Chronic Administration of Exogenous Lactate Increases Energy Expenditure during Exercise through Activation of Skeletal Muscle Energy Utilization Capacity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
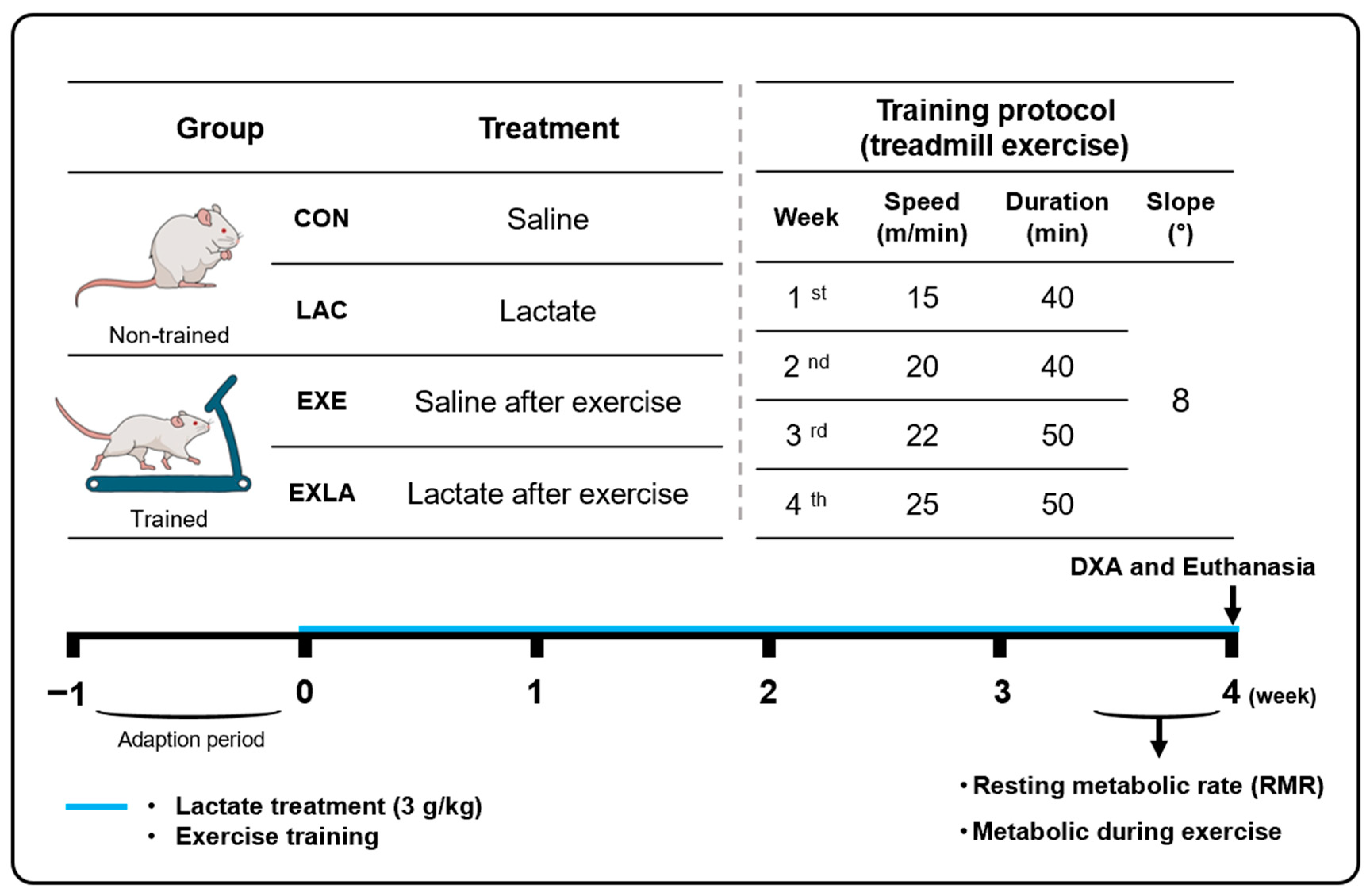
2.2. Study Design
2.3. Metabolic Analysis at Rest and during Exercise
2.4. Body Composition Analysis
2.5. Immunoblotting Analysis
2.6. Statistical Analysis
3. Results
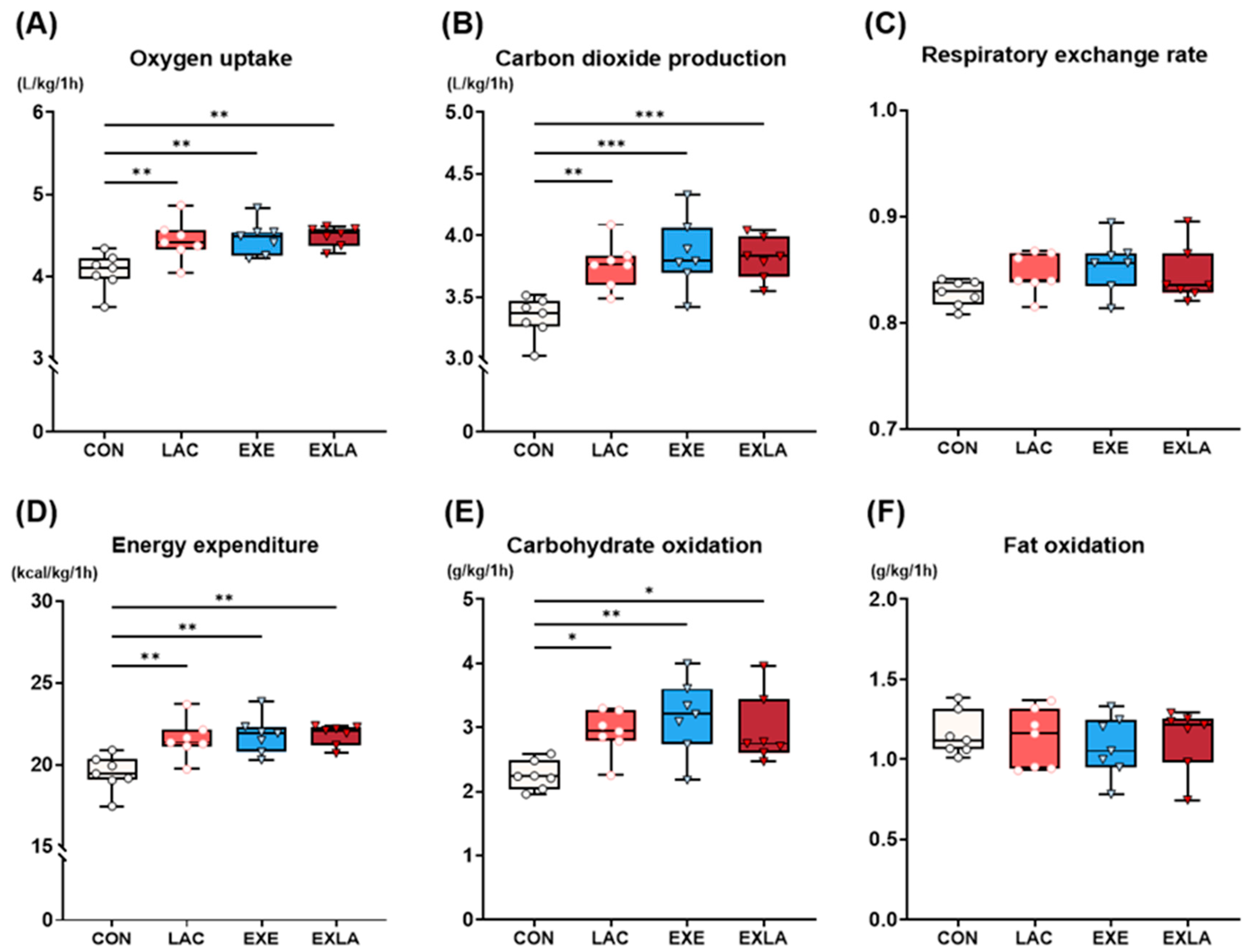
3.1. Administration of Exogenous Lactate Increases Carbohydrate Utilization as an Energy Substrate during Exercise, Leading to Increased Energy Expenditure
3.2. Exercise Training Induces Positive Changes in Body Composition but Not in Body Weight
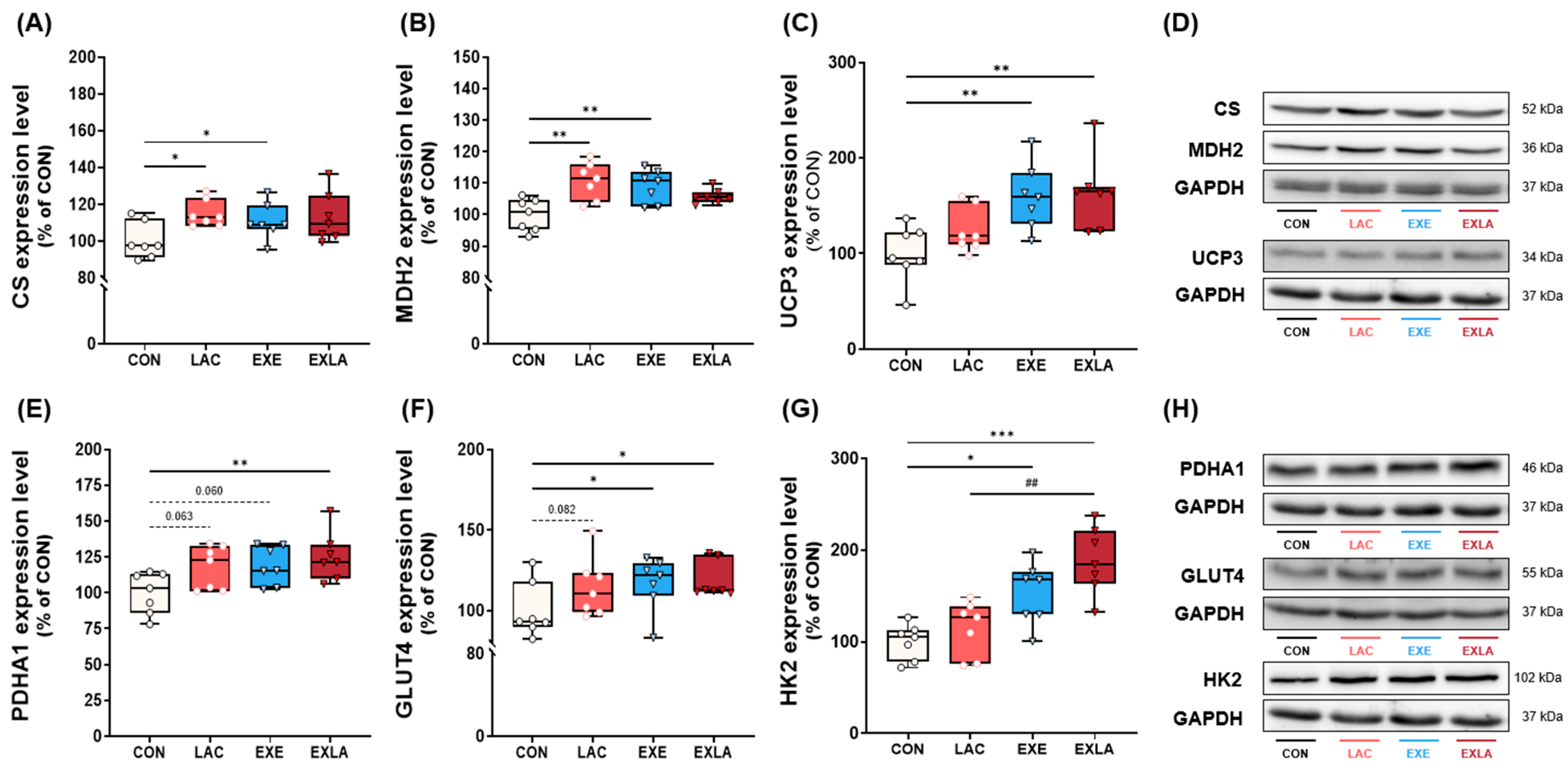
3.3. Administration of Exogenous Lactate Activates Oxidative Metabolism in Skeletal Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K. The Diseasome of Physical Inactivity—And the Role of Myokines in Muscle-Fat Cross Talk. J. Physiol. 2009, 587, 5559–5568. [Google Scholar] [CrossRef]
- Biolo, G.; Ciocchi, B.; Stulle, M.; Piccoli, A.; Lorenzon, S.; Dal Mas, V.; Barazzoni, R.; Zanetti, M.; Guarnieri, G. Metabolic Consequences of Physical Inactivity. J. Ren. Nutr. 2005, 15, 49–53. [Google Scholar] [CrossRef]
- Aakko, J.; Uomilehto, T.; Aana, J.; Indström, L.; Ohan, J.; Riksson, G.E.; Alle, I.T.V.; Ämäläinen, E.H.; Arikka, L.-P.; Irkka, K.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar]
- Nocon, M.; Hiemann, T.; Mü Ller-Riemenschneider, F.; Thalau, F.; Roll, S.; Willich, S.N. Association of Physical Activity with All-Cause and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2008, 15, 239–246. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A.; Lee, I.M. Physical Activity and Colon Cancer Prevention: A Meta-Analysis. Br. J. Cancer 2009, 100, 611–616. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Lee, I.M.; Leung, R. Physical Activity and Personal Charac-Teristics Associated with Depression and Suicide in American College Men. Acta Psychiatr. Scand. 1994, 89, 16–22. [Google Scholar] [CrossRef]
- Moghetti, P.; Bacchi, E.; Brangani, C.; Donà, S.; Negri, C. Metabolic Effects of Exercise. Front. Horm. Res. 2016, 47, 44–57. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Bruce, C.R.; Thrush, A.B.; Mertz, V.A.; Bezaire, V.; Chabowski, A.; Heigenhauser, G.J.F.; Dyck, D.J. Endurance Training in Obese Humans Improves Glucose Tolerance and Mitochondrial Fatty Acid Oxidation and Alters Muscle Lipid Content. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 99–107. [Google Scholar] [CrossRef]
- Mutin-Carnino, M.; Carnino, A.; Roffino, S.; Chopard, A. Effect of Muscle Unloading, Reloading and Exercise on Inflammation during a Head-down Bed Rest. Int. J. Sports Med. 2014, 35, 28–34. [Google Scholar] [CrossRef]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 467932. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a Myokine and Exerkine: Drivers and Signals of Physiology and Metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Sahlin, K. Metabolic Factors in Fatigue. Sports Med. 1992, 13, 99–107. [Google Scholar] [CrossRef]
- Stevenson, R.W.; Mitchell, D.R.; Hendrick, G.K.; Rainey, R.; Cherrington, A.D.; Tyler Frizzell, R. Lactate as Substrate for Glycogen Resynthesis after Exercise. J. Appl. Physiol. 1987, 62, 2237–2240. [Google Scholar] [CrossRef]
- Emhoff, C.-A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and Hepatic Glycogenolysis during Exercise at the Lactate Threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef]
- Cori, C.F.; Cori, G.T. Carbohydrate Metabolism. Annu. Rev. Biochem. 1946, 15, 193–218. [Google Scholar]
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef]
- Cerda-Kohler, H.; Henríquez-Olguín, C.; Casas, M.; Jensen, T.E.; Llanos, P.; Jaimovich, E. Lactate Administration Activates the ERK1/2, MTORC1, and AMPK Pathways Differentially According to Skeletal Muscle Type in Mouse. Physiol. Rep. 2018, 6, e13800. [Google Scholar] [CrossRef]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-Β2 Is an Exercise-Induced Adipokine That Regulates Glucose and Fatty Acid Metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Kyun, S.; Yoo, C.; Hashimoto, T.; Tomi, H.; Teramoto, N.; Kim, J.; Lim, K. Effects of Exogenous Lactate Administration on Fat Metabolism and Glycogen Synthesis Factors in Rats. Phys. Act. Nutr. 2020, 24, 1–5. [Google Scholar] [CrossRef]
- Jang, I.; Kim, J.; Kyun, S.; Hwang, D.; Lim, K. Acute Administration of Exogenous Lactate Increases Carbohydrate Metabolism during Exercise in Mice. Metabolites 2021, 11, 553. [Google Scholar] [CrossRef]
- Yeu, J.; Ko, H.J.; Kim, D.; Ahn, Y.; Kim, J.; Lee, W.; Jung, I.S.; Suh, J.; Lee, S.J. Evaluation of INSiGHT VET DXA (Dual-Energy X-ray Absorptiometry) for Assessing Body Composition in Obese Rats Fed with High Fat Diet: A Follow-up Study of Diet Induced Obesity Model for 8 Weeks. Lab. Anim. Res. 2019, 35, 2. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of Carbohydrate and Lipid Utilization during Exercise: The “Crossover” Concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Kiens, B. Skeletal Muscle Lipid Metabolism in Exercise and Insulin Resistance. Physiol. Rev. 2006, 86, 205–243. [Google Scholar] [CrossRef]
- Ghanassia, E.; Brun, J.F.; Mercier, J.; Raynaud, E. Oxidative Mechanisms at Rest and during Exercise. Clin. Chim. Acta 2007, 383, 1–20. [Google Scholar] [CrossRef]
- Hood, D.A.; Zak, R.; Pette, D. Chronic Stimulation of Rat Skeletal Muscle Induces Coordinate Increases in Mitochondrial and Nuclear MRNAs of Cytochrome-c-oxidase Subunits. Eur. J. Biochem. 1989, 179, 275–280. [Google Scholar] [CrossRef]
- Eigentler, A.; Draxl, A.; Wiethüchter, A.; Av, K.; Lassing, B.; Gnaiger, E. Laboratory Protocol: Citrate Synthase A Mitochondrial Marker Enzyme. Mitochondrial Physiol. Netw. 2015, 17, 1–11. [Google Scholar]
- Molinié, T.; Cougouilles, E.; David, C.; Cahoreau, E.; Portais, J.C.; Mourier, A. MDH2 Produced OAA Is a Metabolic Switch Rewiring the Fuelling of Respiratory Chain and TCA Cycle. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148532. [Google Scholar] [CrossRef]
- Zorzano, A.; Palacín, M.; Gumà, A. Mechanisms Regulating GLUT4 Glucose Transporter Expression and Glucose Transport in Skeletal Muscle. Acta Physiol. Scand 2005, 183, 43–58. [Google Scholar] [CrossRef]
- Huppertz, C.; Fischer, B.M.; Kim, Y.B.; Kotani, K.; Vidal-Puig, A.; Slieker, L.J.; Sloop, K.W.; Lowell, B.B.; Kahn, B.B. Uncoupling Protein 3 (UCP3) Stimulates Glucose Uptake in Muscle Cells through a Phosphoinositide 3-Kinase-Dependent Mechanism. J. Biol. Chem. 2001, 276, 12520–12529. [Google Scholar] [CrossRef]
- Wang, S.; Subramaniam, A.; Cawthorne, M.A.; Clapham, J.C. Increased Fatty Acid Oxidation in Transgenic Mice Overexpressing UCP3 in Skeletal Muscle. Diabetes Obes. Metab. 2003, 5, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ke, Q.; Fang, Y.; Wen, P.; Chen, H.; Yuan, Q.; Luo, J.; Zhang, Y.; Sun, Q.; Lv, Y.; et al. Sodium–Glucose Cotransporter 2 Inhibition Suppresses HIF-1α-Mediated Metabolic Switch from Lipid Oxidation to Glycolysis in Kidney Tubule Cells of Diabetic Mice. Cell Death Dis. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Zhu, S.; Song, K.; He, C. HK2: A Potential Regulator of Osteoarthritis via Glycolytic and Non-Glycolytic Pathways. Cell Commun. Signal. 2022, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.Q.; Ren, N.; Jin, L.; Cheng, K.; Kash, S.; Chen, R.; Wright, S.D.; Taggart, A.K.P.; Waters, M.G. Role of GPR81 in Lactate-Mediated Reduction of Adipose Lipolysis. Biochem. Biophys. Res. Commun. 2008, 377, 987–991. [Google Scholar] [CrossRef]
- Ahmed, K.; Tunaru, S.; Tang, C.; Müller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An Autocrine Lactate Loop Mediates Insulin-Dependent Inhibition of Lipolysis through GPR81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E. Skeletal Muscle Fat Oxidation: Timing and Flexibility Are Everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Hensrud, D.D.; Romanski, S.; Levine, J.A.; Burguera, B.; Jensen, M.D. Body composition and resting energy expenditure in humans: Role of fat, fat-free mass and extracellular fluid. Int. J. Obes. 2000, 24, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Gallagher, D.; Kotler, D.P.; Wang, Z.; Allison, D.B.; Heshka, S. Body-Size Dependence of Resting Energy Expenditure Can Attributed to Nonenergetic Homogeneity of Fat-Free Mass. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E132–E138. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; Lambourne, K.; Szabo, A.N.; Herrmann, S.D.; Honas, J.J.; Donnelly, J.E. Does Increased Prescribed Exercise Alter Non-exercise Physical Activity/Energy Expenditure in Healthy Adults? A Systematic Review. Clin. Obes. 2014, 4, 1–20. [Google Scholar] [CrossRef]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Bowker-Kinley, M.M.; Davis, W.I.; Wu, P.; Harris, R.A.; Popov, K.M. Evidence for Existence of Tissue-Specific Regulation of the Mammalian Pyruvate Dehydrogenase Complex. Biochem. J. 1998, 329, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.H.; Lee, J.S.; Yang, Y.H.; Nam, J.M.; Kim, B.W.; Ko, Y.G. Mitochondrial Oxidative Phosphorylation Complexes Exist in the Sarcolemma of Skeletal Muscle. BMB Rep. 2016, 49, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, T.Y.; Jung, C.H.; Nirmala, F.S.; Park, S.Y.; Huh, Y.H.; Ahn, J. Mitochondrial Dysfunction in Skeletal Muscle Contributes to the Development of Acute Insulin Resistance in Mice. J. Cachexia Sarcopenia Muscle 2021, 12, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Warfel, J.D.; Bermudez, E.M.; Mendoza, T.M.; Ghosh, S.; Zhang, J.; Elks, C.M.; Mynatt, R.; Vandanmagsar, B. Mitochondrial Fat Oxidation Is Essential for Lipid-Induced Inflammation in Skeletal Muscle in Mice. Sci. Rep. 2016, 6, 37941. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Gerhart-Hines, Z.; Puigserver, P. Metabolic Adaptations through the PGC-1α and SIRT1 Pathways. FEBS Lett. 2008, 582, 46–53. [Google Scholar] [CrossRef]
- Kou, G.; Li, Z.; Wu, C.; Liu, Y.; Hu, Y.; Guo, L.; Xu, X.; Zhou, Z. Citrus Tangeretin Improves Skeletal Muscle Mitochondrial Biogenesis via Activating the AMPK-PGC1-α Pathway In Vitro and In Vivo: A Possible Mechanism for Its Beneficial Effect on Physical Performance. J. Agric. Food Chem. 2018, 66, 11917–11925. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, I.; Kyun, S.; Hwang, D.; Kim, T.; Lim, K.; Park, H.-Y.; Kim, S.-W.; Kim, J. Chronic Administration of Exogenous Lactate Increases Energy Expenditure during Exercise through Activation of Skeletal Muscle Energy Utilization Capacity in Mice. Metabolites 2024, 14, 220. https://doi.org/10.3390/metabo14040220
Jang I, Kyun S, Hwang D, Kim T, Lim K, Park H-Y, Kim S-W, Kim J. Chronic Administration of Exogenous Lactate Increases Energy Expenditure during Exercise through Activation of Skeletal Muscle Energy Utilization Capacity in Mice. Metabolites. 2024; 14(4):220. https://doi.org/10.3390/metabo14040220
Chicago/Turabian StyleJang, Inkwon, Sunghwan Kyun, Deunsol Hwang, Taeho Kim, Kiwon Lim, Hun-Young Park, Sung-Woo Kim, and Jisu Kim. 2024. "Chronic Administration of Exogenous Lactate Increases Energy Expenditure during Exercise through Activation of Skeletal Muscle Energy Utilization Capacity in Mice" Metabolites 14, no. 4: 220. https://doi.org/10.3390/metabo14040220
APA StyleJang, I., Kyun, S., Hwang, D., Kim, T., Lim, K., Park, H.-Y., Kim, S.-W., & Kim, J. (2024). Chronic Administration of Exogenous Lactate Increases Energy Expenditure during Exercise through Activation of Skeletal Muscle Energy Utilization Capacity in Mice. Metabolites, 14(4), 220. https://doi.org/10.3390/metabo14040220







