Alterations in Plasma Lipid Profile before and after Surgical Removal of Soft Tissue Sarcoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Collection and Preparation of Samples
2.3. Metabolomics Analysis
2.4. Putative Identification of Metabolites
2.5. Statistical Analysis
3. Results
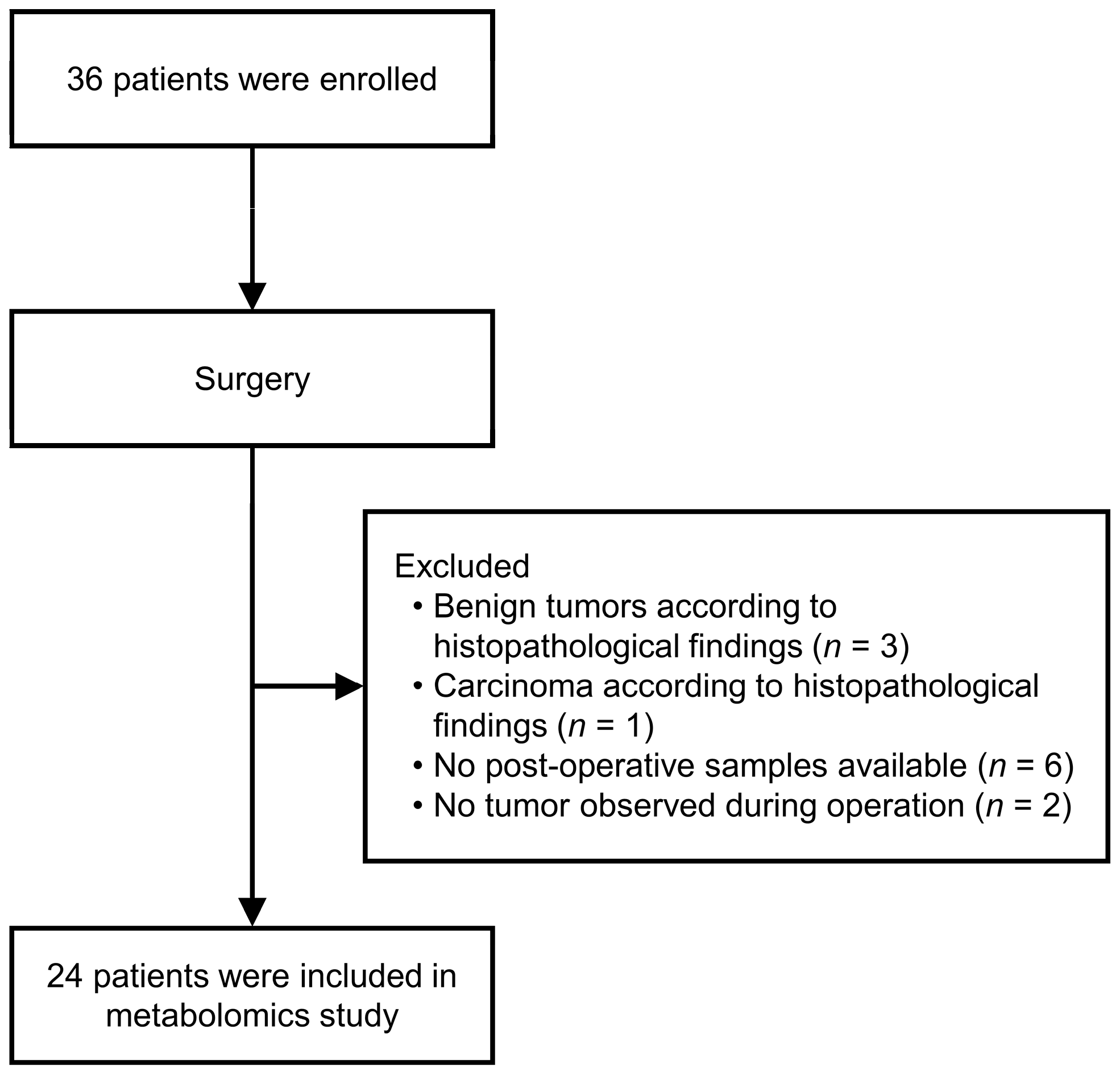
3.1. Patient Characteristics
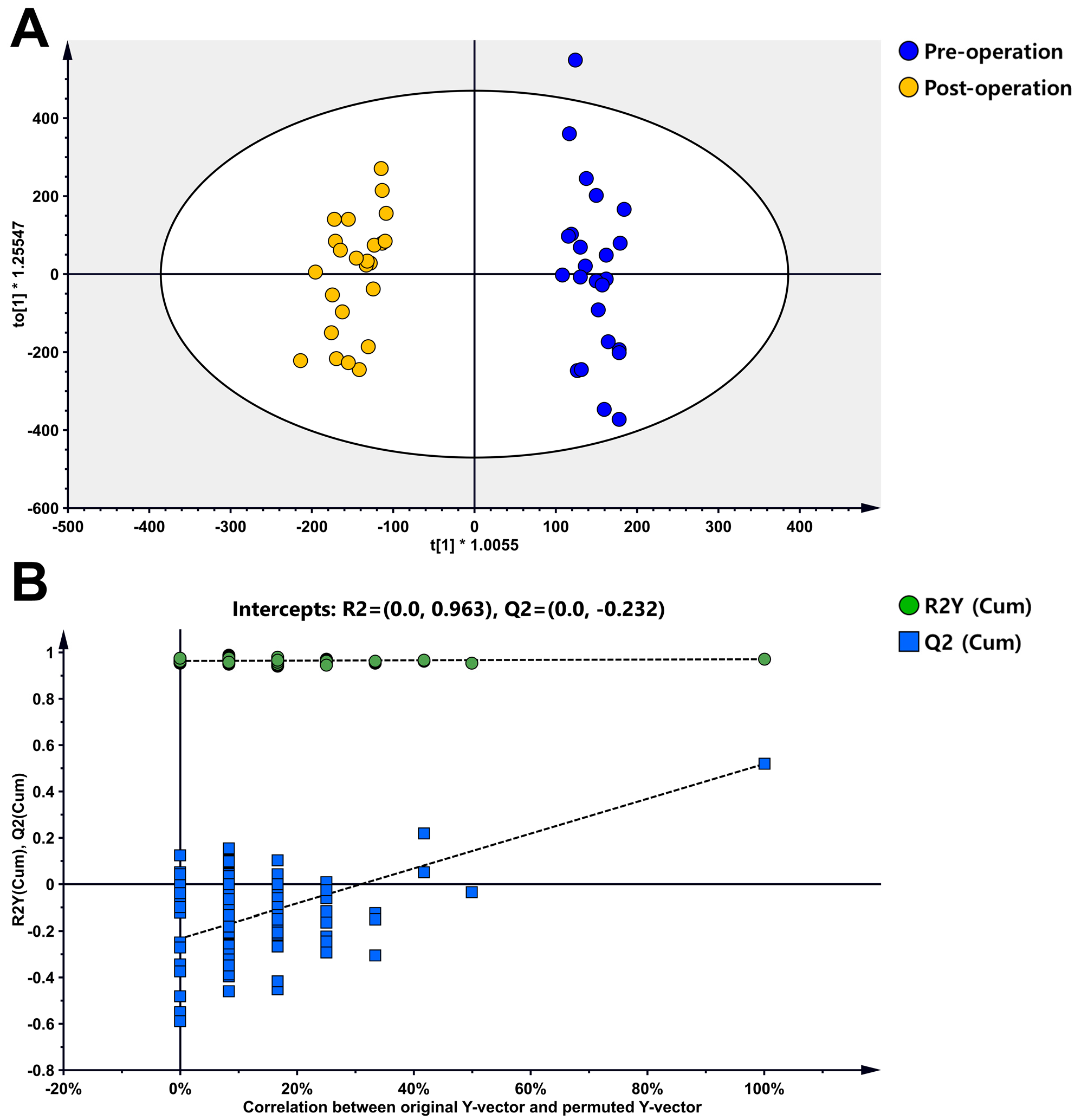
3.2. Metabolite Profiles of Sarcoma Patients
3.3. Metabolite Profiles of Sarcoma Patients
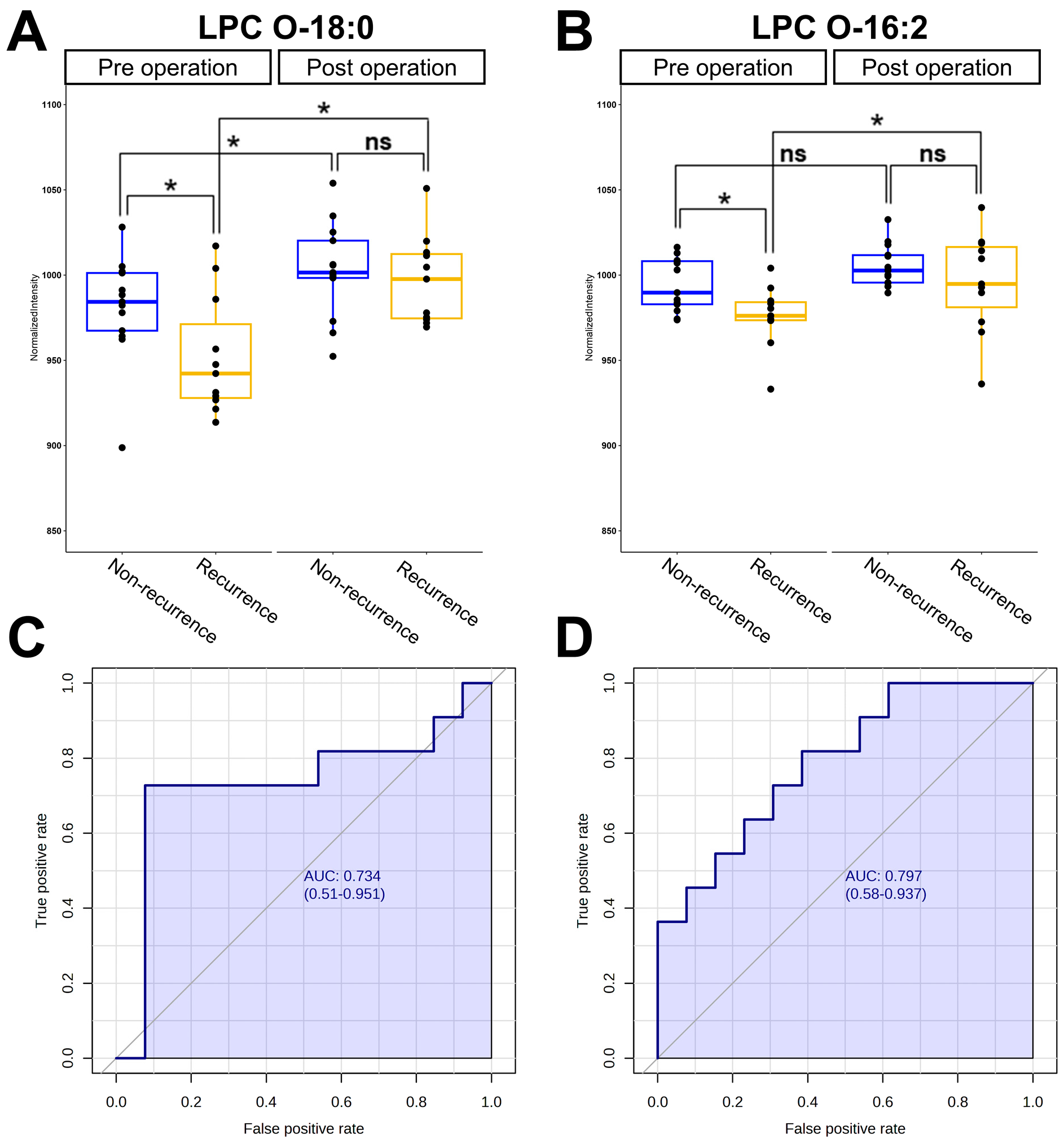
3.4. Analysis of Receiver Operating Characteristics for Potential Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef]
- Damerell, V.; Pepper, M.S.; Prince, S. Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct. Target. Ther. 2021, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Seetharam, M. First-Line Therapy for Metastatic Soft Tissue Sarcoma. Curr. Treat. Options Oncol. 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e928. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Penney, K.L.; Tyekucheva, S.; Rosenthal, J.; El Fandy, H.; Carelli, R.; Borgstein, S.; Zadra, G.; Fanelli, G.N.; Stefanizzi, L.; Giunchi, F.; et al. Metabolomics of prostate cancer gleason score in tumor tissue and serum. Mol. Cancer Res. 2021, 19, 475–484. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454–29464. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; An, K.-Y.; Jeon, J.; Kim, N.K.; Lee, J.W.; Hong, J.; Chung, B.C. Untargeted Metabolomics and Polyamine Profiling in Serum before and after Surgery in Colorectal Cancer Patients. Metabolites 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Lyu, Y.; Chen, J.; He, C.; Lyu, X.; Zhang, Y.; Chen, L.; Jiang, Y.; Xiang, J.; Liu, B.; et al. Prognostic Implication of Plasma Metabolites in Gastric Cancer. Int. J. Mol. Sci. 2023, 24, 12774. [Google Scholar] [CrossRef]
- Dangoor, A.; Seddon, B.; Gerrand, C.; Grimer, R.; Whelan, J.; Judson, I. UK guidelines for the management of soft tissue sarcomas. Clin. Sarcoma Res. 2016, 6, 20. [Google Scholar] [CrossRef]
- Crago, A.M.; Dickson, M.A. Liposarcoma: Multimodality Management and Future Targeted Therapies. Surg. Oncol. Clin. N. Am. 2016, 25, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Xia, J.; Broadhurst, D.I.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2013, 9, 280–299. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles: Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of their Biogenesis and Functions. J. Lipid Res. 2018, 59, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Palinski, W.; Monti, M.; Camerlingo, R.; Iacobucci, I.; Bocella, S.; Pinto, F.; Iannuzzi, C.; Mansueto, G.; Pignatiello, S.; Fazioli, F.; et al. Lysosome purinergic receptor P2X4 regulates neoangiogenesis induced by microvesicles from sarcoma patients. Cell Death Dis. 2021, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Zini, N.; Massa, A.; Baldini, N. Extracellular Nanovesicles Secreted by Human Osteosarcoma Cells Promote Angiogenesis. Cancers 2019, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, Q.; Halim, A.; Song, G. Targeting lipid metabolism of cancer cells: A promising therapeutic strategy for cancer. Cancer Lett. 2017, 401, 39–45. [Google Scholar] [CrossRef]
- Sadri, N.; Zhang, P.J. Hypoxia-inducible factors: Mediators of cancer progression; prognostic and therapeutic targets in soft tissue sarcomas. Cancers 2013, 5, 320–333. [Google Scholar] [CrossRef]
- Kim, J.I.; Choi, K.U.; Lee, I.S.; Choi, Y.J.; Kim, W.T.; Shin, D.H.; Kim, K.; Lee, J.H.; Kim, J.Y.; Sol, M.Y. Expression of hypoxic markers and their prognostic significance in soft tissue sarcoma. Oncol. Lett. 2015, 9, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 2012, 33, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Knific, T.; Vouk, K.; Smrkolj, Š.; Prehn, C.; Adamski, J.; Rižner, T.L. Models including plasma levels of sphingomyelins and phosphatidylcholines as diagnostic and prognostic biomarkers of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2018, 178, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Adriaensens, P.; Guedens, W.; Bigirumurame, T.; Baeten, K.; Vanhove, K.; Vandeurzen, K.; Darquennes, K.; Vansteenkiste, J.; Dooms, C.; et al. Detection of Lung Cancer through Metabolic Changes Measured in Blood Plasma. J. Thorac. Oncol. 2016, 11, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xiao, Y.; Elson, P.; Tan, H.; Plummer, S.J.; Berk, M.; Aung, P.P.; Lavery, I.C.; Achkar, J.P.; Li, L.; et al. Plasma lysophosphatidylcholine levels: Potential biomarkers for colorectal cancer. J. Clin. Oncol. 2007, 25, 2696–2701. [Google Scholar] [CrossRef]
- Joo, E.J.; Weyers, A.; Li, G.; Gasimli, L.; Li, L.; Choi, W.J.; Lee, K.B.; Linhardt, R.J. Carbohydrate-containing molecules as potential biomarkers in colon cancer. Omics A J. Integr. Biol. 2014, 18, 231–241. [Google Scholar] [CrossRef]
- Sinkala, M.; Mulder, N.; Patrick Martin, D. Metabolic gene alterations impact the clinical aggressiveness and drug responses of 32 human cancers. Commun. Biol. 2019, 2, 414. [Google Scholar] [CrossRef]
- Liu, B.; He, S.; Li, C.; Feng, C.; Wang, H.; Zhang, H.; Tu, C.; Li, Z. Integration analysis based on fatty acid metabolism robustly predicts prognosis, dissecting immunity microenvironment and aiding immunotherapy for soft tissue sarcoma. Front. Genet. 2023, 14, 1161791. [Google Scholar] [CrossRef]
- Kühn, T.; Floegel, A.; Sookthai, D.; Johnson, T.; Rolle-Kampczyk, U.; Otto, W.; von Bergen, M.; Boeing, H.; Kaaks, R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016, 14, 13. [Google Scholar] [CrossRef]
- Maija, D.; Marina, M.-K.; Janis, K.; Reinis, V.; Didi, N.; Misty, M.A.; Stefan, S.; Zumrut Duygu, S.; An Chi, G.; Eponine, O.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506. [Google Scholar] [CrossRef]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]
- Kozar, N.; Kruusmaa, K.; Bitenc, M.; Argamasilla, R.; Adsuar, A.; Takač, I.; Arko, D. Identification of Novel Diagnostic Biomarkers in Breast Cancer Using Targeted Metabolomic Profiling. Clin. Breast Cancer 2021, 21, e204–e211. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, L.; Yin, P.; Lu, X.; Wang, X.; Niu, J.; Gao, P.; Xu, G. Serum Metabolic Profiling Study of Hepatocellular Carcinoma Infected with Hepatitis B or Hepatitis C Virus by Using Liquid Chromatography–Mass Spectrometry. J. Proteome Res. 2012, 11, 5433–5442. [Google Scholar] [CrossRef]
- Enooku, K.; Nakagawa, H.; Fujiwara, N.; Kondo, M.; Minami, T.; Hoshida, Y.; Shibahara, J.; Tateishi, R.; Koike, K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019, 9, 10663. [Google Scholar] [CrossRef] [PubMed]
- His, M.; Viallon, V.; Dossus, L.; Gicquiau, A.; Achaintre, D.; Scalbert, A.; Ferrari, P.; Romieu, I.; Onland-Moret, N.C.; Weiderpass, E.; et al. Prospective analysis of circulating metabolites and breast cancer in EPIC. BMC Med. 2019, 17, 178. [Google Scholar] [CrossRef]
- Li, Y.J.; Fahrmann, J.F.; Aftabizadeh, M.; Zhao, Q.; Tripathi, S.C.; Zhang, C.; Yuan, Y.; Ann, D.; Hanash, S.; Yu, H. Fatty acid oxidation protects cancer cells from apoptosis by increasing mitochondrial membrane lipids. Cell Rep. 2022, 39, 110870. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Cheng, C.; Tan, Y.; Jiang, N.; Liao, C.; Liao, W.; Cao, Y.; Luo, X. Acyl-CoA synthetase long-chain 3-mediated fatty acid oxidation is required for TGFβ1-induced epithelial-mesenchymal transition and metastasis of colorectal carcinoma. Int. J. Biol. Sci. 2022, 18, 2484–2496. [Google Scholar] [CrossRef] [PubMed]
- Radif, Y.; Ndiaye, H.; Kalantzi, V.; Jacobs, R.; Hall, A.; Minogue, S.; Waugh, M.G. The endogenous subcellular localisations of the long chain fatty acid-activating enzymes ACSL3 and ACSL4 in sarcoma and breast cancer cells. Mol. Cell. Biochem. 2018, 448, 275–286. [Google Scholar] [CrossRef]
- Patel, A.V.; Johansson, G.; Colbert, M.C.; Dasgupta, B.; Ratner, N. Fatty acid synthase is a metabolic oncogene targetable in malignant peripheral nerve sheath tumors. Neuro-Oncology 2015, 17, 1599–1608. [Google Scholar] [CrossRef]
- Reilly, P.T.; Mak, T.W. Molecular pathways: Tumor cells Co-opt the brain-specific metabolism gene CPT1C to promote survival. Clin. Cancer Res. 2012, 18, 5850–5855. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Jeong, S.H.; Jang, C.; Bae, H.; Kim, Y.H.; Park, I.; Kim, S.K.; Koh, G.Y. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 2019, 363, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Sinturel, F.; Spaleniak, W.; Dibner, C. Circadian rhythm of lipid metabolism. Biochem. Soc. Trans. 2022, 50, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Falchook, G.; Infante, J.; Arkenau, H.-T.; Patel, M.R.; Dean, E.; Borazanci, E.; Brenner, A.; Cook, N.; Lopez, J.; Pant, S.; et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. eClinicalMedicine 2021, 34, 100797. [Google Scholar] [CrossRef]


| Pathology | Chemosensitivity | Anatomical Location of Primary Lesion | Patients (n) |
|---|---|---|---|
| Angiosarcoma | Moderately chemosensitive | Hip | 1 |
| Dedifferentiated liposarcoma | Relatively chemo-insensitive | Calf, thigh, retroperitoneum | 3 |
| Leiomyosarcoma | Moderately chemosensitive | Upper arm, hip, thigh (4) | 6 |
| MPNST | Relatively chemo-insensitive | Shoulder | 1 |
| Myxofibrosarcoma | Relatively chemo-insensitive | Upper arm, forearm, thigh (3) | 5 |
| Myxoid liposarcoma | Chemosensitive | Thigh | 2 |
| Pleomorphic leiomyosarcoma | NE | Thigh | 1 |
| Pleomorphic liposarcoma | Moderately chemo-sensitive | Thigh | 1 |
| Undifferentiated pleomorphic sarcoma | Relatively chemo-insensitive | Calf, hip, thigh | 3 |
| Well differentiated liposarcoma | Relatively chemo-insensitive | Hip | 1 |
| Metabolites | VIP | RT (min) | m/z | Formula | Trend a | p-Value |
|---|---|---|---|---|---|---|
| Porphyrin Metabolism; Bile Secretion | ||||||
| Bilirubin | 2.62 | 5.28 | 585.2713 | C33H36N4O6 | ↓ | 0.003 |
| Fatty Acid Metabolism | ||||||
| N-Palmitoyl threonine | 1.00 | 5.98 | 358.2932 | C20H39NO4 | ↓ | 0.245 |
| 13Z-Docosenamide | 1.34 | 12.96 | 338.3430 | C22H43NO | ↓ | 0.004 |
| Nervonamide | 1.20 | 15.89 | 366.3738 | C24H47NO | ↓ | 0.072 |
| cis-4-Decenoylcarnitine | 2.63 | 5.30 | 314.2328 | C17H31NO4 | ↓ | 0.002 |
| cis-5-Dodecenoylcarnitine | 3.04 | 5.64 | 342.2636 | C19H35NO4 | ↓ | 0.001 |
| 5Z,8Z-Tetradecadienoylcarnitine | 2.63 | 5.79 | 368.2790 | C21H37NO4 | ↓ | 0.022 |
| cis-5-Tetradecenoylcarnitine | 3.19 | 6.04 | 370.2948 | C21H39NO4 | ↓ | 0.001 |
| alpha-Linolenic acid | 1.38 | 9.13 | 279.2312 | C18H30O2 | ↓ | 0.009 |
| Docosahexaenoic acid | 2.39 | 9.38 | 329.2480 | C22H32O2 | ↓ | 0.014 |
| Glycerophospholipid Metabolism | ||||||
| LPC 16:1 | 1.48 | 6.84 | 494.3263 | C24H48NO7P | ↑ | 0.001 |
| LPC 17:0 | 1.16 | 8.14 | 510.3557 | C25H52NO7P | ↑ | 0.002 |
| LPC 17:1 | 1.30 | 7.31 | 508.3422 | C25H50NO7P | ↑ | 0.003 |
| LPC 18:0 | 1.20 | 8.56 | 524.3714 | C26H54NO7P | ↑ | 0.000 |
| LPC 18:3 | 1.53 | 6.65 | 518.3248 | C26H48NO7P | ↑ | 0.026 |
| LPC 20:1 | 1.78 | 8.98 | 550.3888 | C28H56NO7P | ↑ | 0.000 |
| LPC 20:2 | 1.37 | 8.09 | 548.3735 | C28H54NO7P | ↑ | 0.005 |
| LPC O-16:2 | 1.28 | 30.95 | 478.3303 | C24H48NO6P | ↑ | 0.007 |
| LPC O-18:0 | 1.65 | 9.19 | 510.3930 | C26H56NO6P | ↑ | 0.000 |
| LPC O-18:1 | 1.62 | 9.14 | 508.3753 | C26H54NO6P | ↑ | 0.001 |
| LPC P-18:0 | 1.18 | 8.13 | 508.3740 | C26H54NO6P | ↑ | 0.002 |
| LPE 18:2 | 1.01 | 7.10 | 478.2935 | C23H44NO7P | ↑ | 0.011 |
| LPE 22:5 | 1.19 | 7.25 | 528.3093 | C27H46NO7P | ↑ | 0.068 |
| LPE P-18:0 | 1.79 | 9.12 | 466.3305 | C23H48NO6P | ↑ | 0.000 |
| LPS O-18:0 | 1.68 | 6.80 | 512.3363 | C24H50NO8P | ↑ | 0.001 |
| PC 16:0/20:5 | 1.24 | 17.81 | 780.5541 | C44H78NO8P | ↑ | 0.500 |
| PC 18:0/20:4 | 1.86 | 25.36 | 810.6000 | C46H84NO8P | ↑ | 0.000 |
| PC 18:1/18:1 | 1.02 | 31.71 | 786.5999 | C44H84NO8P | ↑ | 0.018 |
| PC 18:2/18:3 | 1.21 | 15.43 | 780.5511 | C44H78NO8P | ↑ | 0.069 |
| PC 18:3/18:3 | 1.71 | 15.31 | 778.5419 | C44H76NO8P | ↑ | 0.001 |
| PC 18:4/18:2 | 1.11 | 18.75 | 778.5348 | C44H76NO8P | ↑ | 0.039 |
| PC 32:1 | 1.48 | 22.76 | 732.5541 | C40H78NO8P | ↑ | 0.001 |
| PC 34:3 | 1.87 | 19.57 | 756.5552 | C42H78NO8P | ↑ | 0.003 |
| PC 36:4 | 1.17 | 31.18 | 782.5684 | C44H80NO8P | ↑ | 0.007 |
| PC 38:6 | 1.14 | 25.01 | 806.5683 | C46H80NO8P | ↑ | 0.003 |
| PC 38:7 | 1.17 | 15.53 | 804.5562 | C46H78NO8P | ↑ | 0.000 |
| PC 40:7 | 1.11 | 19.64 | 832.5880 | C48H82NO8P | ↑ | 0.000 |
| PC 40:8 | 1.51 | 16.12 | 830.5694 | C48H80NO8P | ↑ | 0.001 |
| PE 36:4 | 1.45 | 22.07 | 740.5210 | C41H74NO8P | ↑ | 0.014 |
| Metabolites | AUC Value | Sensitivity | Specificity |
|---|---|---|---|
| LPC O-18:0 | 0.734 (0.510–0.951) | 0.727 | 0.923 |
| LPC O-16:2 | 0.797 (0.580–0.937) | 0.727 | 0.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Gwon, M.-R.; Kim, J.-I.; Hwang, S.-y.; Seong, S.-J.; Yoon, Y.-R.; Kim, M.; Kim, H. Alterations in Plasma Lipid Profile before and after Surgical Removal of Soft Tissue Sarcoma. Metabolites 2024, 14, 250. https://doi.org/10.3390/metabo14050250
Lee J-H, Gwon M-R, Kim J-I, Hwang S-y, Seong S-J, Yoon Y-R, Kim M, Kim H. Alterations in Plasma Lipid Profile before and after Surgical Removal of Soft Tissue Sarcoma. Metabolites. 2024; 14(5):250. https://doi.org/10.3390/metabo14050250
Chicago/Turabian StyleLee, Jae-Hwa, Mi-Ri Gwon, Jeung-Il Kim, Seung-young Hwang, Sook-Jin Seong, Young-Ran Yoon, Myungsoo Kim, and Hyojeong Kim. 2024. "Alterations in Plasma Lipid Profile before and after Surgical Removal of Soft Tissue Sarcoma" Metabolites 14, no. 5: 250. https://doi.org/10.3390/metabo14050250
APA StyleLee, J.-H., Gwon, M.-R., Kim, J.-I., Hwang, S.-y., Seong, S.-J., Yoon, Y.-R., Kim, M., & Kim, H. (2024). Alterations in Plasma Lipid Profile before and after Surgical Removal of Soft Tissue Sarcoma. Metabolites, 14(5), 250. https://doi.org/10.3390/metabo14050250






