Metabolomic and Physiological Effects of a Cardiorenal Protective Diet Intervention in African American Adults with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.3. Health Outcomes
2.4. Metabolomic Analysis
2.5. Statistical Analysis
2.5.1. Health Outcomes and FV Intake
2.5.2. Metabolomic Analysis
3. Results
3.1. Demographic Data
3.2. Fruit and Vegetable Intake
3.3. Health Outcomes
3.4. Metabolomic Analysis
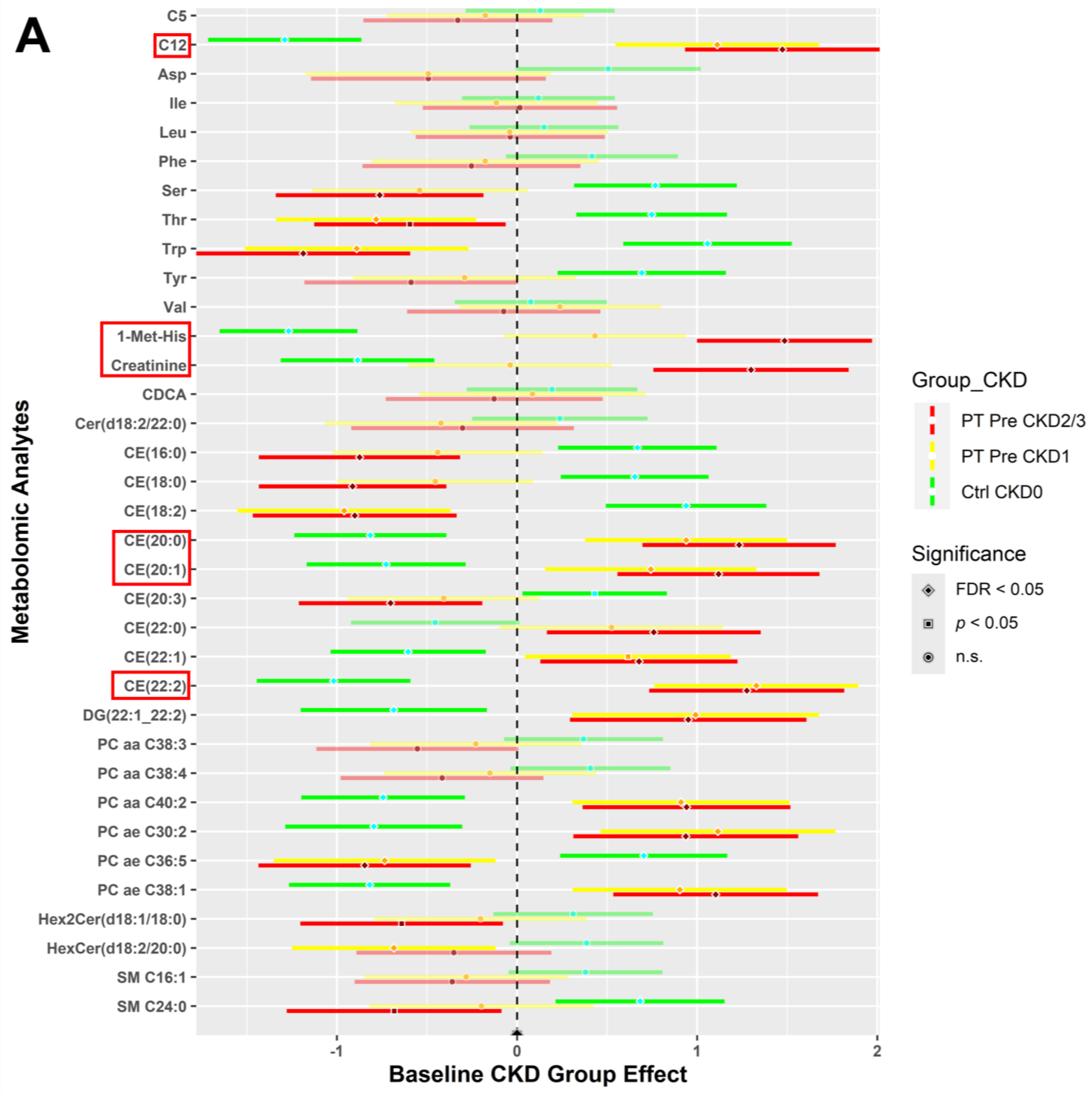
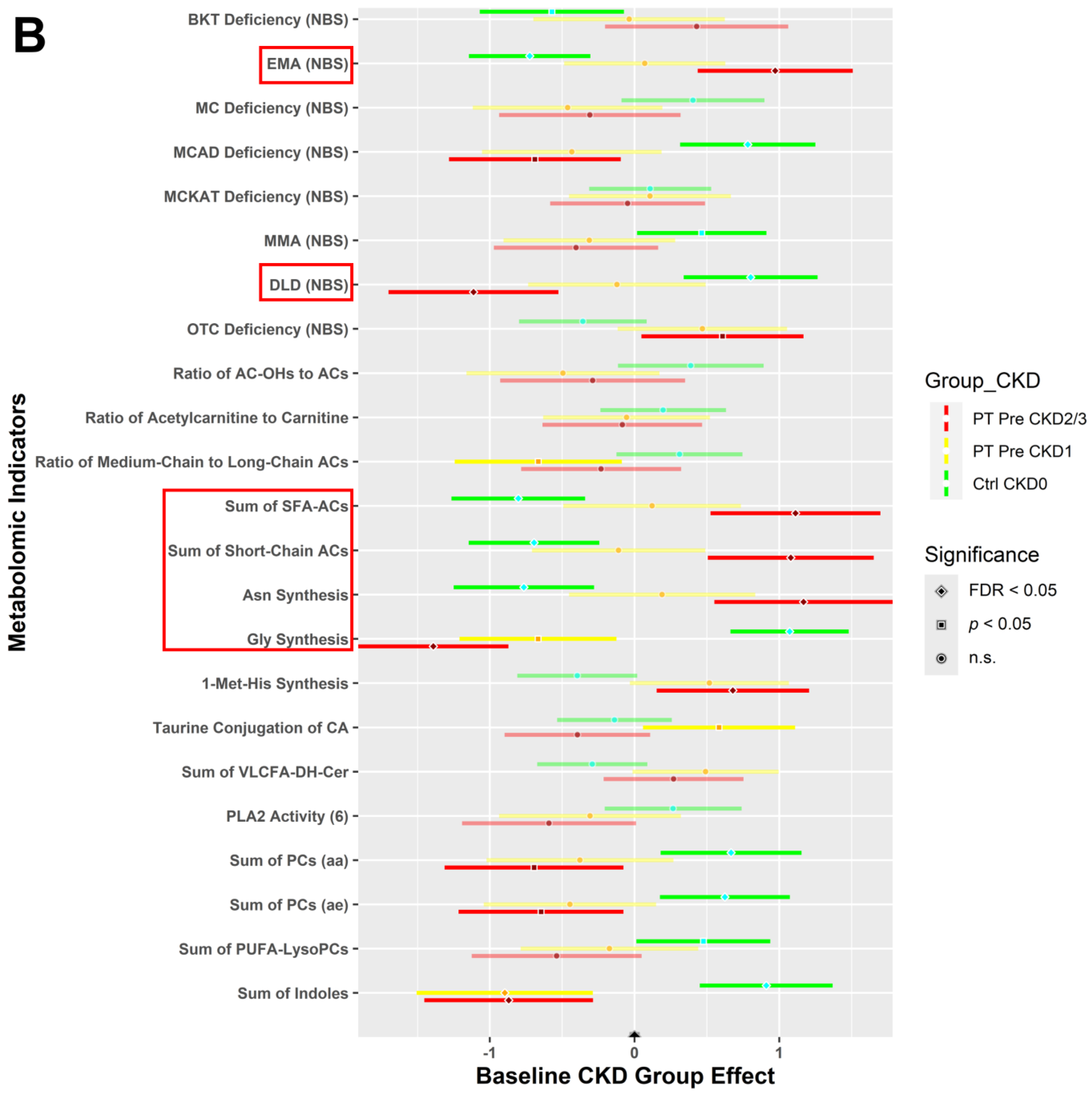
3.4.1. CKD Effects for All Study Participants at Baseline
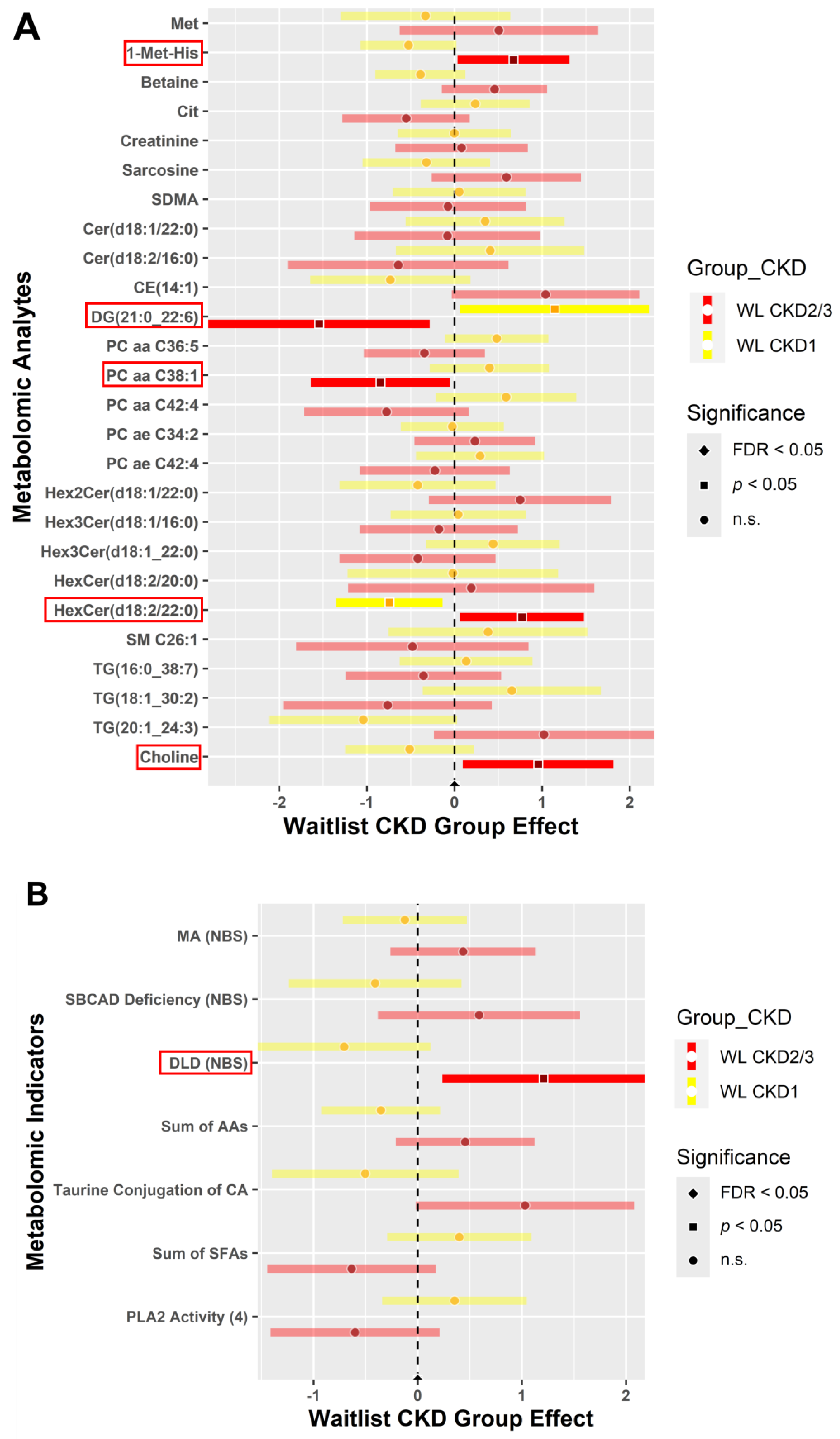
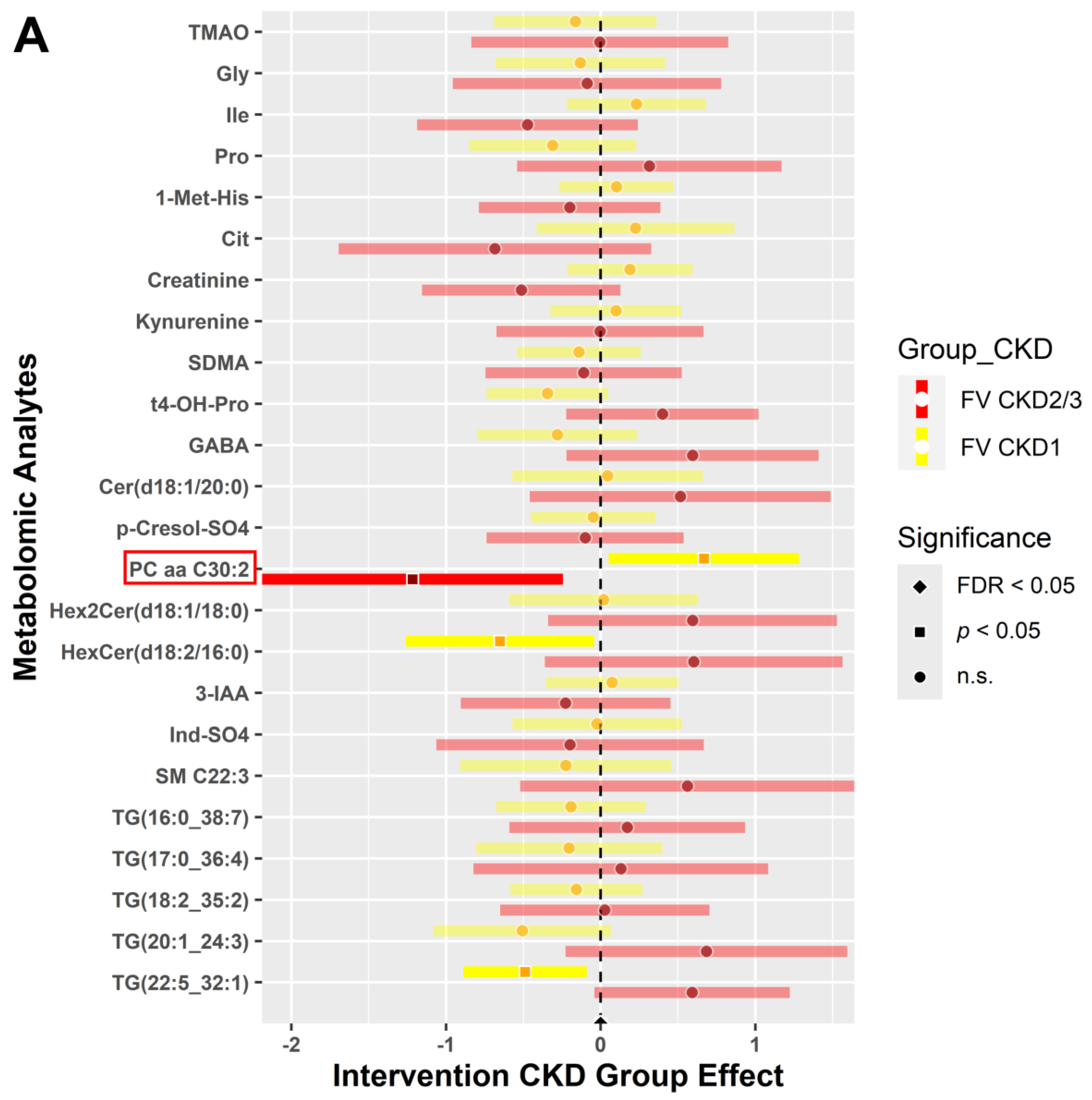
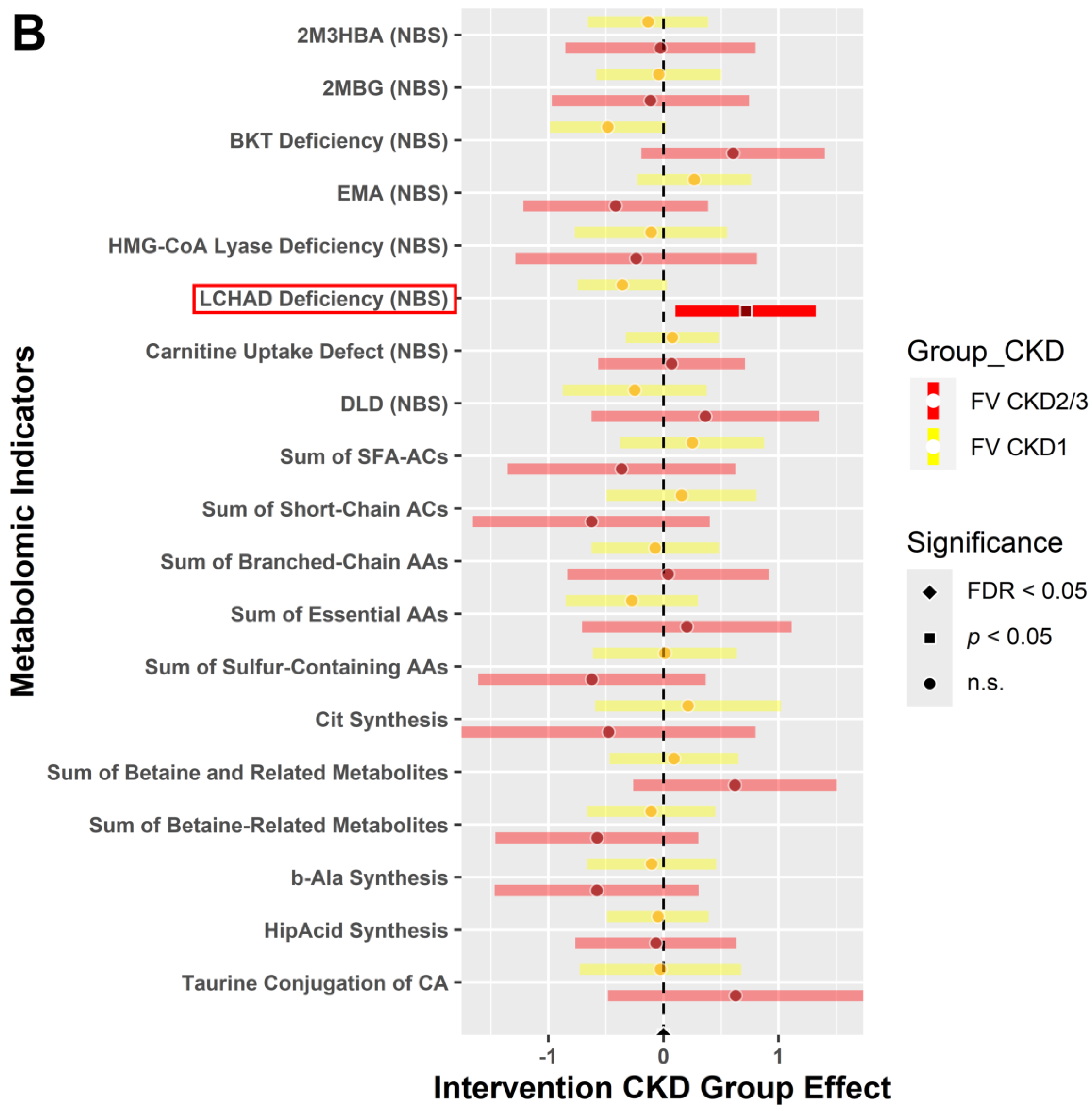
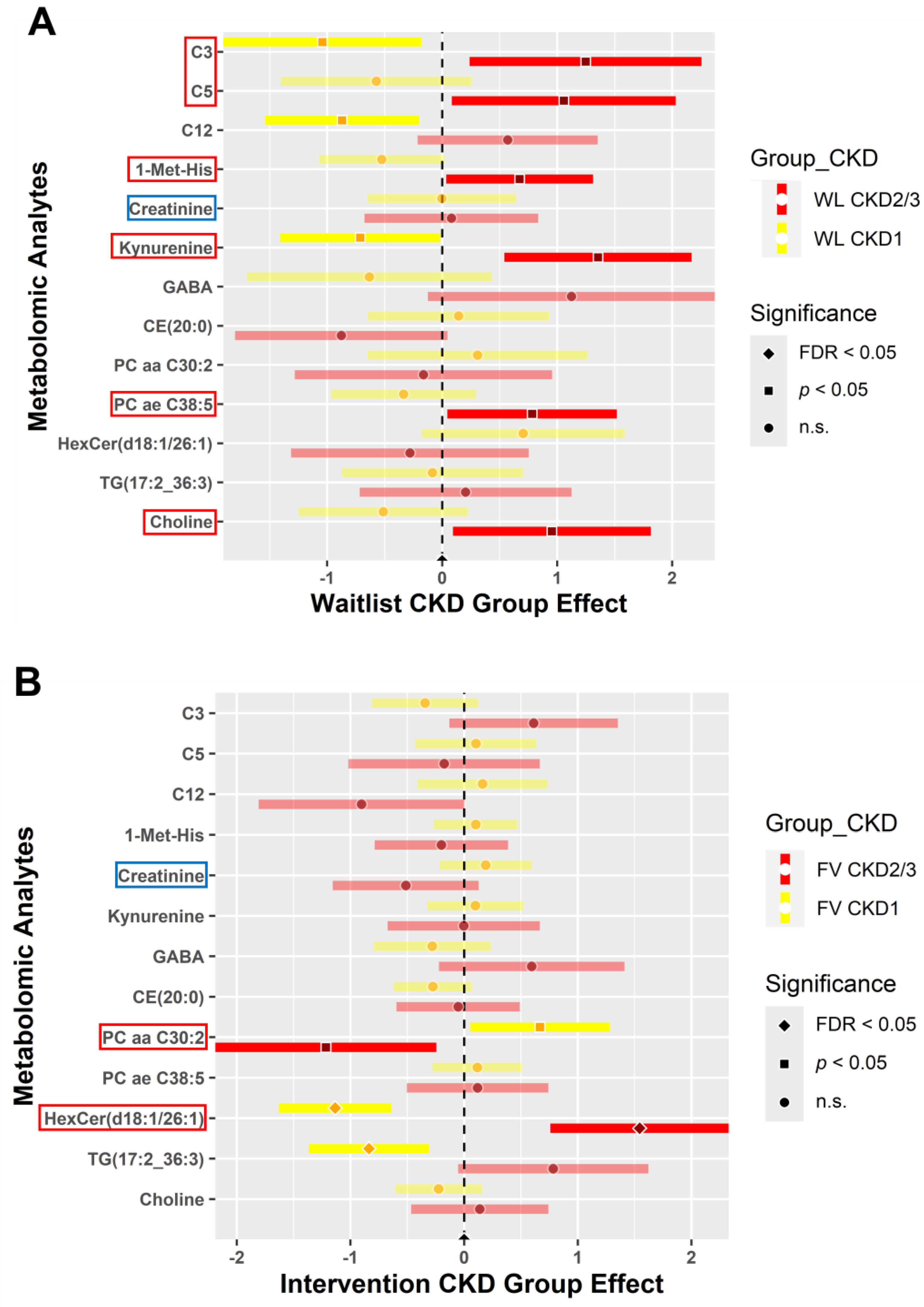
3.4.2. CKD within Group Effects at Baseline vs. 6 Weeks Post-Intervention
3.4.3. CKD between Group Effects at Baseline vs. Post-Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vallianou, N.G.; Mitesh, S.; Gkogkou, A.; Geladari, E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relation-ship? Curr. Cardiol. Rev. 2019, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Weldegiorgis, M.; Woodward, M. The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 506. [Google Scholar] [CrossRef]
- Malhotra, R.; Nguyen, H.A.; Benavente, O.; Mete, M.; Howard, B.V.; Mant, J.; Odden, M.C.; Peralta, C.A.; Cheung, A.K.; Nadkarni, G.N.; et al. Association Between More Intensive vs Less Intensive Blood Pressure Lowering and Risk of Mortality in Chronic Kidney Disease Stages 3 to 5: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2017, 177, 1498–1505. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Lu, J.L.; Molnar, M.Z.; Ma, J.Z.; Canada, R.B.; Streja, E.; Kalantar-Zadeh, K.; Bleyer, A.J. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern. Med. 2014, 174, 1442–1449. [Google Scholar] [CrossRef]
- Al Kibria, G.M. Racial/ethnic disparities in prevalence, treatment, and control of hypertension among US adults following application of the 2017 American College of Cardiology/American Heart Association guideline. Prev. Med. Rep. 2019, 14, 100850. [Google Scholar] [CrossRef]
- Laster, M.; Shen, J.I.; Norris, K.C. Kidney Disease Among African Americans: A Population Perspective. Am. J. Kidney Dis. 2018, 72, S3–S7. [Google Scholar] [CrossRef]
- Hounkpatin, H.O.; Fraser, S.D.S.; Honney, R.; Dreyer, G.; Brettle, A.; Roderick, P.J. Ethnic minority disparities in progression and mortality of pre-dialysis chronic kidney disease: A systematic scoping review. BMC Nephrol. 2020, 21, 217. [Google Scholar] [CrossRef]
- Wu, S.; Kong, M.; Song, Y.; Peng, A. Ethnic disparities in bidirectional causal effects between serum uric acid concentrations and kidney function: Trans-ethnic Mendelian randomization study. Heliyon 2023, 9, e21108. [Google Scholar] [CrossRef]
- Reynolds, K.M.; Horimoto, A.R.V.R.; Lin, B.M.; Zhang, Y.; Kurniansyah, N.; Yu, B.; Boerwinkle, E.; Qi, Q.; Kaplan, R.; Daviglus, M.; et al. Ancestry-driven metabolite variation provides insights into disease states in admixed populations. Genome Med. 2023, 15, 52. [Google Scholar] [CrossRef]
- Ma, L.; Palmer, N.D.; Choi, Y.A.; Murea, M.; Snipes, J.A.; Parks, J.S.; Langefeld, C.D.; Freedman, B. APOL1 Risk Variants Impair Multiple Mitochondrial Pathways in a Metabolomics Analysis. Kidney360 2020, 1, 1353–1362. [Google Scholar] [CrossRef]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Dharmarajan, S.; Saran, R.; Burrows, N.R.; Saydah, S.; Powe, N.R.; Hsu, C.-Y.; Bibbins-Domingo, K.; et al. Food Insecurity, CKD, and Subsequent ESRD in US Adults. Am. J. Kidney Dis. 2017, 70, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Suarez, J.J.; Isakova, T.; Anderson, C.A.; Boulware, L.E.; Wolf, M.; Scialla, J.J. Food Access, Chronic Kidney Disease, and Hypertension in the U.S. Am. J. Prev. Med. 2015, 49, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Gao, H.-K.; Kengne, A.P. Food Patterns are Associated with Likelihood of CKD in US Adults. Sci. Rep. 2018, 8, 10696. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Tuot, D.S.; Pavkov, M.E.; Burrows, N.R.; Stack, A.G.; Saran, R.; Bragg-Gresham, J.; Powe, N.R.; Hsu, C.-Y.; et al. Poor accordance to a DASH dietary pattern is associated with higher risk of ESRD among adults with moderate chronic kidney disease and hypertension. Kidney Int. 2019, 95, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Valle-Hita, C.; Becerra-Tomás, N.; Díaz-López, A.; Vázquez-Ruiz, Z.; Megías, I.; Corella, D.; Goday, A.; Martínez, J.A.; Alonso-Gómez, M.; Wärnberg, J.; et al. Longitudinal association of dietary acid load with kidney function decline in an older adult population with metabolic syndrome. Front. Nutr. 2022, 9, 986190. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Crews, D.C.; Wesson, D.E.; Tilea, A.M.; Saran, R.; Ríos-Burrows, N.; Williams, D.E.; Powe, N.R. High Dietary Acid Load Predicts ESRD among Adults with CKD. J. Am. Soc. Nephrol. 2015, 26, 1693–1700. [Google Scholar] [CrossRef]
- Clegg, D.J.; Gallant, K.M.H. Plant-Based Diets in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 141–143. [Google Scholar] [CrossRef]
- Joshi, S.; McMacken, M.; Kalantar-Zadeh, K. Plant-Based Diets for Kidney Disease: A Guide for Clinicians. Am. J. Kidney Dis. 2021, 77, 287–296. [Google Scholar] [CrossRef]
- Wen, D.; Zheng, Z.; Surapaneni, A.; Yu, B.; Zhou, L.; Zhou, W.; Xie, D.; Shou, H.; Avila-Pacheco, J.; Kalim, S.; et al. Metabolite profiling of CKD progression in the chronic renal insufficiency cohort study. JCI Insight. 2022, 7, e161696. [Google Scholar] [CrossRef]
- Cedars, A.; Manlhiot, C.; Ko, J.-M.; Bottiglieri, T.; Arning, E.; Weingarten, A.; Opotowsky, A.; Kutty, S. Metabolomic Profiling of Adults with Congenital Heart Disease. Metabolites 2021, 11, 525. [Google Scholar] [CrossRef]
- Liu, X.; Locasale, J.W. Metabolomics: A Primer. Trends Biochem. Sci. 2017, 42, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. Reading the metabolic fine print. The application of metabolomics to diagnostics, drug research and nutrition might be integral to improved health and personalized medicine. Embo Rep. 2009, 10, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [PubMed]
- German, J.B.; Hammock, B.D.; Watkins, S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics 2005, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Kalecký, K.; Huang, N.K.; Petersen, K.; Singh, V.; Ross, A.C.; Neuberger, T.; Bottiglieri, T. A very-low carbohydrate content in a high-fat diet modifies the plasma metabolome and impacts systemic inflammation and experimental atherosclerosis. J. Nutr. Biochem. 2024, 126, 109562. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kubant, R.; Cho, C.E.; Kranenburg, E.; Beaudry, J.; Bottiglieri, T.; Anderson, G.H. Micronutrients in High-Fat Diet Modify Insulin Resistance and Its Regulatory Genes in Adult Male Mice. Mol. Nutr. Food Res. 2023, 67, e2300199. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.G.; Koehle, A.M.; Paudel, D.; Neuberger, T.; Ross, A.C.; Singh, V.; Bottiglieri, T.; Castro, R. Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice. Nutrients 2024, 16, 330. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Aliaga, I.; de Roos, B.; Sailer, M.; McLoughlin, G.A.; Boekschoten, M.V.; van Erk, M.; Bachmair, E.-M.; van Schothorst, E.M.; Keijer, J.; Coort, S.L.; et al. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol. Genom. 2011, 43, 408–416. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, Y.; Alexander, D.; Morrison, A.C.; Coresh, J.; Boerwinkle, E. Genetic determinants influencing human serum metabolome among African Americans. PLoS Genet. 2014, 10, e1004212. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, Y.; Nettleton, J.A.; Alexander, D.; Coresh, J.; Boerwinkle, E. Serum Metabolomic Profiling and Incident CKD among African Americans. Clin. J. Am. Soc. Nephrol. 2014, 9, 1410–1417. [Google Scholar] [CrossRef]
- Hu, J.-R.; Coresh, J.; Inker, L.A.; Levey, A.S.; Zheng, Z.; Rebholz, C.M.; Tin, A.; Appel, L.J.; Chen, J.; Sarnak, M.J.; et al. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018, 94, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Surapaneni, A.; Levey, A.S.; Sarnak, M.J.; Inker, L.A.; Appel, L.J.; Coresh, J.; Grams, M.E. The Serum Metabolome Identifies Biomarkers of Dietary Acid Load in 2 Studies of Adults with Chronic Kidney Disease. J. Nutr. 2019, 149, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Mwasongwe, S.E.; Young, B.; Bidulescu, A.; Sims, M.; Correa, A.; Musani, S.K. Relation of multi-marker panel to incident chronic kidney disease and rapid kidney function decline in African Americans: The Jackson Heart Study. BMC Nephrol. 2018, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C. Biomarkers in chronic kidney disease: Utility and issues towards better understanding. Curr. Opin. Nephrol. Hypertens. 2005, 14, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ntrinias, T.; Papasotiriou, M.; Balta, L.; Kalavrizioti, D.; Vamvakas, S.; Papachristou, E.; Goumenos, D.S. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Prilozi 2019, 40, 27–39. [Google Scholar] [CrossRef]
- Weekley, C.C.; Peralta, C.A. Advances in the use of multimarker panels for renal risk stratification. Curr. Opin. Nephrol. Hypertens. 2012, 21, 301–308. [Google Scholar] [CrossRef]
- Provenzano, M.; Andreucci, M.; De Nicola, L.; Garofalo, C.; Battaglia, Y.; Borrelli, S.; Gagliardi, I.; Faga, T.; Michael, A.; Mastroroberto, P.; et al. The Role of Prognostic and Predictive Biomarkers for Assessing Cardiovascular Risk in Chronic Kidney Disease Patients. BioMed Res. Int. 2020, 2020, 2314128. [Google Scholar] [CrossRef]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am. J. Nephrol. 2019, 49, 438–448. [Google Scholar] [CrossRef]
- James, P.A.; Oparil, S.; Carter, B.L.; Cushman, W.C.; Dennison-Himmelfarb, C.; Handler, J.; Lackland, D.T.; Lefevre, M.L.; MacKenzie, T.D.; Ogedegbe, O.; et al. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014, 311, 507–520. [Google Scholar] [CrossRef]
- Scialla, J.J.; Parekh, R.S.; Eustace, J.A.; Astor, B.C.; Plantinga, L.; Jaar, B.G.; Shafi, T.; Coresh, J.; Powe, N.R.; Melamed, M.L. Race, Mineral Homeostasis and Mortality in Patients with End-Stage Renal Disease on Dialysis. Am. J. Nephrol. 2015, 42, 25–34. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Judd, S.E.; Irvin, M.R.; Zhi, D.; Limdi, N.; Palmer, N.D.; Rich, S.S.; Sale, M.M.; Freedman, B.I. APOL1 nephropathy risk variants are associated with altered high-density lipoprotein profiles in African Americans. Nephrol. Dial. Transplant. 2015, 31, 602–608. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Parsa, A.; Isakova, T.; Scialla, J.J.; Chen, J.; Flack, J.M.; Nessel, L.C.; Gupta, J.; Bellovich, K.A.; Steigerwalt, S.; et al. Genetic African Ancestry and Markers of Mineral Metabolism in CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, O.M.; Rostand, S.G. Chronic kidney disease in African Americans: Past, present, and future: Introduction. Semin. Nephrol. 2013, 33, 407–408. [Google Scholar] [CrossRef]
- Kalecký, K.; German, D.C.; Montillo, A.A.; Bottiglieri, T. Targeted Metabolomic Analysis in Alzheimer’s Disease Plasma and Brain Tissue in Non-Hispanic Whites. J. Alzheimer’s Dis. 2022, 86, 1875–1895. [Google Scholar] [CrossRef]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jing, W.; Qian, W.; Fan, L.; Cheng, J. Association between dietary patterns and cardiovascular diseases: A review. Curr. Probl. Cardiol. 2024, 49, 102412. [Google Scholar] [CrossRef] [PubMed]
- Henning, R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: A review of the pathophysiology and treatment of obesity. Am. J. Cardiovasc. Dis. 2021, 11, 504–529. [Google Scholar]
- Saeed, D.; Reza, T.; Shahzad, M.W.; Mandokhail, A.K.; Bakht, D.; Qizilbash, F.H.; O Silloca-Cabana, E.; Ramadhan, A.; Bokhari, S.F.H. Navigating the Crossroads: Understanding the Link Between Chronic Kidney Disease and Cardiovascular Health. Cureus 2023, 15, e51362. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Cardiovascular-Kidney-Metabolic (CKM) syndrome: A state-of-the-art review. Curr. Probl. Cardiol. 2024, 49, 102344. [Google Scholar] [CrossRef]
- Minhas, A.M.K.; Talha, K.M.; Abramov, D.; Johnson, H.M.; Antoine, S.; Rodriguez, F.; Fudim, M.; Michos, E.D.; Misra, A.; Abushamat, L.; et al. Racial and ethnic disparities in cardiovascular disease—Analysis across major US national databases. J. Natl. Med. Assoc. 2024. [Google Scholar] [CrossRef]
- Goraya, N.; Simoni, J.; Jo, C.; Wesson, D.E. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012, 81, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, H.; Montgomery, A.H.; Khan, M.; Mamun, A.; Tecson, K.M.; Allison, P.; Simoni, J.; Wesson, D.E. The Fruit and Veggies for Kidney Health Study: A Prospective Randomized Trial. Kidney Med. 2023, 5, 100736. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Munoz-Maldonado, Y.; Simoni, J.; Wesson, D.E. Treatment of Chronic Kidney Disease-Related Metabolic Acidosis with Fruits and Vegetables Compared to NaHCO3 Yields More and Better Overall Health Outcomes and at Comparable Five-Year Cost. J. Ren. Nutr. 2021, 31, 239–247. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.K.; Symons, M.; Demark-Wahnefried, W.; Polhamus, B.; Bernhardt, J.M.; McClelland, J.W.; Washington, C. Stages of change and psychosocial correlates of fruit and vegetable consumption among rural African-American church members. Am. J. Health Promot. 1998, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Osei-Kwasi, H.; Mohindra, A.; Booth, A.; Laar, A.; Wanjohi, M.; Graham, F.; Pradeilles, R.; Cohen, E.; Holdsworth, M. Factors influencing dietary behaviours in urban food environments in Africa: A systematic mapping review. Public Health Nutr. 2020, 23, 2584–2601. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; Giacco, R.; Mennella, I.; Ferracane, R.; Pons, N.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut 2020, 69, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Joshi, S.; Schlueter, R.; Cooke, J.; Brown-Tortorici, A.; Donnelly, M.; Schulman, S.; Lau, W.-L.; Rhee, C.M.; Streja, E.; et al. Plant-Dominant Low-Protein Diet for Conservative Management of Chronic Kidney Disease. Nutrients 2020, 12, 1931. [Google Scholar] [CrossRef]
- Kuznik, A.; Mardekian, J.; Tarasenko, L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: An analysis of national health and nutritional examination survey data, 2001–2010. BMC Nephrol. 2013, 14, 132. [Google Scholar] [CrossRef]
- Rosenstein, K.T.L.; Tannock, L.R. Dyslipidemia in Chronic Kidney Disease. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305899/ (accessed on 18 May 2023).
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Guo, C.; Geng, X.; Yao, Y.; Guo, J.; Zhang, Y.; Zhang, J.; Mi, S. Effects of fruits and vegetables on gut microbiota in a mouse model of metabolic syndrome induced by high-fat diet. Food Sci. Nutr. 2023, 11, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, R.E.; Elvestad, M.; Molin, M.; Aune, D. Fruit and vegetable consumption and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis of prospective studies. BMJ Nutr. Prev. Health 2021, 4, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Tyni, T.; Pihko, H. Long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Acta Paediatr. 1999, 88, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, G.; Saini, A.; de Alba, C.G.; Gregor, A.; Harding, C.O.; Gillingham, M.B.; Vinocur, J.M. Cardiac phenotype in adolescents and young adults with long-chain 3-hydroxyacyl CoA dehydrogenase (LCHAD) deficiency. Anesthesia Analg. 2024, 26, 101123. [Google Scholar] [CrossRef] [PubMed]
- Wongkittichote, P.; Cuddapah, S.R.; Master, S.R.; Grange, D.K.; Dietzen, D.; Roper, S.M.; Ganetzky, R.D. Biochemical characterization of patients with dihydrolipoamide dehydrogenase deficiency. JIMD Rep. 2023, 64, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.J.; Pimpão, A.B.; Fernandes, D.G.F.; Morello, J.; Sequeira, C.O.; Calado, J.; Antunes, A.M.M.; Almeida, M.S.; Branco, P.; Monteiro, E.C.; et al. Cysteine as a Multifaceted Player in Kidney, the Cysteine-Related Thiolome and Its Implications for Precision Medicine. Molecules 2022, 27, 1416. [Google Scholar] [CrossRef]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J. Am. Hear. Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Bottlieri, T.; Tecson, K.M.; Kluger, A.Y.; McCullough, P.A. Alteration of lipid metabolism in chronic kidney disease, the role of novel antihyperlipidemic agents, and future directions. Rev. Cardiovasc. Med. 2018, 19, 77–88. [Google Scholar] [CrossRef]
- Bassi, R.; Niewczas, M.A.; Biancone, L.; Bussolino, S.; Merugumala, S.; Tezza, S.; D’addio, F.; Ben Nasr, M.; Valderrama-Vasquez, A.; Usuelli, V.; et al. Metabolomic Profiling in Individuals with a Failing Kidney Allograft. PLoS ONE 2017, 12, e0169077. [Google Scholar] [CrossRef]
- Arjmand, B.; Dehghanbanadaki, H.; Yoosefi, M.; Rezaei, N.; Fateh, S.M.; Ghodssi-Ghassemabadi, R.; Najjar, N.; Hosseinkhani, S.; Tayanloo-Beik, A.; Adibi, H.; et al. Association of plasma acylcarnitines and amino acids with hypertension: A nationwide metabolomics study. PLoS ONE 2023, 18, e0279835. [Google Scholar] [CrossRef] [PubMed]
- Toback, F.G. Phosphatidylcholine metabolism during renal growth and regeneration. Am. J. Physiol. Physiol. 1984, 246, F249–F259. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Corbacho, M.J.; Jenkins, R.W.; Clarke, C.J.; Hannun, Y.A.; Obeid, L.M.; Snider, A.J.; Siskind, L.J. Accumulation of long-chain glycosphingolipids during aging is prevented by caloric restriction. PLoS ONE 2011, 6, e20411. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, J.; Krueger, K.; Wong, D.W.; Klinkhammer, B.M.; Buhl, E.M.; Dehairs, J.; Swinnen, J.V.; Noels, H.; Jankowski, J.; Lebherz, C.; et al. 2,8-Dihydroxyadenine-induced nephropathy causes hexosylceramide accumulation with increased mTOR signaling, reduced levels of protective SirT3 expression and impaired renal mitochondrial function. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 166825. [Google Scholar] [CrossRef]
- Pignanelli, M.; Bogiatzi, C.; Gloor, G.; Allen-Vercoe, E.; Reid, G.; Urquhart, B.L.; Ruetz, K.N.; Velenosi, T.J.; Spence, J.D. Moderate Renal Impairment and Toxic Metabolites Produced by the Intestinal Microbiome: Dietary Implications. J. Ren. Nutr. 2019, 29, 55–64. [Google Scholar] [CrossRef]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.B.; Ortiz, A.; Sanchez-Niño, M.D. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef]
- Mutengo, K.H.; Masenga, S.K.; Mweemba, A.; Mutale, W.; Kirabo, A. Gut microbiota dependant trimethylamine N-oxide and hypertension. Front. Physiol. 2023, 14, 1075641. [Google Scholar] [CrossRef]
- El Chamieh, C.; Larabi, I.A.; De Pinho, N.A.; Lambert, O.; Combe, C.; Fouque, D.; Frimat, L.; Jacquelinet, C.; Laville, M.; Laville, S.; et al. Study of the association between serum levels of kynurenine and cardiovascular outcomes and overall mortality in chronic kidney disease. Clin. Kidney J. 2024, 17, sfad248. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Kalaska, B.; Pawlak, D. Kynurenine Pathway in Chronic Kidney Disease: What’s Old, What’s New, and What’s Next? Int. J. Tryptophan Res. 2020, 13, 1178646920954882. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, M.; Sun, J.; Lin, F.; Xu, D.; Chen, Y.; Song, W.; Li, Q.; Jiang, Y.; Gu, J.; et al. Identification of novel serum metabolic signatures to predict chronic kidney disease among Chinese elders using UPLC-Orbitrap-MS. J. Nutr. Health Aging 2024, 28, 100036. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Zampino, M.; Moaddel, R.; Chen, T.K.; Tian, Q.; Ferrucci, L.; Semba, R.D. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging. Metabolomics 2021, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Bazzano, L.A.; He, J.; Whelton, S.P.; Rebholz, C.M.; Fernandez, C.; Krousel-Wood, M.; Li, C.; Shi, M.; Nierenberg, J.L.; et al. Race modifies the association between animal protein metabolite 1-methylhistidine and blood pressure in middle-aged adults: The Bogalusa Heart Study. J. Hypertens. 2020, 38, 2435–2442. [Google Scholar] [CrossRef]
- Mitry, P.; Wawro, N.; Rohrmann, S.; Giesbertz, P.; Daniel, H.; Linseisen, J. Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. Eur. J. Clin. Nutr. 2019, 73, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, Q.; Wang, Y.; Lv, Y.; Liu, Q.Q. Metabolomics combined with clinical analysis explores metabolic changes and potential serum metabolite biomarkers of antineutrophil cytoplasmic antibody-associated vasculitis with renal impairment. PeerJ 2023, 11, e15051. [Google Scholar] [CrossRef]
- Gounden, V.B.H.; Jialal, I. Renal Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 18 May 2023).
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef] [PubMed]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Kalantar-Zadeh, K.; Fouque, D.; Wee, P.T.; Kovesdy, C.P.; Price, S.R.; Kopple, J.D. Precision Medicine for Nutritional Management in End-Stage Kidney Disease and Transition to Dialysis. Semin. Nephrol. 2018, 38, 383–396. [Google Scholar] [CrossRef]
- Betts, J.A.; Gonzalez, J.T. Personalised nutrition: What makes you so special? Nutr. Bull. 2016, 41, 353–359. [Google Scholar] [CrossRef]
- Mathews, A.T.; Famodu, O.A.; Olfert, M.D.; Murray, P.J.; Cuff, C.F.; Downes, M.T.; Haughe, N.J.; Colby, S.E.; Olfert, I.M.; McFadden, J.W. Fruit and Vegetable Intervention Lowers Circulating Ceramide Levels and Improves Estimated Insulin Sensitivity in Young Adults at Risk of Developing Metabolic Syndrome: A FRUVEDomic Pilot Study. FASEB J. 2016, 30, 1260–1263. [Google Scholar] [CrossRef]
- Joshi, S.; Hashmi, S.; Shah, S.; Kalantar-Zadeh, K. Plant-based diets for prevention and management of chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, P.; Koppe, L.; Combe, C.; Lasseur, C.; Trolonge, S.; Aparicio, M. Vegetarian diets and chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Manjarrez, N.; Ulaszewska, M.; Garcia-Aloy, M.; Mattivi, F.; Praticò, G.; Dragsted, L.O.; Manach, C. Biomarkers of intake for tropical fruits. Genes Nutr. 2020, 15, 11. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; Brandl, B.; Buso, M.E.C.; Skurk, T.; Manach, C. Food intake biomarkers for green leafy vegetables, bulb vegetables, and stem vegetables: A review. Genes Nutr. 2020, 15, 7. [Google Scholar] [CrossRef]


| Variable | All | Intervention (FV) | Control (WL) |
|---|---|---|---|
| N | 91 | 46 | 45 |
| Age, mean (SD) | 58.3 (10.1) | 58.5 (8.5) | 58.2 (11.6) |
| Sex, n (%) | |||
| Female | 60 (66) | 26 (57) | 34 (46) |
| Male | 31 (34) | 20 (43) | 11 (24) |
| Income, n (%) | |||
| USD0 to less than USD15,000 | 30 (33.0) | 17 (37.0) | 13 (28.8) |
| USD15,000 to less than USD25,000 | 14 (15.4) | 7 (15.2) | 7 (15.6) |
| USD25,000 to less than USD50,000 | 15 (16.5) | 6 (13.0) | 9 (20.0) |
| USD50,000 to 75,000 | 15 (16.5) | 8 (17.4) | 7 (15.6) |
| More than USD75,000 | 7 (7.6) | 3 (6.5) | 4 (8.9) |
| Missing | 10 (11.0) | 5 (10.9) | 5 (11.1) |
| Education, n (%) | |||
| Some high school | 16 (17.6) | 8 (17.4) | 8 (17.8) |
| High school diploma | 13 (14.3) | 7 (15.2) | 6 (13.3) |
| Technical degree | 3 (3.3) | 1 (2.2) | 2 (4.4) |
| Some college | 20 (22.0) | 9 (19.6) | 11 (24.4) |
| College degree | 28 (30.8) | 15 (32.6) | 13 (28.9) |
| Missing | 11 (12.0) | 6 (13.0) | 5 (11.1) |
| Marital status, n (%) | |||
| Married | 27 (29.7) | 14 (30.4) | 13 (28.9) |
| Divorced | 11 (12.1) | 6 (13.0) | 5 (11.1) |
| Separated | 4 (4.4) | 3 (6.5) | 1 (2.2) |
| Single | 32 (35.2) | 15 (32.6) | 17 (37.8) |
| Widowed | 4 (4.4) | 2 (4.3) | 2 (4.4) |
| Living with partner/Unmarried | 6 (6.6) | 3 (6.5) | 3 (6.6) |
| Missing | 7 (7.6) | 3 (6.5) | 4 (8.9) |
| Health Outcomes, Mean (SD) | |||
| SBP (mmHg) | 136.0 (18.1) | 139.4 (19.5) | |
| DBP (mmHg) | 82.5 (13.1) | 81.8 (12.3) | |
| HDL (mg/dL) | 54.9 (18.3) | 52.0 (19.1) | |
| LDL (mg/dL) | 100.2 (41.4) | 92.9 (34.5) | |
| TC (mg/dL) | 175.0 (41.3) | 168.2 (43.9) | |
| TG (mg/dL) | 115.5 (47.5) | 118.6 (62.8) | |
| BMI (kg/m2) | 35.8 (7.3) | 34.3 (4.3) | |
| HbA1C (%) | 6.7 (1.6) | 6.4 (1.2) | |
| ACR (mg/g) | 20.8 (22.1) | 28.2 (34.0) |
| Variables (Mean (SD)) | Intervention (FV) (6 Weeks—Baseline), n = 38 | Control (Waitlist) (6 Weeks—Baseline), n = 30 | p Value |
|---|---|---|---|
| SBP (mmHg) | −2.9 (16.4) | −2.4 (16.0) | 0.89 |
| DBP (mmHg) | 0.88 (13.1) | −0.09 (13.3) | 0.76 |
| HDL (mg/dL) | 1.5 (10.6) | 1.7 (7.4) | 0.94 |
| LDL (mg/dL) | 3.0 (24.0) | 2.2 (22.2) | 0.91 |
| TC (mg/dL) | −1.3 (23.8) | 0.30 (22.4) | 0.78 |
| TG (mg/dL) | 3.6 (49.8) | −3.0 (51.6) | 0.63 |
| BMI (kg/m2) | −0.17 (0.96) | −0.25 (0.82) | 0.76 |
| HbA1C (%) | 0.02 (0.38) | −0.06 (0.42) | 0.45 |
| ACR (mg/g) | −5.5 (19.9) | 1.8 (24.8) | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.J.; Emerenini, C.; Wang, X.; Bottiglieri, T.; Kitzman, H. Metabolomic and Physiological Effects of a Cardiorenal Protective Diet Intervention in African American Adults with Chronic Kidney Disease. Metabolites 2024, 14, 300. https://doi.org/10.3390/metabo14060300
Patel MJ, Emerenini C, Wang X, Bottiglieri T, Kitzman H. Metabolomic and Physiological Effects of a Cardiorenal Protective Diet Intervention in African American Adults with Chronic Kidney Disease. Metabolites. 2024; 14(6):300. https://doi.org/10.3390/metabo14060300
Chicago/Turabian StylePatel, Meera J., Chiamaka Emerenini, Xuan Wang, Teodoro Bottiglieri, and Heather Kitzman. 2024. "Metabolomic and Physiological Effects of a Cardiorenal Protective Diet Intervention in African American Adults with Chronic Kidney Disease" Metabolites 14, no. 6: 300. https://doi.org/10.3390/metabo14060300
APA StylePatel, M. J., Emerenini, C., Wang, X., Bottiglieri, T., & Kitzman, H. (2024). Metabolomic and Physiological Effects of a Cardiorenal Protective Diet Intervention in African American Adults with Chronic Kidney Disease. Metabolites, 14(6), 300. https://doi.org/10.3390/metabo14060300









