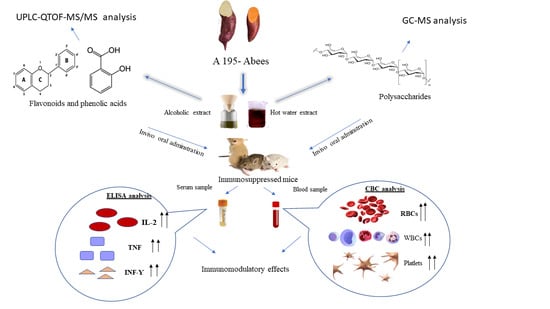
A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytochemical Study
2.1.1. Collection of Plant Material
2.1.2. Preparation of Extracts and Polysaccharide Fractions
2.1.3. UHPLC-QTOF-MS/MS Profiling of Ethanolic Extracts
2.1.4. Gas Chromatography Mass Spectroscopy (GC-MS) Analysis of the Polysaccharides
2.2. Immunostimulant Activity of the Ethanolic Extracts and Polysaccharide Fractions
2.2.1. Ethics Statement
2.2.2. In Vivo Evaluation of the Immunostimulant Activity of the Ethanolic Extracts and Polysaccharide Fractions
3. Results
3.1. Phytochemical Study
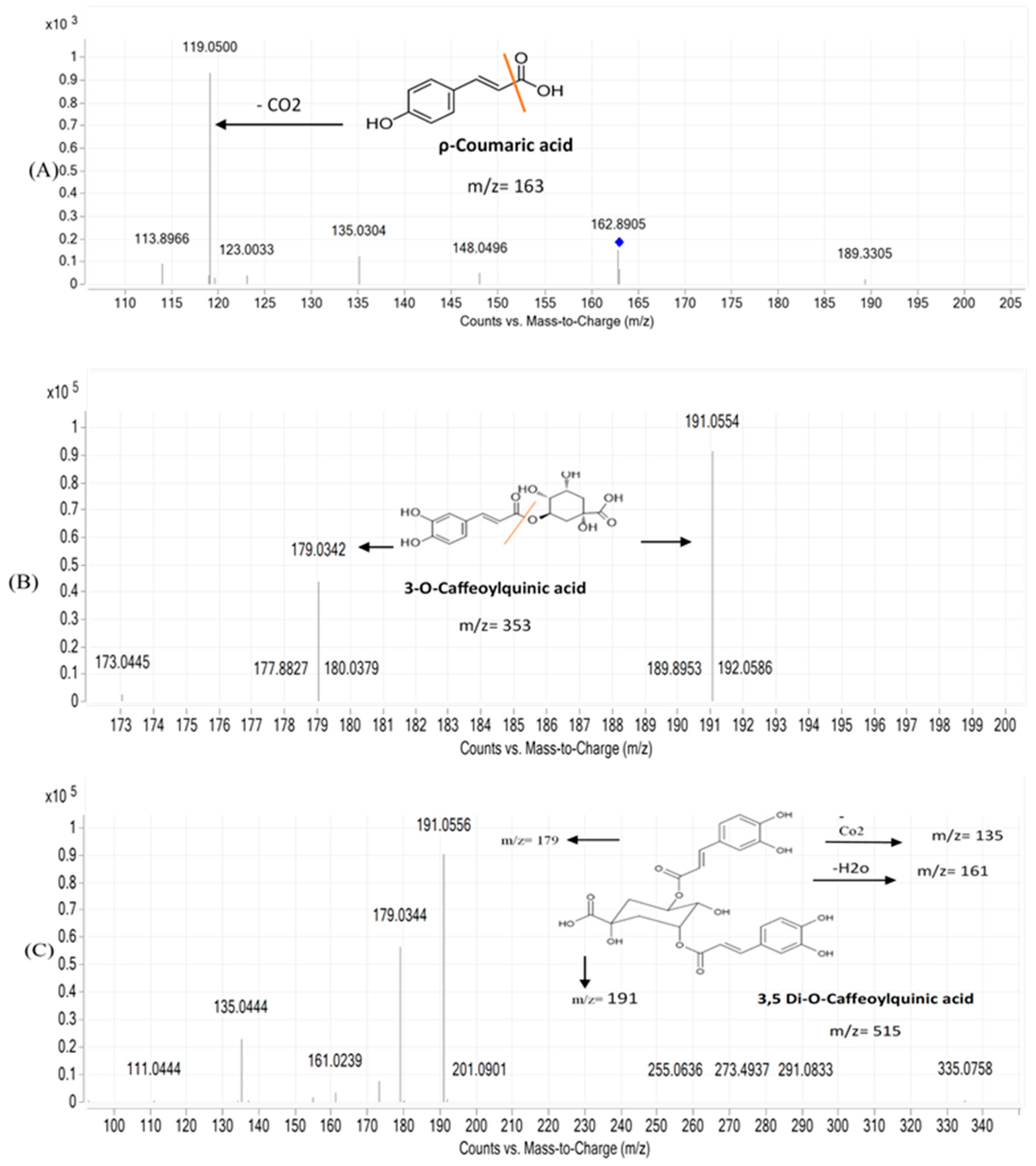
3.1.1. UPLC-MS/MS Analysis
3.1.2. Chemical Characterization of Polysaccharides Fractions
3.2. Imunostimulant Activity of the Ethanolic Extracts and Polysaccharides Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Ditu, L.M.; Pircalabioru, G.G.; Gheorghe, I.; Curutiu, C.; Holban, A.M.; Picu, A.; Petcu, L.; Chifiriuc, M.C. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front. Immunol. 2018, 9, 354337. [Google Scholar] [CrossRef] [PubMed]
- Vorobiov, A.A. Principles of classification and strategies for the use of immunomodulators in medicine. Microbiol. Biotechnol. 2002, 4, 93–98. [Google Scholar]
- Walaa, N.A. Immunomodulatory and Natural Immunomodulators. J. Allergy Inflamm. 2017, 1, e101. [Google Scholar]
- Wagner, H. Immunostimulants of Plant Origin. Croat. Chem. Acta 1995, 68, 615–626. [Google Scholar]
- Ishida, H.; Suzuno, H.; Sugiyama, N.; Innami, S.; Tadokoro, T.; Maekawa, A. Nutritive Evaluation on Chemical Components of Leaves, Stalks and Stems of Sweet Potatoes (Ipomoea batatas poir). Food Chem. 2000, 68, 359–367. [Google Scholar] [CrossRef]
- Loebenstein, G.; Thottappilly, G. The Sweetpotato; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Mazyad, A.A.; El-Attar, A.K.; Amer, W.M.; Ahmed, A.A.; Ismail, M.H. Investigations on the prevalence of two sweet potato viruses and their potential weed reservoirs in Egypt. Egypt. J. Bot. 2021, 61, 105–125. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Zhou, B.; Jin, C.; Liu, J.; Gou, Y.; Chen, H.; Kan, J.; Qian, C.; Zhang, N. Immunomodulatory effects of polysaccharides from purple sweet potato on lipopolysaccharide treated RAW 264.7 macrophages. J. Food Biochem. 2018, 42, e12535. [Google Scholar] [CrossRef]
- Haskell, M.J.; Jamil, K.M.; Hassan, F.; Peerson, J.M.; Hossain, M.I.; Fuchs, G.J.; Brown, K.H. Daily Consumption of Indian Spinach (Basella alba) or Sweet Potatoes Has a Positive Effect on Total-Body Vitamin A Stores in Bangladeshi Men 1–3. 2004. Available online: https://academic.oup.com/ajcn/article-abstract/80/3/705/4690550 (accessed on 1 February 2024).
- Kim, O.K.; Nam, D.E.; Yoon, H.G.; Baek, S.J.; Jun, W.; Lee, J. Immunomodulatory and Antioxidant Effects of Purple Sweet Potato Extract in LP-BM5 Murine Leukemia Virus-Induced Murine Acquired Immune Deficiency Syndrome. J. Med. Food 2015, 18, 882–889. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yoon, E.J.; Cha, J.; Lee, H.G. Antitumor activity of levan polysaccharides from selected microorganisms. Int. J. Biol. Macromol. 2004, 34, 37–41. [Google Scholar] [CrossRef]
- Park, S.D.; Lai, Y.S.; Kim, C.H. Immunopontentiating and antitumor activities of the purified polysaccharides from Phellodendron chinese SCHNEID. Life Sci. 2004, 75, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Lestari, L.A.; Soesatyo, M.H.N.E.; Ravati, S.; Harmayani, E. Characterization of Bestak sweet potato (Ipomoea batatas) variety from Indonesian origin as prebiotic. Int. Food Res. J. 2013, 20, 2241–2245. [Google Scholar]
- Kumalasari, I.D.; Sugahara, T.; Nishi, K. Immunostimulating effect of sweet potato fiber extract on IgM production by HB4C5 cells. IOP Conf. Ser. Mater. Sci. Eng. 2020, 821, 012028. [Google Scholar] [CrossRef]
- Takamine, K.; Abe, J.-I.; Iwaya, A.; Maseda, S.; Hizukuri, S. A New Manufacturing Process for Dietary Fiber from Sweetpotato Residue and Its Physical Characteristics. J. Appl. Glycosci. 2000, 47, 67–72. [Google Scholar] [CrossRef]
- Manthey, J.A.; Buslig, B.S. Flavonoids in the Living System; Springer: Boston, MA, USA, 1998; Volume 439. [Google Scholar]
- Hanieh, H.; Gerile, C.; Narabara, K.; Gu, Z.; Abe, A.; Kondo, Y. In vivo immunomodulatory effects of dietary purple sweet potato after immunization in chicken. Anim. Sci. J. 2010, 81, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.S.; Lee, K.J.; Chang, J.H.; Park, J.H.; Cho, J.H.; Park, J.H.; Kim, K.M.; Lee, M.S. In vitro antioxidant, anti-allergic and anti-inflammatory effects of ethanol extracts from Korean sweet potato leaves and stalks. J. Korean Soc. Food Sci. Nutr. 2013, 42, 369–377. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.Y.; Yang, J.W.; Lee, J.S.; Oh, S.D.; Oh, S.; Lee, S.M.; Lim, M.H.; Park, S.K.; Jang, J.S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas L.) tubers. Food Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef]
- Zhao, G.; Kan, J.; Li, Z.; Chen, Z. Characterization and immunostimulatory activity of an (1→6)-a-D-glucan from the root of Ipomoea batatas. Int. Immunopharmacol. 2005, 5, 1436–1445. [Google Scholar] [CrossRef]
- Hamed, A.A.; El-Shiekh, R.A.; Mohamed, O.G.; Aboutabl, E.A.; Fathy, F.I.; Fawzy, G.A.; Al-Taweel, A.M.; Elsayed, T.R.; Tripathi, A.; Al-Karmalawy, A.A. Cholinesterase Inhibitors from an Endophytic Fungus Aspergillus niveus Fv-er401: Metabolomics, Isolation and Molecular Docking. Molecules 2023, 28, 2559. [Google Scholar] [CrossRef] [PubMed]
- Kirk, S.; Sawyer, R. Pearson’s Composition and Analysis of Foods; Longman Scientific and Technical: Harlow, UK, 1991. [Google Scholar]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE 2019, 13, e0204152. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wang, G.; You, L.; Zhang, L.; Ren, H.; Hu, W.; Qiang, Q.; Wang, X.; Ji, L.; Gu, Z.; et al. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef] [PubMed]
- Heroor, S.; Beknal, A.K.; Mahurkar, N. Immunomodulatory activity of methanolic extracts of fruits and bark of Ficus glomerata Roxb. in mice and on human neutrophils. Indian J. Pharmacol. 2013, 45, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, M.I.; Hassan, M.; Abdelhalim, M.A.; El-Desoky, A.M.; Mohamed, S.O.; Ezzat, S.M. Immunomodulatory effect of Noni fruit and its isolates: Insights into cell-mediated immune response and inhibition of LPS-induced THP-1 macrophage inflammation. Food Funct. 2021, 12, 3170–3179. [Google Scholar] [CrossRef]
- Khaliq, H.A.; Ortiz, S.; Alhouayek, M.; Muccioli, G.G.; Quetin-Leclercq, J. Dereplication and Quantification of Major Compounds of Convolvulus arvensis L. Extracts and Assessment of Their Effect on LPS-Activated J774 Macrophages. Molecules 2022, 27, 963. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhu, M.; Luo, Y.; Liu, Y.; Li, R.; Kou, M.; Wang, X.; Zhang, Y.; Meng, X.; Zheng, Y.; et al. A sweet potato cinnamate 4-hydroxylase gene, IbC4H, increases phenolics content and enhances drought tolerance in tobacco. Acta Physiol. Plant 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Batiga, S.; Valli, M.; Zeraik, M.L.; Fraige, K.; Leme, G.M.; Pitangui, N.S.; Almeida, A.M.F.; Michel, S.; Young, M.C.M.; Bolzani, V.S. Chemical composition and biological properties of Ipomoea procumbens. Rev. Bras. Farmacogn. 2019, 29, 191–197. [Google Scholar] [CrossRef]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Su, J.; Tan, C.; Gao, Y.; Feng, Y. Four phenolic acids from purple sweet potato and their effects on physicochemical, digestive and structural characteristics of starch. Int. J. Food Sci. Technol. 2021, 56, 1896–1904. [Google Scholar] [CrossRef]
- Jung, J.-K.; Lee, S.-U.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 2011, 24, 29–37. [Google Scholar] [CrossRef]
- Ambika, A.P.; Nair, S.N. Wound healing activity of plants from the convolvulaceae family. Adv. Wound Care 2019, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ryu, C.M.; Sumner, L.W.; Paré, P.W. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 2006, 67, 2262–2268. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Ma, L.; Zhang, Z.; Cao, L.; Liu, J.; Zeng, X. Preparation, preliminary characterization and immunostimulatory activity of polysaccharide fractions from the peduncles of Hovenia dulcis. Food Chem. 2013, 138, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.S.; Kim, W.J.; Kim, S.M.; Park, J.K.; Lee, S.M.; Kim, S.O.; Synytsya, A.; Park, Y.I. Purification, characterization and immunostimulating activity of water-soluble polysaccharide isolated from Capsosiphon fulvescens. Int. Immunopharmacol. 2010, 10, 364–370. [Google Scholar] [CrossRef]
- Mohanraj, R.; Sivasankar, S. Sweet potato (Ipomoea batatas [L.] Lam)—A valuable medicinal food: A review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef]
- Harrison, H.F.; Mitchell, T.R.; Peterson, J.K.; Wechter, W.P.; Majetich, G.F.; Snook, M.E. Contents of Caffeoylquinic Acid Compounds in the Storage Roots of Sixteen Sweetpotato Genotypes and Their Potential Biological Activity. J. Am. Soc. Hortic. Sci. 2008, 133, 492–500. [Google Scholar] [CrossRef]
- Fischer, G.; Cleff, M.B.; Dummer, L.A.; Paulino, N.; Paulino, A.S.; de Oliveira Vilela, C.; Campos, F.S.; Storch, T.; Vargas, G.D.A.; de Oliveira Hübner, S.; et al. Adjuvant effect of green propolis on humoral immune response of bovines immunized with bovine herpesvirus type 5. Vet. Immunol. Immunopathol. 2007, 116, 79–84. [Google Scholar] [CrossRef]
- Hikosaka, K.; El-Abasy, M.; Koyama, Y.; Motobu, M.; Koge, K.; Isobe, T.; Kang, C.B.; Hayashidani, H.; Onodera, T.; Wang, P.C.; et al. Immunostimulating Effects of the Polyphenol-Rich Fraction of Sugar Cane (Saccharum officinarum L.) Extract in Chickens. Phytother. Res. 2007, 21, 120–125. [Google Scholar] [CrossRef]
- Kilani-Jaziri, S.; Mokdad-Bzeouich, I.; Krifa, M.; Nasr, N.; Ghedira, K.; Chekir-Ghedira, L. Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: A structure–activity relationship study. Drug Chem. Toxicol. 2017, 40, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Ilina, T.; Kashpur, N.; Granica, S.; Bazylko, A.; Shinkovenko, I.; Kovalyova, A.; Goryacha, O.; Koshovyi, O. Phytochemical profiles and in vitro immunomodulatory activity of ethanolic extracts from Galium aparine L. Plants 2019, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Lee, T.T.; Huang, C.C.; Shieh, X.H.; Chen, C.L.; Chen, L.J.; Yu, B.I. Flavonoid, Phenol and Polysaccharide Contents of Echinacea Purpurea L. and Its Immunostimulant Capacity In Vitro. Int. J. Environ. Sci. Dev. 2010, 1, 5–9. [Google Scholar] [CrossRef]
- Bendjeddou, D.; Lalaoui, K.; Satta, D. Immunostimulating activity of the hot water-soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galanga and Citrullus colocynthis. J. Ethnopharmacol. 2003, 88, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kiddane, A.T.; Kim, G.D. Anticancer and Immunomodulatory Effects of Polysaccharides. Nutr. Cancer 2021, 73, 2219–2231. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Irfan, M.; Quah, Y.; Saba, E.; Kim, S.D.; Park, S.C.; Jeong, M.G.; Kwak, Y.S.; Rhee, M.H. The increasing hematopoietic effect of the combined treatment of Korean Red Ginseng and Colla corii asini on cyclophosphamide-induced immunosuppression in mice. J. Ginseng Res. 2021, 45, 591–598. [Google Scholar] [CrossRef]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef]
- Shin, J.M.; Son, Y.J.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Song, D.G.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022, 22, 64. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, G.; Wen, Y.; Liu, S.; Li, C.; Yang, R.; Li, W. Intestinal microbiota are involved in the immunomodulatory activities of longan polysaccharide. Mol. Nutr. Food Res. 2017, 61, 1700466. [Google Scholar] [CrossRef] [PubMed]



| Group (n = 6) | Treatment |
|---|---|
| A (healthy control) | Vehicle treatment (distilled water) |
| B (Model control) | Cyclophosphamide (70 mg kg−1/i.p.) |
| C (ethanolic extract of Abees) | R1 extract (450 mg kg−1/orally) |
| D (ethanolic extract of A 195) | R2 extract (450 mg kg−1/orally) |
| E (polysaccharide fraction of Abees) | A1 extract (450 mg kg−1/orally) |
| F (polysaccharide fraction of A195) | A2 extract (450 mg kg−1/orally) |
| No. | Compound | Rt (min) | Molecular Formula | (M+H)/(M-H) | Fragments | Error | Abees | A 195 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sucrose | 0.434 | C12H22O11 | 341.1100 | 179.0314 | −1.3 | + | + | [30] |
| 161.0233 | |||||||||
| 101.0233 | |||||||||
| 59.0141 | |||||||||
| 2 | Quinic acid | 0.489 | C7H12O6 | 191.0553 | 191.0553 | −1.91 | + | + | [30] |
| 85.0291 | |||||||||
| 59.0141 | |||||||||
| 3 | Succinic acid | 0.517 | C4H6O4 | 117.0178 | 73.0289 | −2.52 | − | + | [30] |
| 99.9249 | |||||||||
| 4 | Caffeic acid | 0.831 | C9H8O4 | 179.0341/181.0492 | 135.0454 | 1.28 | + | + | [30] |
| 5 | Protocatechuic acid | 0.850 | C7H6O4 | 153.0189 | 109.0286 | 3.12 | + | + | [31] |
| 6 | Salicylic acid | 1.189 | C7H6O3 | 137.0241 | 93.3250 | 1.97 | + | + | [30] |
| 7 | Protocatechualdehyde | 1.216 | C7H6O3 | 137.0240 | 119.0117 | 3.07 | + | + | [31] |
| 108.0196 | |||||||||
| 93.0339 | |||||||||
| 8 | Scopoletin | 1.442 | C10H8O4 | 193.0498 | 178.0237 | −1.27 | + | + | [32] |
| 150.0293 | |||||||||
| 133.0577 | |||||||||
| 9 | 4-Acetylphenylcaffeic acid | 1.577 | C14H18O7 | 297.0980 | 179.0381 | −3.31 | + | + | [31] |
| 161.0250 | |||||||||
| 135.0452 | |||||||||
| 10 | 3-O-ρ-coumaroyl quinic acid | 2.214 | C16H18O8 | 337.0915/339.1067 | 191.0588 | 2.87 | + | + | [31] |
| 163.0385 | |||||||||
| 11 | Feruloyl quinic acid | 2.408 | C17H20O9 | 367.1021 | 193.0490 | 3.82 | + | + | [30] |
| 191.0527 | |||||||||
| 173.0447 | |||||||||
| 12 | ρ-Coumaric acid | 2.410 | C9H8O3 | 163.0392 | 119.0500 | 5.59 | + | + | [31] |
| 135.0304 | |||||||||
| 13 | Diosmin | 2.519 | C28H32O15 | 607.1660 | 299.1540 | 0.72 | − | + | [33] |
| 14 | 4-Hydroxybenzoic acid | 2.740 | C7H6O3 | 137.0238 | 93.0336 | 4.6 | + | + | [34] |
| 65.0381 | |||||||||
| 15 | Azelaic acid | 2.879 | C9H16O4 | 187.0967 | 125.0969 | −2.74 | + | + | [30] |
| 97.0642 | |||||||||
| 16 | Umbelliferone | 2.938 | C9H6O3 | 163.0388 | 135.0434 | 0.58 | + | + | [32] |
| 117.0327 | |||||||||
| 89.0386 | |||||||||
| 107.0480 | |||||||||
| 17 | 3,4-di-O-caffeoylquinic acid | 2.999 | C25H24O12 | 515.1185/517.1346 | 353.0830 | 2.53 | + | + | [30] |
| 335.0735 | |||||||||
| 18 | 3,5-di-O-caffeoylquinic acid | 3.046 | C25H24O12 | 515.1179 | 353.0870 | 2.03 | + | + | [30] |
| 191.0553 | |||||||||
| 179.0341 | |||||||||
| 19 | 1,3-Dicaffeoylquinic acid | 3.073 | C25H24O12 | 515.1188 | 335.0754 | 1.6 | + | + | [35] |
| 179.0342 | |||||||||
| 20 | 1,5-Dicaffeoylquinic acid | 3.088 | C25H24O12 | 515.1187 | 335.5230 | 2.05 | + | + | [35] |
| 179.5046 | |||||||||
| 21 | 3-O-Caffeoylquinic acid | 3.100 | C16H18O9 | 353.0868 | 191.0559 | 2.13 | + | + | [35] |
| 179.0350 | |||||||||
| 173.0455 | |||||||||
| 135.0452 | |||||||||
| 22 | 4,5-di-O-caffeoylquinic acid | 3.212 | C25H24O12 | 515.1179 | 353.0854 | 3 | + | + | [30] |
| 299.1596 | |||||||||
| 179.0341 | |||||||||
| 23 | N-trans-p-coumaroyltyramine | 3.406 | C17H17NO3 | 282.1124 | 162.0553 | 3.19 | + | + | [30] |
| 145.0285 | |||||||||
| 119.0494 | |||||||||
| 24 | Caffeoyl-feruloyl quinic acid | 3.433 | C26H26O12 | 529.1341 | 367.1016 | 2.11 | + | + | [30] |
| 193.0495 | |||||||||
| 179.0342 | |||||||||
| 173.0446 | |||||||||
| 25 | 3,4Dimethoxycinnamic acid | 3.571 | C11H12O4 | 207.0655/209.0809 | 207.0653 | 3.61 | + | + | [31] |
| 163.7717 | |||||||||
| 135.0446 | |||||||||
| 133.0289 | |||||||||
| 26 | Trihydroxy-10,15-octadecadienoic acid | 3.599 | C18H32O5 | 327.2163 | 291.1937 | 2.8 | + | + | [30] |
| 229.1438 | |||||||||
| 211.1325 | |||||||||
| 171.1014 | |||||||||
| 27 | Diosmetin | 4.015 | C16H12O6 | 299.0565 | 255.0296 | −1.19 | − | + | [33] |
| 284.0333 | |||||||||
| 28 | Jaceosidin | 4.042 | C17H14O7 | 329.0671/331.0814 | 299.0196 | −0.68 | − | + | [33] |
| 314.0432 | |||||||||
| 29 | Trihydroxy-10-octadecenoic acid | 4.043 | C18H34O5 | 329.2334 | 329.2326 | −0.18 | + | + | [30] |
| 311.2222 | |||||||||
| 293.2110 | |||||||||
| 229.1442 | |||||||||
| 211.1336 | |||||||||
| 171.1024 | |||||||||
| 30 | Oleanolic acid | 5.99 | C30H48O3 | 455.1524 | 455.0423 | 1.96 | + | + | [36] |
| No. | RT | Identification | Abees | A 195 |
|---|---|---|---|---|
| 1 | 7.913 | Glyoxylic acid | - | 1.15 |
| 2 | 16.877 | D-Arabinose | 3.586 | 2.83 |
| 3 | 17.398 | D-Fructofuranose | 4.36 | - |
| 4 | 17.596 | 1,2,3-Propanetricarboxylic acid | - | 14.75 |
| 5 | 17.782 | L-Rhamnose | 4.88 | 1.81 |
| 6 | 18.424 | L-Gluconic acid | - | 3.62 |
| 7 | 18.489 | D-Allopyranose | 1.8 | - |
| 8 | 18.943 | Fructose oxime | 6.66 | - |
| 9 | 18.993 | D-Psicose | 5.53 | 0.58 |
| 10 | 19.287 | D-Talopyranose | 4.04 | 2.67 |
| 11 | 19.430 | Glucose | 4.96 | 13.88 |
| 12 | 19.723 | Galactose | 7.6 | 9.36 |
| 13 | 19.785 | Galacturonic acid | - | 4.02 |
| 14 | 20.052 | Glucaric acid | 17.82 | 17.98 |
| 15 | 20.450 | Myo-Inositol | 1.62 | 3.99 |
| 16 | 25.517 | 2-Deoxy-galactopyranose | - | 1.30 |
| 17 | 25.975 | D-Xylopyranose | 2.42 | - |
| 18 | 26.392 | D-Glucuronic acid | - | 2.17 |
| 19 | 27.225 | 3-alpha-Mannobiose | 2.33 | 1.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgabry, R.M.; Hassan, M.; Fawzy, G.A.; Meselhy, K.M.; Mohamed, O.G.; Al-Taweel, A.M.; Sedeek, M.S. A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities. Metabolites 2024, 14, 222. https://doi.org/10.3390/metabo14040222
Elgabry RM, Hassan M, Fawzy GA, Meselhy KM, Mohamed OG, Al-Taweel AM, Sedeek MS. A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities. Metabolites. 2024; 14(4):222. https://doi.org/10.3390/metabo14040222
Chicago/Turabian StyleElgabry, Rehab M., Mariam Hassan, Ghada A. Fawzy, Khaled M. Meselhy, Osama G. Mohamed, Areej M. Al-Taweel, and Mohamed S. Sedeek. 2024. "A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities" Metabolites 14, no. 4: 222. https://doi.org/10.3390/metabo14040222
APA StyleElgabry, R. M., Hassan, M., Fawzy, G. A., Meselhy, K. M., Mohamed, O. G., Al-Taweel, A. M., & Sedeek, M. S. (2024). A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities. Metabolites, 14(4), 222. https://doi.org/10.3390/metabo14040222