height (cm) | 67.31 ± 2.63 | 66.36 ± 3.42 | 65.53 ± 5.05 | 0.081 | 67.31 ± 2.63 | 67.50 ± 3.15 | 64.74 ± 4.64 | <0.001 |
| BMI (kg/m2) | 17.36 ± 1.25 | 17.44 ± 2.05 | 16.38 ± 1.55 | 0.005 | 17.36 ± 1.25 | 17.64 ± 1.60 | 16.39 ± 1.97 | 0.001 |
| Breastfeeding ≥ 6 months | 28 (56.0%) | 15 (31.2%) | 19 (46.3%) | 0.046 | 28 (56.0%) | 14 (35.0%) | 20 (40.8%) | 0.110 |
| GA < 28 Wks vs. GA ≥ 37 Wks | GA 28–32 Wks vs. GA ≥ 37 Wks | GA < 28 Wks vs. GA 28–32 Wks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Chemical Shift, ppm (Multiplicity) | VIP Score * | Fold Change † | p ‡ | VIP Score | Fold Change | p | VIP Score | Fold Change | p |
| N-Phenylacetylglycine | 7.404–7.448 (m) | 2.17 | 1.73 | <0.001 | 1.72 | 1.37 | 0.005 | 1.93 | 1.27 | 0.019 |
| Creatine | 3.928–3.941 (s) | 2.16 | 1.43 | <0.001 | 2.12 | 1.20 | 0.023 | 1.48 | 1.19 | 0.158 |
| Acetylsalicylate | 2.337–2.357 (s) | 2.04 | 1.96 | <0.001 | 2.16 | 1.57 | 0.004 | 1.13 | 1.25 | 0.266 |
| Hippurate | 7.529–7.580 (m) | 1.89 | 1.55 | <0.001 | 1.61 | 1.28 | 0.006 | 1.59 | 1.21 | 0.039 |
| 4-Hydroxyphenylacetate | 6.855–6.883 (ddd) | 1.75 | 1.66 | <0.001 | 1.10 | 1.42 | 0.145 | 1.73 | 1.17 | 0.082 |
| 3-Methyl-2-oxovalerate | 1.098–1.122 (d) | 1.74 | 1.47 | <0.001 | 1.34 | 1.28 | 0.027 | 1.54 | 1.15 | 0.044 |
| Indoxyl sulfate | 7.693–7.719 (d) | 1.47 | 1.43 | <0.001 | 0.72 | 1.12 | 0.232 | 1.81 | 1.27 | 0.024 |
| 1-Methylnicotinamide | 9.245–9.320 (s) | 1.23 | 1.41 | 0.003 | 1.47 | 1.38 | 0.016 | 0.29 | 1.02 | 0.726 |
| Ribose | 5.373–5.389 (d) | 1.51 | 1.84 | 0.004 | 1.13 | 1.48 | 0.131 | 1.29 | 1.24 | 0.221 |
| 3-Hydroxy-3-methylglutarate | 1.317–1.327 (s) | 1.01 | 0.84 | 0.005 | 0.76 | 0.94 | 0.158 | 0.79 | 0.89 | 0.275 |
| Betaine | 3.260–3.274 (s) | 1.40 | 0.79 | 0.009 | 0.94 | 0.90 | 0.222 | 1.32 | 0.87 | 0.205 |
| Valine | 1.045–1.057 (d) | 0.91 | 1.16 | 0.011 | 1.54 | 1.43 | 0.009 | 0.51 | 0.81 | 0.490 |
| N,N-Dimethylglycine | 2.920–2.938 (s) | 0.95 | 0.87 | 0.018 | 0.64 | 0.96 | 0.251 | 0.89 | 0.90 | 0.255 |
| Urea | 5.650–6.056 (s) | 0.92 | 1.23 | 0.023 | 0.72 | 1.09 | 0.195 | 0.85 | 1.13 | 0.234 |
| Carnitine | 3.224–3.237 (s) | 1.02 | 1.34 | 0.030 | 1.09 | 1.18 | 0.090 | 0.55 | 1.13 | 0.516 |
| Maltose | 5.402–5.410 (d) | 0.86 | 1.30 | 0.032 | 0.70 | 1.08 | 0.148 | 0.82 | 1.20 | 0.266 |
| Gluconate | 4.643–4.670 (d) | 0.94 | 2.11 | 0.033 | 0.23 | 1.02 | 0.603 | 1.51 | 2.08 | 0.060 |
| Tyrosine | 6.890–6.915 (ddd) | 0.83 | 1.14 | 0.035 | 0.14 | 1.06 | 0.817 | 1.25 | 1.08 | 0.101 |
| Trimethylamine N-oxide | 3.274–3.282 (s) | 0.76 | 1.17 | 0.044 | 1.13 | 1.16 | 0.033 | 0.01 | 1.01 | 0.991 |
| Allantoin | 5.391–5.402 (s) | 0.46 | 1.17 | 0.327 | 1.75 | 1.38 | 0.007 | 1.26 | 0.85 | 0.154 |
| Succinate | 2.400–2.418 (s) | 0.39 | 1.19 | 0.388 | 1.22 | 1.25 | 0.045 | 0.72 | 0.96 | 0.393 |
| M + S BPD vs. HC | No + Mild BPD vs. HC | M + S BPD vs. No + Mild BPD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | Chemical Shift, ppm (Multiplicity) | VIP Score * | Fold Change † | p ‡ | VIP Score | Fold Change | p | VIP Score | Fold Change | p |
| N-Phenylacetylglycine | 7.404–7.448 (m) | 2.14 | 1.74 | <0.001 | 1.61 | 1.29 | 0.019 | 0.63 | 1.34 | 0.008 |
| Acetylsalicylate | 2.337–2.357 (s) | 2.09 | 1.96 | <0.001 | 2.08 | 1.49 | 0.012 | 1.29 | 1.31 | 0.143 |
| Creatine | 3.928–3.941 (s) | 2.03 | 1.37 | <0.001 | 2.35 | 1.22 | 0.026 | 0.77 | 1.12 | 0.340 |
| Hippurate | 7.529–7.580 (m) | 1.87 | 1.57 | <0.001 | 1.53 | 1.21 | 0.019 | 0.52 | 1.30 | 0.019 |
| 4-Hydroxyphenylacetate | 6.855–6.883 (ddd) | 1.84 | 1.77 | <0.001 | 0.69 | 1.23 | 0.406 | 2.47 | 1.44 | 0.012 |
| 3-Methyl-2-oxovalerate | 1.098–1.122 (d) | 1.79 | 1.59 | <0.001 | 1.04 | 1.09 | 0.104 | 2.15 | 1.45 | 0.002 |
| 1-Methylnicotinamide | 9.245–9.320 (s) | 1.46 | 1.49 | <0.001 | 1.09 | 1.28 | 0.107 | 0.92 | 1.16 | 0.135 |
| Indoxyl sulfate | 7.693–7.719 (d) | 1.40 | 1.40 | <0.001 | 0.63 | 1.10 | 0.352 | 0.14 | 1.27 | 0.026 |
| Valine | 1.045–1.057 (d) | 1.08 | 1.31 | 0.004 | 1.40 | 1.31 | 0.027 | 0.21 | 1.00 | 0.692 |
| Dimethylamine | 2.718–2.732 (s) | 0.58 | 1.08 | 0.008 | 0.18 | 1.04 | 0.666 | 0.75 | 1.04 | 0.152 |
| Ribose | 5.373–5.389 (d) | 1.37 | 1.83 | 0.009 | 1.26 | 1.43 | 0.123 | 0.75 | 1.28 | 0.324 |
| Maltose | 5.402–5.410 (d) | 0.94 | 1.24 | 0.010 | 0.49 | 1.11 | 0.402 | 0.24 | 1.11 | 0.134 |
| Trimethylamine N-oxide | 3.274–3.282 (s) | 0.91 | 1.20 | 0.012 | 0.94 | 1.13 | 0.115 | 0.07 | 1.06 | 0.407 |
| Gluconate | 4.643–4.670 (d) | 0.97 | 1.95 | 0.020 | 0.08 | 0.99 | 0.878 | 0.18 | 1.97 | 0.028 |
| Pantothenate | 0.928–0.940 (d) | 0.97 | 1.28 | 0.021 | 0.71 | 1.09 | 0.288 | 0.89 | 1.18 | 0.207 |
| Glutamine | 2.431–2.461 (dt) | 0.80 | 1.22 | 0.025 | 0.10 | 1.01 | 0.861 | 1.47 | 1.21 | 0.037 |
| Carnitine | 3.224–3.237 (s) | 1.03 | 1.37 | 0.026 | 1.06 | 1.12 | 0.128 | 0.35 | 1.22 | 0.375 |
| Urea | 5.650–6.056 (s) | 0.86 | 1.20 | 0.026 | 0.77 | 1.10 | 0.219 | 0.87 | 1.09 | 0.330 |
| Betaine | 3.260–3.274 (s) | 1.08 | 0.81 | 0.031 | 1.43 | 0.89 | 0.109 | 0.62 | 0.91 | 0.882 |
| 3-Hydroxyisobutyrate | 1.064–1.09 (d) | 0.86 | 1.39 | 0.032 | 0.36 | 1.05 | 0.546 | 0.82 | 1.33 | 0.122 |
| N,N-Dimethylglycine | 2.920–2.938 (s) | 0.77 | 0.89 | 0.042 | 0.90 | 0.96 | 0.162 | 1.99 | 0.93 | 0.719 |
| Allantoin | 5.391–5.402 (s) | 0.91 | 1.33 | 0.048 | 1.23 | 1.21 | 0.085 | 0.15 | 1.10 | 0.731 |
| 3-Hydroxy-3-methylglutarate | 1.317–1.327 (s) | 0.49 | 0.94 | 0.170 | 1.65 | 0.84 | 0.005 | 0.53 | 1.12 | 0.185 |
| Propylene glycol | 1.130–1.146 (d) | 0.67 | 0.71 | 0.220 | 1.89 | 0.58 | 0.033 | 0.60 | 1.22 | 0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, C.-Y.; Chiang, M.-C.; Chiang, M.-H.; Lien, R.; Fu, R.-H.; Hsu, K.-H.; Chu, S.-M. Metabolomic Analysis Reveals the Association of Severe Bronchopulmonary Dysplasia with Gut Microbiota and Oxidative Response in Extremely Preterm Infants. Metabolites 2024, 14, 219. https://doi.org/10.3390/metabo14040219
Chiu C-Y, Chiang M-C, Chiang M-H, Lien R, Fu R-H, Hsu K-H, Chu S-M. Metabolomic Analysis Reveals the Association of Severe Bronchopulmonary Dysplasia with Gut Microbiota and Oxidative Response in Extremely Preterm Infants. Metabolites. 2024; 14(4):219. https://doi.org/10.3390/metabo14040219
Chicago/Turabian StyleChiu, Chih-Yung, Ming-Chou Chiang, Meng-Han Chiang, Reyin Lien, Ren-Huei Fu, Kai-Hsiang Hsu, and Shih-Ming Chu. 2024. "Metabolomic Analysis Reveals the Association of Severe Bronchopulmonary Dysplasia with Gut Microbiota and Oxidative Response in Extremely Preterm Infants" Metabolites 14, no. 4: 219. https://doi.org/10.3390/metabo14040219
APA StyleChiu, C.-Y., Chiang, M.-C., Chiang, M.-H., Lien, R., Fu, R.-H., Hsu, K.-H., & Chu, S.-M. (2024). Metabolomic Analysis Reveals the Association of Severe Bronchopulmonary Dysplasia with Gut Microbiota and Oxidative Response in Extremely Preterm Infants. Metabolites, 14(4), 219. https://doi.org/10.3390/metabo14040219








