Is Lipid Metabolism of Value in Cancer Research and Treatment? Part I- Lipid Metabolism in Cancer
Abstract
:1. Introduction
2. Lipid Categories and Their Major Biological Functions [5,6,7,8,9,10,11]
- Fatty acyls: The molecules in fatty acyl groups are synthesized by chain elongation of acetyl coenzyme A (CoA) primers with malonyl–CoA groups. They may contain cyclic functional groups and/or be substituted by heteroatoms. The structure of fatty acyls is the simplest, as they are characterized by a repeating series of methylene groups. Given their simplicity, fatty acyls function as the basic components of other, more complex lipids.
- Prenol lipids: Five-carbon isoprene units represent the basic building blocks for prenol lipids. They may contain linear, cyclic or branched isoprene chains, any of which may consist of fatty acids, characteristic side-chains, functional groups, sugars, and double bonds. Given the range of permutations, these basic structures may form thousands of unique lipids. Prenol lipids are based on one or multiple five-carbon isoprene units, the building blocks of prenol lipids. The isoprene chain can be linear, cyclic or branched and features fatty acids, characteristic side-chains, functional groups, sugars and double bonds. The flexibility of the prenol lipids’ structure gives rise to thousands of different lipid species.
- Glycerolipids: The glycerolipid groups include monoacylglycerol, diacylglycerol, triacylglycerol (TAG) and glycolipids, and they are characterized by the presence of a glycerol backbone with fatty acyl chains connected to the hydroxyl groups of glycerol. Glycerolipids can be hydrolyzed into glycerol, non-esterified fatty acid (NEFA) and/or alkyl variants; they contribute to energy storage, energy metabolism and signal transduction.
- Glycerophospholipids: Glycerophospholipids (GPLs) contain at least one glycerol hydroxyl group esterified with one phosphate or phosphonate group. This category includes phosphatidylcholine (PC), phosphatidyl ethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidylglycerol (PG), phosphatidic acid (PA), cardiolipin, bis(monoacylglycerol) phosphate and lysophospholipids, etc. GPLs represent the bulk of cellular and plasma membranes and are one of their key components. GPLs contribute to second messenger generation and are involved in cellular metabolism and signal transduction.
- Sphingolipids: Sphingolipid groups act to control cellular signaling; they are components of plasma membranes. They are characterized by their core structure, specifically long-chain sphingoids such as ceramide (Cer), sphingomyelin, cerebroside, sulfatide and gangliosides.
- Saccharolipids: The saccharolipids are a group of lipids which contain a sugar backbone directly connected with fatty acids (as opposed to the glycerol backbone in glycerolipids and glycerophospholipids). They exist of glycans or phosphorylated derivative forms.
- Sterol lipids: Sterol lipids are compounds containing four fused rings. Among the subcategories of sterol lipids are cholesterol and its derivatives, steroids, bile acids and their derivatives, etc. Cholesterol, along with its derivatives, is a vital component of cellular membranes. Steroids function as hormones and signaling molecules and are important to a variety of additional biological and metabolic processes.
- Polyketides: The polyketides are a group of metabolites with characteristics similar to lipids. They come from plant and microbial sources. This category of lipids functions as cellular membrane components, energy storage depots and signaling molecules (Table 1).
3. Lipidomics Studies in Cancer Samples (MS Tech)
3.1. Sample Preparation and Lipid Extraction
- Modified Bligh and Dyer. Chloroform/methanol/H2O (1:1:0.9, v:v:v) for extraction of a small amount of biological sample (e.g., <50 mg of tissue). It is well-established and broadly practiced. The disadvantages include the use of hazardous chloroform and the collection of chloroform extract from the bottom layer, which may cause the carry-over of water-soluble impurities and difficulty in automation.
- Modified Folch. Chloroform/methanol (2:1, v:v), 0.9% NaCl (0.2 volume) is used to extract biological tissue (e.g., ~0.1 g). Water or 0.9% NaCl (0.2 volume) is then added to wash the solvent extract. It has similar advantages and disadvantages to the modified Bligh and Dyer method.
- Methyl tert-butyl ether (MTBE) method. The ratios are MTBE/methanol/water (5:1.5:1.45, v:v:v). This method resolves some of the difficulties in chloroform-involved methods because MTBE is present in the top layer after phase separation and therefore is more feasible for high throughput and automation. A disadvantage is that the MTBE phase contains a significant amount of aqueous components that may produce carry-over of water-soluble contaminants.
- Butanol/methanol method. Start with a volume of butanol/methanol (BUME, 3:1, v/v) added to a small volume of aqueous phase. Next, an equal volume of heptane/ethyl acetate (3:1, v/v) is added, and then 1% acetic acid is added (equal volume to BUME) to induce phase separation. This method may compensate for the issue with the previous method, with less water-soluble contaminants being carried over in the organic phase. Its drawback is the difficulty in evaporation of the butanol component in the organic phase.
3.2. Lipid Separation by HPLC
- Reversed-phase LC. This type of LC works by separating lipids based on their hydrophobicity. Although retention times vary between individual lipids, this remains the most commonly used method for working with complex lipids. Lipidomics research generally employs short columns (range of 50–150 mm; typically, 100); particle sizes either less than 2 µm or fused-core between 2.6 and 2.8 µm; and sorbents modified with C18 or C8
- Normal-phase LC. This type of LC is a good complement to reversed-phase LC. It uses highly non-polar solvents with low ionization capacity to separate lipids based on their polarities, which makes it an excellent separation mechanism for phospholipids, especially PA. With its longer retention times, generally between 30 and 60 min, it improves separation of large particle sizes on long columns. Lipid classes may be separated using this technique.
- Hydrophilic interaction liquid chromatography. This technique is more reproducible and robust, with the added benefit of greater compatibility with MS. However, the physical properties of either reversed-phase or normal-phase columns are somewhat compromised.
- Supercritical fluid chromatography. Using columns packed with sub-2 µm particles, this method is a newly emerging technique that can be used for fast lipid profiling (<20 min).
- Two-dimensional liquid chromatography. In this technique, the injected sample is separated by passing through two different separation stages. This is accomplished through sequentially connecting two different chromatographic columns, with the effluent from the first system being transferred onto the second column. It can optimize the separation conditions of complex lipidomes. One drawback is that it requires more labor and time.
3.3. Summary of Modern MS-Based Lipidomics Approaches (Table 3)
- LC-MS/MS
- Shotgun lipidomics
- MALDI-MS
- MS imaging
- Ion-mobility MS
- Global Lipidomic Analysis: This type of approach, referred to as a “shotgun” technique, offers the capability to identify and quantify large numbers of cellular lipids on a high-throughput basis. Several of these techniques are widely used for analyses of wide-ranging pathways and networks involved with metabolism, trafficking and homeostasis of lipids. Recent developments in mapping techniques provide support for research into spatial and temporal relationships among lipids.
- These methods use LC-MS and LC-MS/MS to identify a Targeted Lipidomic Analysis: much smaller set of lipids of interest.
- Novel Lipid Discovery: Techniques using LC coupled with MS work with different enrichment technologies with the aim of finding novel classes and molecular species of lipids. This type of analysis involving lipid mediators in biological samples is complicated by their low concentrations in biological samples, their transient nature and limited half-lives.
| Parameter | Type of MS | ||||
|---|---|---|---|---|---|
| Shotgun MS | LC-MS/MS | MALDI-MS | MS Imaging | Ion-Mobility MS | |
| Reproducibility | High | High | Medium | Medium | High |
| Sensitivity | Low | High | Medium | Medium | High |
| Resolution | Low | High | High | High | High |
| Quantitativity | Yes | Yes, with IS | Yes | Yes | Yes, with IS |
| Sample preparation | Minimal | Extensive sample preparation steps | Minimal | Minimal | Extensive sample preparation steps |
| Structural information | Yes | Yes | No | No | Yes |
| High throughput | Yes, but dynamic range might be affected with coexistence of other major lipids | Yes | No | No | Yes |
| Information/Key point | - Uses lipid chemistry and physics for analysis under constant concentrations - Infuse sample directly into the MS - Less time-consuming and low cost - High reproducibility - Low sensitivity incapable of distinguishing isomers - Application-typical building blocks include glycerol, sphingoid bases, polar head groups and fatty acyl substituents (or other aliphatic chains). | - Uses pre-separation of lipids - Resolves lipid classes and/or molecular species before MS analysis - Powerful for targeted analysis of very low abundance lipid class, but fairly slow for comprehensive analysis of cellular lipidomes - The most commonly used method in lipidomics; able to analyze a wide variety of lipids - High separation efficiency and sensitivity - Organic solvent Consumption Metabolite target analysis - Metabolite profiling Lipidomics | - Provides rapid screening and can identify lipid classes with high sensitivity - Comprehensive analysis is limited | - Useful for determining spatial distribution of lipids - Largely qualitative comparison - Ionize sample by coating with matrix and irradiating laser - High resolution; the most established technique - Requires matrix pretreatment | - A post-ionization separation technique based on the masses, charges, sizes and shapes of lipids - Might enable the analysis of isomeric and isobaric species - Comprehensive analysis under development |
3.4. Ionization Technique
- ESI MS. This technique works with the gaseous ions generated from polar, thermally labile and mainly non-volatile molecules and is useful for a variety of lipids. As a soft-ionization method, its effects on the sample chemistry are minimal prior to analysis. There are a number of ESI-MS techniques which enable analysis of a variety of classes, subclasses and individual lipid species within biological extracts. One technique, referred to as “shotgun lipodomics”, has been developed by Han and his team. For this method, a crude lipid extract is infused directly into an ESI source which is tuned for separation of lipids using their intrinsic electrical properties.
- APCI MS. While it is similar to ESI, this method relies on the generation of ions as the heated analyte solvent interacts with a corona discharge needle set for a high electrical potential. As primary ions form in the immediate vicinity of the needle, their interaction with the solvent forms secondary ions, which, in turn, ionize the sample. This is a very good technique for the analysis of nonpolar lipids such as triacylglycerols, sterols and fatty acid esters.
- DESI MS. DESI mass spectrometry is an ambient ionization technique which combines desorption ionization with ESI. This is accomplished by directing an electrically charged mist to the sample surface that is a few millimeters away, making it an excellent tool for mapping lipid distribution contained in biological samples. A major advantage is that, because no matrix is required for tissue preparation, DESI MS allows for multiple consecutive measurements on the same tissue specimen.
- MALDI MS. This technique, typically employed in the analysis of large proteins, has proven beneficial in lipid studies. A mixture containing a matrix such as 2,5-dihydroxybenzoic acid and the lipid sample is placed on the sample holder in a small spot. When a laser is fired at the spot, the matrix absorbs the energy, subsequently transferring it to the analyte, resulting in ionization of the molecule. MALDI–time-of-flight (MALDI-TOF) MS has shown great promise as an approach for lipidomics studies, particularly for the imaging of lipids from tissue slides.
4. The Importance of Lipid Metabolism in Oncology
4.1. Fatty Acid (FA)
4.2. Cholesterol
4.3. Triacylglycerol and Lipid Droplets
4.4. Phospholipid
4.5. Glycosphingolipids (GSLs)
5. Lipid Metabolism in Cancer Cells
6. Lipid Drug Candidates for Cancer
7. Challenges for Lipidomics
- The unique nature, complexity and specificity of lipodomics research make it exciting and challenging. For example, mapping of lipidomes is not possible with present equipment and techniques, which are unable to provide the detailed information necessary to quantify individual lipid molecules present in an organism. The diversity of lipid classes and lipid molecular species complicates efforts for the structural identification of lipids by mass spectrometry and renders it impossible to accommodate all lipid classes with a common method for extraction, chromatography and detection. Several methods need to be applied.
- LC-MS is gaining favor over “shotgun” or direct infusion methods in lipodomics research, given its enhanced specificity and sensitivity. The greater specificity of MS/MS is invaluable for elucidating additional levels of detail which is crucial for identifying individual acyl chains.
- One of the challenges related to MS-based lipidomics is the complexity of the sample, with a small mass range between 300 and 900 Da. The classical quadrupoles have a limited mass resolution, and, usually, multiple lipid species with similar masses (referred to as isobaric species) are co-detected, hampering the exact assignment of a mass to a specific individual lipid species. The use of mass spectrometers with higher resolution of mass offers a partial solution to this issue. One example is the time-of-flight (TOF) analyzer, which uses the m/z-dependent acceleration of ions in a flight tube; another is the Orbitrap, where ions oscillate around an inner rod. Use of a Fourier transformation with the measured ion oscillations gives the Orbitrap excellent resolution of much smaller mass differences.
- The differences in ionization efficiency between species present a clear challenge in lipodomics research, though it is enhanced in some situations by the addition of salts such as ammonium acetate. Prior to extraction, one or multiple internal standards at known concentrations for each lipid class of interest are spiked.
- Reference standards and relevant internal standards remain limited.
- With the acquisition of the massive output of data generated, and subsequently requiring processing, the voracious demand for computer power and bioinformation clearly represents another major difficulty in MS-based lipodomics research. Spectral alignment and statistical evaluation of fluctuating signal intensities demands significant effort during the collection of chromatographic and MS data. The causes for these discrepancies include biological variations, sample handling issues and analytical accuracy; reliable resolution of lipid levels within a complex mixture may require additional replications.
- Software packages offer help for identifying correlations between lipid metabolites and physiological phenotypes, especially for developing lipid-based biomarkers. Given the extreme complexity of some lipid pathways, such as the mammalian glycosphingolipid pathway, information technology in lipodomics should help in constructing metabolic maps from data on lipid structures and lipid-related proteins and genes.
- There is a need to create searchable and interactive databases of lipids and lipid-related genes/proteins. Such databases can be integrated with MS and other experimental data, as well as with metabolic networks. These combined resources will lead to improved therapeutic strategies to prevent or reverse these pathological states involving dysfunction of lipid-related processes.
8. Common Software and Databases Used in Lipidomics
- METLIN, data analysis based on MS/MS spectra, ISF 0eV, https://enigma.lbl.gov/metlin-new-metabolite-identification/
- MZmine 3.3.0, LC-MS data analysis workflow, http://mzmine.sourceforge.net/
- Lipid View software 1.2 EULA, analysis based on electrospray MS data, https://sciex.com/products/software/lipidview-software
- LipidXplorer 1.2.8.1, data analysis based on MS and MS/MS spectra, https://lifs-tools.org/lipidxplorer.html
- LipidSearch 5.1 Software, automatically identify and relatively quantify LC-MS data, http://www.thermoscientific.com/content/tfs/en/product/lipidsearch-software.html
- LIPID MAPS, website, lipid structure, annotation, classification and pathways, analytical methods, http://www.lipidmaps.org
- Lipid Bank, data base, lipid structure, name, spectra and literature information, http://lipidbank.jp
- Lipid Library, lipid chemical, biological and analytical, http://lipidlibrary.co.uk
- LipidBlast, in silico tandem MS database for lipid identification, https://fiehnlab.ucdavis.edu/projects/lipidblast/
- CyberLipids, lipid structure and analytical methods, http://www.cyberlipid.org
- KEGG, synthesis and degradation of fatty acid, metabolic pathways of lipid, http://www.genome.jp/kegg/
- Metlin, MS/MS database, https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage
- Cholesterol ester: http://ctdbase.org/detail.go?type=chem&acc=D002788&view=disease
- Glycosyltransferases, https://www.genenames.org/data/genegroup/#!/group/424
- Lipid Identification and Characterization using SimLipid. This is a high-throughput lipid identification and quantification software. It can process raw data from hundreds of LC-MS and MS/MS experimental runs for LC-peak detection, peak-picking, molecular feature finding and retention time alignment.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffths, W.J.; Ogundare, M.; Williams, C.M.; Wang, Y. On the future of ‘omics’: Lipidomics. J. Inherit. Metab. Dis. 2011, 34, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipidomics: Comprehensive Mass Spectrometry of Lipids/Xianlin Han; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bach D and Wachtel E, Phospholipid/cholesterol model membranes: Formation of cholesterol crystallites. Biochim. Biophys. Acta 2003, 1610, 187–197. [CrossRef] [PubMed]
- Han, X.; Gross, R.W. The Foundations and Development of Lipidomics. J. Lipid Res. 2022, 63, 100164. [Google Scholar] [CrossRef] [PubMed]
- Tsai MJ and O’Malley BW, Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994, 63, 451–486. [CrossRef] [PubMed]
- Kuzuyama, T.; Seto, H. Diversity of the biosynthesis of the isoprene units. Nat. Prod. Rep. 2003, 20, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Beloribi-Djefafia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Addepalli, R.V.; Mullangi, R. A concise review on lipidomics analysis in biological samples. ADMET DMPK 2021, 9, 1–22. [Google Scholar]
- Sorgi, C.A.; Peti, A.P.; Petta, T.; Meirelles, A.F.; Fontanari, C.; Moraes, L.A.; Faccioli, L.H. Comprehensive high-resolution multiple-reaction monitoring mass spectrometry for targeted eicosanoid assays. Sci. Data 2018, 5, 180167. [Google Scholar] [CrossRef]
- Züllig, T.; Trötzmüller, M.; Köfeler, H.C. Lipidomics from sample preparation to data analysis: A primer. Anal. Bioanal. Chem. 2020, 412, 2191–2209. [Google Scholar] [CrossRef]
- Seppanen-Laakso, T.; Oresic, M. How to study lipidomes. J. Mol. Endocrinol. 2009, 42, 185–190. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Del Boccio, P.; Pieragostino, D.; di Ioia, M.; Petrucci, F.; Lugaresi, A.; De Luca, G.; Gambi, D.; Onofrj, M.; Di Ilio, C.; Sacchetta, P.; et al. Lipidomic investigations for the characterization of circulating serum lipids in multiple sclerosis. J. Proteom. 2011, 74, 2826–2836. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, applications, and outcomes related to biomedical sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Wang, M.; Han, X. Multidimensional mass spectrometry-based shotgun lipidomics. Methods Mol. Biol. 2014, 1198, 203–220. [Google Scholar]
- Blanksby, S.J.; Mitchell, T.W. Advances in Mass Spectrometry for Lipidomics. Annu. Rev. Anal. Chem. 2010, 3, 433–465. [Google Scholar] [CrossRef]
- Harkewicz, R.; Dennis, E.A. Applications of Mass Spectrometry to Lipids and Membranes. Annu. Rev. Biochem. 2011, 80, 301–325. [Google Scholar]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Sandra, K.; Sandra, P. Lipidomics from an analytical perspective. Curr. Opin. Chem. Biol. 2013, 17, 847–853. [Google Scholar] [CrossRef]
- Massey, K.A.; Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 2011, 39, 1240–1246. [Google Scholar] [CrossRef]
- Sun, T.; Chen, J.; Yang, F.; Zhang, G.; Chen, J.; Wang, X.; Zhang, J. Lipidomics reveals new lipid-based lung adenocarcinoma early diagnosis model. EMBO Mol. Med. 2024, 16, 854–869. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Han, X. Applications of mass spectrometry for cellular lipid analysis. Mol. BioSyst. 2015, 11, 698–713. [Google Scholar] [CrossRef]
- Pulfer, M.; Murphy, R.C. Electrospray mass spectrometry of phospholipids. Mass Spectrom. Rev. 2003, 22, 332–364. [Google Scholar] [CrossRef]
- Fuchs, B.; Suss, R.; Schiller, J. An update of MALDI-TOF mass spectrometry in lipid research. Prog. Lipid Res. 2011, 49, 450–475. [Google Scholar] [CrossRef]
- Serna, J.; Garcia-Seisdedos, D.; Alcazar, A.; Lasuncion, M.A.; Busto, R.; Pastor, O. Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2015, 189, 7–18. [Google Scholar] [CrossRef]
- Han, X.; Yang, K.; Gross, R.W. Multi-dimensional Mass Spectrometry-based Shotgun Lipidomics and Novel Strategies for Lipidomic Analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel Advances in Shotgun Lipidomics for Biology and Medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J. Mass-spectrometry-based Lipidomics. J. Sep. Sci. 2018, 41, 351–372. [Google Scholar] [CrossRef]
- Eberlin, L.S.; Dill, A.L.; Costa, A.B.; Ifa, D.R.; Cheng, L.; Masterson, T.; Koch, M.; Ratliff, T.L.; Cooks, R.G. Cholesterol Sulfate Imaging in Human Prostate Cancer Tissue by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2010, 82, 3430–3434. [Google Scholar] [CrossRef]
- Angerer, T.B.; Magnusson, Y.; Landberg, G.; Fletcher, J.S. Lipid Heterogeneity Resulting from Fatty Acid Processing in the Human Breast Cancer Microenvironment Identified by GCIB-ToF-SIMS Imaging. Anal. Chem. 2016, 88, 11946–11954. [Google Scholar] [CrossRef]
- Banerjee, S.; Zare, R.N.; Tibshirani, R.J.; Kunder, C.A.; Nolley, R.; Fan, R.; Brooks, J.D.; Sonn, G.A. Diagnosis of Prostate Cancer by Desorption Electrospray Ionization Mass Spectrometric Imaging of Small Metabolites and Lipids. Proc. Natl. Acad. Sci. USA 2017, 114, 3334–3339. [Google Scholar] [CrossRef]
- Ling, Y.S.; Liang, H.; Lin, M.; Tang, C.; Wu, K.; Kuo, M.; Lin, C.Y. Two-dimensional LC-MS/MS to Enhance Ceramide and Phosphatidylcholine Species Profiling in Mouse Liver. Biomed. Chromatogr. 2014, 28, 1284–1293. [Google Scholar] [CrossRef]
- Sarafian, M.H.; Gaudin, M.; Lewis, M.R.; Martin, F.P.; Holmes, E.; Nicholson, J.K.; Dumas, M.E. Objective Set of Criteria for Optimization of Sample Preparation Procedures for Ultra-High Throughput Untargeted Blood Plasma Lipid Profiling by Ultra Performance Liquid Chromatography−Mass Spectrometry. Anal. Chem. 2014, 86, 5766–5774. [Google Scholar] [CrossRef]
- Nassar, A.-E.F.; Talaat, R. Strategies for Dealing with Metabolite Elucidation in Drug Discovery and Development. Drug Discov. Today 2004, 9, 317–327. [Google Scholar] [CrossRef]
- Shimma, S.; Sugiura, Y.; Hayasaka, T.; Hoshikawa, Y.; Noda, T.; Setou, M. MALDI-based imaging mass spectrometry revealed abnormal distribution of phospholipids in colon cancer liver metastasis. J. Chromatogr. 2007, B855, 98–103. [Google Scholar] [CrossRef]
- Kendall, A.C.; Koszyczarek, M.M.; Jones, E.A.; Hart, P.J.; Towers, M.; Griffiths, C.E.; Morris, M.; Nicolaou, A. Lipidomics for translational skin research: A primer for the uninitiated. Exp. Dermatol. 2018, 27, 721–728. [Google Scholar] [CrossRef]
- Goto-Inoue, N.; Hayasaka, T.; Zaima, N.; Setou, M. Imaging mass spectrometry for lipidomics. Biochim. Et Biophys. Acta –Mol. Cell Biol. Lipids 2011, 1811, 961–969. [Google Scholar] [CrossRef]
- Bokhart, M.T.; Muddiman, D.C. Infrared matrix-assisted laser desorption electrospray ionization mass spectrometry imaging analysis of biospecimens. Analyst 2016, 141, 5236–5245. [Google Scholar] [CrossRef]
- Meier, F.; Garrard, K.P.; Muddiman, D.C. Silver dopants for targeted and untargeted direct analysis of unsaturated lipids via infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI). Rapid Commun. Mass Spectrom. 2014, 28, 2461–2470. [Google Scholar] [CrossRef]
- JonesJones, E.A.; Simon, D.; Karancsi, T.; Balog, J.; Pringle, S.D.; Takats, Z. Matrix assisted rapid evaporation ionisation mass spectrometry. Anal. Chem. 2019, 91, 9784–9791. [Google Scholar] [CrossRef]
- Nassar, A.F.; Wu, T.; Nassar, S.F.; Wisnewski, A.V. UPLC–MS for metabolomics: A giant step forward in support of pharmaceutical research. Drug Discov. Today 2016, 22, 463–470. [Google Scholar] [CrossRef]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar]
- Hall, Z.; Ament, Z.; Wilson, C.H.; Burkhart, D.L.; Ashmore, T.; Koulman, A.; Littlewood, T.; Evan, G.I.; Griffin, J.L. Myc Expression drives aberrant lipid metabolism in lung cancer. Cancer Res. 2016, 76, 4608–4618. [Google Scholar] [CrossRef]
- Lee, G.K.; Lee, H.S.; Park, Y.S.; Lee, J.H.; Lee, S.C.; Lee, J.H.; Lee, S.J.; Shanta, S.R.; Park, H.M.; Kim, H.R.; et al. Lipid MALDI profile classifies non-small cell lung cancers according to the histologic type. Lung Cancer 2012, 76, 197–203. [Google Scholar] [CrossRef]
- Pirman, D.A.; Efuet, E.; Ding, X.P.; Pan, Y.; Tan, L.; Fischer, S.M.; DuBois, R.N.; Yang, P. Changes in cancer cell metabolism revealed by direct sample analysis with MALDI mass spectrometry. PLoS ONE 2013, 8, e61379. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Qiu, L.; Qin, X.; Liu, H.; Wang, Y.; Li, F.; Wang, X.; Chen, G.; Song, G.; et al. Probing gender-specific lipid metabolites and diagnostic biomarkers for lung cancer using Fourier transform ion cyclotron resonance mass spectrometry. Clin. Chim. Acta 2012, 414, 135–141. [Google Scholar] [CrossRef]
- Marien, E.; Meister, M.; Muley, T.; Fieuws, S.; Bordel, S.; Derua, R.; Spraggins, J.; van de Plas, R.; Dehairs, J.; Wouters, J.; et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int. J. Cancer 2015, 137, 1539–1548. [Google Scholar] [CrossRef]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Muller, B.; Brockmoller, S.; Seppanen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel theranostic opportunities offered by characterization of altered membrane lipid metabolism in breast cancer progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, S.C.; Park, Y.S.; Jeon, Y.E.; Lee, J.H.; Jung, S.Y.; Park, I.H.; Jang, S.H.; Park, H.M.; Yoo, C.W.; et al. Protein and lipid MALDI profiles classify breast cancers according to the intrinsic subtype. BMC Cancer 2011, 11, 465–474. [Google Scholar] [CrossRef]
- Chughtai, K.; Jiang, L.; Greenwood, T.R.; Glunde, K.; Heeren, R.M. Mass spectrometry images acylcarnitines, phosphatidylcholines, and sphingomyelin in MDA-MB-231 breast tumor models. J. Lipid Res. 2013, 54, 333–344. [Google Scholar] [CrossRef]
- Min, H.K.; Kong, G.; Moon, M.H. Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography—tandem mass spectrometry: II. Negative ion mode analysis of four phospholipid classes. Anal. Bioanal. Chem. 2010, 396, 1273–1280. [Google Scholar] [CrossRef]
- Kawashima, M.; Iwamoto, N.; Kawaguchi-Sakita, N.; Sugimoto, M.; Ueno, T.; Mikami, Y.; Terasawa, K.; Sato, T.A.; Tanaka, K.; Shimizu, K.; et al. High-resolution imaging mass spectrometry reveals detailed spatial distribution of phosphatidylinositols in human breast cancer. Cancer Sci. 2013, 104, 1372–1379. [Google Scholar] [CrossRef]
- Doria, M.L.; Cotrim, C.Z.; Simoes, C.; Macedo, B.; Domingues, P.; Domingues, M.R.; Helguero, L.A. Lipidomic analysis of phospholipids from human mammary epithelial and breast cancer cell lines. J. Cell. Physiol. 2013, 228, 457–468. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, K.M.; Kim, S.H.; Kwon, Y.J.; Chun, Y.J.; Choi, H.K. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget 2016, 7, 67111–67128. [Google Scholar] [CrossRef]
- Cifkova, E.; Holcapek, M.; Lisa, M.; Vrana, D.; Gatek, J.; Melichar, B. Determination of lipidomic differences between human breast cancer and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. Anal. Bioanal. Chem. 2015, 407, 991–1002. [Google Scholar] [CrossRef]
- Hilvo, M.; Gade, S.; Hyotylainen, T.; Nekljudova, V.; Seppanen-Laakso, T.; Sysi-Aho, M.; Untch, M.; Huober, J.; von Minckwitz, G.; Denkert, C.; et al. Monounsaturated fatty acids in serum triacylglycerols are associated with response to neoadjuvant chemotherapy in breast cancer patients. Int. J. Cancer 2014, 134, 1725–1733. [Google Scholar] [CrossRef]
- Wei, S.; Liu, L.; Zhang, J.; Bowers, J.; Gowda, G.A.; Seeger, H.; Fehm, T.; Neubauer, H.J.; Vogel, U.; Clare, S.E.; et al. Metabolomics approach for predicting response to neoadjuvant chemotherapy for breast cancer. Mol. Oncol. 2013, 7, 297–307. [Google Scholar] [CrossRef]
- Min, H.K.; Lim, S.; Chung, B.C.; Moon, M.H. Shotgun lipidomics for candidate biomarkers of urinary phospholipids in prostate cancer. Anal. Bioanal. Chem. 2011, 399, 823–830. [Google Scholar] [CrossRef]
- Goto, T.; Terada, N.; Inoue, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Sumiyoshi, S.; Kobayashi, T.; et al. The expression profile of phosphatidylinositol in high spatial resolution imaging mass spectrometry as a potential biomarker for prostate cancer. PLoS ONE 2014, 9, e90242. [Google Scholar] [CrossRef]
- Goto, T.; Terada, N.; Inoue, T.; Kobayashi, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Utsunomiya, N.; et al. Decreased expression of lysophosphatidylcholine (16:0/OH) in high resolution imaging mass spectrometry independently predicts biochemical recurrence after surgical treatment for prostate cancer. Prostate 2015, 75, 1821–1830. [Google Scholar] [CrossRef]
- Patel, N.; Vogel, R.; Chandra-Kuntal, K.; Glasgow, W.; Kelavkar, U. A novel three serum phospholipid panel differentiates normal individuals from those with prostate cancer. PLoS ONE 2014, 9, e88841. [Google Scholar] [CrossRef]
- Duscharla, D.; Bhumireddy, S.R.; Lakshetti, S.; Pospisil, H.; Murthy, P.V.; Walther, R.; Sripadi, P.; Ummanni, R. Prostate cancer associated lipid signatures in serum studied by ESI-tandem mass spectrometryas potential new biomarkers. PLoS ONE 2016, 11, e0150253. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Elson, P.; Tan, H.; Plummer, S.J.; Berk, M.; Aung, P.P.; Lavery, I.C.; Achkar, J.P.; Li, L.; et al. Plasma lysophosphatidylcholine levels: Potential biomarkers for colorectal cancer. J. Clin. Oncol. 2007, 25, 2696–2701. [Google Scholar] [CrossRef]
- Dobrzynska, I.; Szachowicz-Petelska, B.; Sulkowski, S.; Figaszewski, Z. Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol. Cell. Biochem. 2005, 276, 113–119. [Google Scholar] [CrossRef]
- Kurabe, N.; Hayasaka, T.; Ogawa, M.; Masaki, N.; Ide, Y.; Waki, M.; Nakamura, T.; Kurachi, K.; Kahyo, T.; Shinmura, K.; et al. Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci. 2013, 104, 1295–1302. [Google Scholar] [CrossRef]
- Mirnezami, R.; Spagou, K.; Vorkas, P.A.; Lewis, M.R.; Kinross, J.; Want, E.; Shion, H.; Goldin, R.D.; Darzi, A.; Takats, Z.; et al. Chemical mapping of the colorectal cancer microenvironment via MALDI imaging mass spectrometry (MALDI-MSI) reveals novel cancer-associated Feld effects. Mol. Oncol. 2014, 8, 39–49. [Google Scholar] [CrossRef]
- Thomas, A.; Patterson, N.H.; Marcinkiewicz, M.M.; Lazaris, A.; Metrakos, P.; Chaurand, P. Histology-driven data mining of lipid signatures from multiple imaging mass spectrometry analyses: Application to human colorectal cancer liver metastasis biopsies. Anal. Chem. 2013, 85, 2860–2866. [Google Scholar] [CrossRef]
- Coviello, G.; Tutino, V.; Notarnicola, M.; Caruso, M.G. Erythrocyte membrane fatty acids profile in colorectal cancer patients: A preliminary study. Anticancer. Res. 2014, 34, 4775–4779. [Google Scholar]
- Zhang, Y.; Liu, Y.; Li, L.; Wei, J.; Xiong, S.; Zhao, Z. High resolution mass spectrometry coupled with multivariate data analysis revealing plasma lipidomic alteration in ovarian cancer in Asian women. Talanta 2016, 150, 88–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, Q.; Xu, Y. The Lipidomic analyses in low and highly aggressive ovarian cancer cell lines. Lipids 2016, 51, 179–187. [Google Scholar] [CrossRef]
- Kang, S.; Lee, A.; Park, Y.S.; Lee, S.C.; Park, S.Y.; Han, S.Y.; Kim, K.P.; Kim, Y.H.; Yoo, C.W.; Kim, H.K. Alteration in lipid and protein profiles of ovarian cancer: Similarity to breast cancer. Int. J. Gynecol. Cancer 2011, 21, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Sutphen, R.; Xu, Y.; Wilbanks, G.D.; Fiorica, J.; Grendys, E.C., Jr.; LaPolla, J.P.; Arango, H.; Hoffman, M.S.; Martino, M.; Wakeley, K.; et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1185–1191. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Wiper, D.W.; Wu, M.; Morton, R.E.; Elson, P.; Kennedy, A.W.; Belinson, J.; Markman, M.; Casey, G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 1998, 280, 719–723. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, Y.; Kennedy, A.W.; Belinson, J.; Xu, Y. Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses. Ann. N. Y. Acad. Sci. 2000, 905, 242–259. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova, I.; Vavrova, J.; Tosner, J.; Hanousek, L. Lysophosphatidic acid: An ovarian cancer marker. Eur. J. Gynaecol. Oncol. 2008, 29, 511–514. [Google Scholar] [PubMed]
- Sedlakova, I.; Vavrova, J.; Tosner, J.; Hanousek, L. Lysophosphatidic acid (LPA)—A perspective marker in ovarian cancer. Tumour Biol. 2011, 32, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dou, A.; Chen, J.; Lu, X.; Cao, R.; Xu, C.; Xu, G. Study of phospholipid profile of ovarian tumor by high performance liquid chromatography-mass spectrometry. Chin. J. Chromatogr. 2011, 29, 843–850. [Google Scholar]
- Jiang, Y.; DiVittore, N.A.; Young, M.M.; Jia, Z.; Xie, K.; Ritty, T.M.; Kester, M.; Fox, T.E. Altered sphingolipid metabolism in patients with metastatic pancreatic cancer. Biomolecules 2013, 3, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Mu, G.; Zhang, L.; Zhou, W.; Zhang, J.; Yu, H. Lysophosphatidic acid stimulates activation of focal adhesion kinase and paxillin and promotes cell motility, via LPA1–3, in human pancreatic cancer. Dig. Dis. Sci. 2013, 58, 3524–3533. [Google Scholar] [CrossRef] [PubMed]
- Macasek, J.; Vecka, M.; Zak, A.; Urbanek, M.; Krechler, T.; Petruzelka, L.; Stankova, B.; Zeman, M. Plasma fatty acid composition in patients with pancreatic cancer: Correlations to clinical parameters. Nutr. Cancer 2012, 64, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Kikuchi, H.; Miyazaki, S.; Iino, I.; Setoguchi, T.; Hiramatsu, Y.; Ohta, M.; Kamiya, K.; Morita, Y.; Tanaka, H.; et al. Overexpression of lysophosphatidylcholine acyltransferase 1 and concomitant lipid alterations in gastric cancer. Ann. Surg. Oncol. 2016, 23, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Dill, A.L.; Eberlin, L.S.; Costa, A.B.; Zheng, C.; Ifa, D.R.; Cheng, L.; Masterson, T.A.; Koch, M.O.; Vitek, O.; Cooks, R.G. Multivariate statistical identification of human bladder carcinomas using ambient ionization imaging mass spectrometry. Chemistry 2011, 17, 2897–2902. [Google Scholar] [CrossRef]
- Dill, A.L.; Ifa, D.R.; Manicke, N.E.; Costa, A.B.; Ramos-Vara, J.A.; Knapp, D.W.; Cooks, R.G. Lipid profiles of canine invasive transitional cell carcinoma of the urinary bladder and adjacent normal tissue by desorption electrospray ionization imaging mass spectrometry. Anal. Chem. 2009, 81, 8758–8764. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Zhang, R.; Song, Y.; Cao, J.; Bi, N.; Wang, J.; He, J.; Bai, J.; Dong, L.; et al. Global and targeted metabolomics of esophageal squamous cell carcinoma discovers potential diagnostic and therapeutic biomarkers. Mol. Cell. Proteom. 2013, 12, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Hayasaka, T.; Masaki, N.; Watanabe, Y.; Masumoto, K.; Nagata, T.; Katou, F.; Setou, M. Imaging mass spectrometry distinguished the cancer and stromal regions of oral squamous cell carcinoma by visualizing phosphatidylcholine (16:0/16:1) and phosphatidylcholine (18:1/20:4). Anal. Bioanal. Chem. 2014, 406, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Lin, L.; Huang, Z.; Gao, Y.; Chen, Y.; Hang, W.; Xing, J.; Yan, X. LC-MS-based serum metabolic profiling for genitourinary cancer classification and cancer type-specific biomarker discovery. Proteomics 2012, 12, 2238–2246. [Google Scholar] [CrossRef]
- Saito, K.; Arai, E.; Maekawa, K.; Ishikawa, M.; Fujimoto, H.; Taguchi, R.; Matsumoto, K.; Kanai, Y.; Saito, Y. Lipidomic signatures and associated transcriptomic profiles of clear cell renal cell carcinoma. Sci. Rep. 2016, 6, 28932–28944. [Google Scholar] [CrossRef] [PubMed]
- Cifkova, E.; Holcapek, M.; Lisa, M.; Vrana, D.; Melichar, B.; Student, V. Lipidomic differentiation between human kidney tumors and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. J. Chromatogr. B 2015, 1000, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Dill, A.L.; Eberlin, L.S.; Zheng, C.; Costa, A.B.; Ifa, D.R.; Cheng, L.; Masterson, T.A.; Koch, M.O.; Vitek, O.; Cooks, R.G. Multivariate statistical differentiation of renal cell carcinomas based on lipidomic analysis by ambient ionization imaging mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Tateya, I.; Hayasaka, T.; Masaki, N.; Takizawa, Y.; Ohno, S.; Kojima, T.; Kitani, Y.; Kitamura, M.; Hirano, S.; et al. Increased expression of phosphatidylcholine (16:0/18:1) and (16:0/18:2) in thyroid papillary cancer. PLoS ONE 2012, 7, e48873. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiu, L.; Wang, Y.; Qin, X.; Liu, H.; He, M.; Zhang, Y.; Li, Z.; Chen, X. Tissue imaging and serum lipidomic profiling for screening potential biomarkers of thyroid tumors by matrix-assisted laser desorption/ionization—Fourier transform ion cyclotron resonance mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Francesca Perrotti, Consuelo Rosa, Ilaria Cicalini, Paolo Sacchetta, Piero Del Boccio, Domenico Genovesiand Damiana Pieragostino, Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992.
- Schcolnik-Cabrera, A.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Taja-Chayeb, L.; Morales-Barcenas, R.; Trejo-Becerril, C.; Perez-Cardenas, E.; Gonzalez-Fierro, A.; Dueñas-González, A. Orlistat as a FASN inhibitor and multitargeted agent for cancer therapy. Expert. Opin. Investig. Drugs 2018, 2, 475–489. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, I.G.; Bastos, D.C.; Cuadra-Zelaya, F.J.; Teixeira, I.F.; Salo, T.; Della Coletta, R.; Graner, E. Anticancer properties of the fatty acid synthase inhibitor TVB-3166 on oral squamous cell carcinoma cell lines. Arch. Oral Biol. 2020, 113, 104707. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, Y.Y.; Rychahou, P.G.; Le, A.T.; Scott, T.L.; Flight, R.M.; Kim, J.T.; Harris, J.; Liu, J.; Wang, C.; Morris, A.J.; et al. Preclinical evaluation of novel fatty acid synthase inhibitors in primary colorectal cancer cells and a patient-derived xenograft model of colorectal cancer. Oncotarget 2018, 9, 24787–24800. [Google Scholar] [CrossRef]
- Zadra, G.; Ribeiro, C.F.; Chetta, P.; Ho, Y.; Cacciatore, S.; Gao, X.; Syamala, S.; Bango, C.; Photopoulos, C.; Huang, Y.; et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castrationresistant prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 631–640. [Google Scholar] [CrossRef]
- Wang, C.; Xu, C.; Sun, M.; Luo, D.; Liao, D.F.; Cao, D. Acetyl-CoA carboxylasealpha inhibitor TOFA induces human cancer cell apoptosis. Biochem. Biophys. Res. Commun. 2009, 385, 302–306. [Google Scholar] [CrossRef]
- Beckers, A.; Organe, S.; Timmermans, L.; Scheys, K.; Peeters, A.; Brusselmans, K.; Verhoeven, G.; Swinnen, J.V. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007, 67, 8180–8187. [Google Scholar] [CrossRef]
- Svensson, R.U.; Parker, S.J.; Eichner, L.J.; Kolar, M.J.; Wallace, M.; Brun, S.N.; Lombardo, P.S.; Van Nostrand, J.L.; Hutchins, A.; Vera, L.; et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 2016, 22, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Clark, A.K.; Selth, L.A.; Haynes, V.R.; Lister, N.; Rebello, R.; Porter, L.H.; Niranjan, B.; Whitby, S.T.; Lo, J.; et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 2019, 11, eaau5758. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.T.; Hu, P.; Huang, W.C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Dehairs, J.; Rambow, F.; Rogiers, A.; Nittner, D.; Derua, R.; Vanderhoydonc, F.; Duarte, J.A.; Bosisio, F.; Van den Eynde, K.; et al. Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat. Commun. 2018, 9, 2500. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.L.; Robbins, M.D.; Warren, L.C.; Xia, D.; Petras, S.F.; Valentine, J.J.; Varghese, A.H.; Wang, K.; Subashi, T.A.; Shelly, L.D.; et al. Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J. Pharmacol. Exp. Ther. 2008, 326, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Saha, A.K.; Wen, R.; Ruderman, N.B.; Luo, Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem. Biophys. Res. Commun. 2004, 321, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ishida, C.T.; Shang, E.; Shu, C.; Torrini, C.; Zhang, Y.; Bianchetti, E.; Sanchez-Quintero, M.J.; Kleiner, G.; Quinzii, C.M.; et al. Activation of LXRbeta inhibits tumor respiration and is synthetically lethal with Bcl-xL inhibition. EMBO Mol. Med. 2019, 11, e10769. [Google Scholar] [CrossRef]
- Szász, I.; Koroknai, V.; Várvölgyi, T.; Pál, L.; Szűcs, S.; Pikó, P.; Emri, G.; Janka, E.; Szabó, I.L.; Ádány, R.; et al. Identification of Plasma Lipid Alterations Associated with Melanoma Metastasis. Int. J. Mol. Sci. 2024, 25, 4251. [Google Scholar] [CrossRef]
- Poli, G.; Lapillo, M.; Jha, V.; Mouawad, N.; Caligiuri, I.; Macchia, M.; Minutolo, F.; Rizzolio, F.; Tuccinardi, T.; Granchi, C. Computationally driven discovery of phenyl(piperazin-1-yl)methanone derivatives as reversible monoacylglycerol lipase (MAGL) inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Yan, Y.; Xu, Z.; Zeng, S.; Qian, L.; Huo, L.; Li, X.; Sun, L.; Gong, Z. SCD1 Confers Temozolomide Resistance to Human Glioma Cells via the Akt/GSK3beta/beta-Catenin Signaling Axis. Front. Pharmacol. 2017, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Nashed, M.; Chisholm, J.W.; Igal, R.A. Stearoyl-CoA desaturase activity modulates the activation of epidermal growth factor receptor in human lung cancer cells. Exp. Biol. Med. 2012, 237, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, M.E.; Maugeri-Saccà, M.; Fattore, L.; Bruschini, S.; De Vitis, C.; Tabbì, E.; Bellei, B.; Migliano, E.; Kovacs, D.; Camera, E.; et al. Inhibition of Stearoyl-CoA desaturase 1 reverts BRAF and MEK inhibitioninduced selection of cancer stem cells in BRAF-mutated melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 318. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Patel, S.; Affleck, V.S.; Wilson, I.; Turnbull, D.M.; Joshi, A.R.; Maxwell, R.; Stoll, E.A. Fatty acid oxidation is required for the respiration and proliferation of malignant glioma cells. Neuro-Oncology 2016, 19, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Hernández-Esquivel, L.; Marín-Hernández, A.; El Hafidi, M.; Gallardo-Pérez, J.C.; Hernández-Reséndiz, I.; Rodríguez-Zavala, J.S.; Pacheco-Velázquez, S.C.; Moreno-Sánchez, R. Mitochondrial free fatty acid b-oxidation supports oxidative phosphorylation and proliferation in cancer cells. Int. J. Biochem. Cell Biol. 2015, 65, 209–221. [Google Scholar] [CrossRef]
- Deng, F.; Ma, Y.X.; Liang, L.; Zhang, P.; Feng, J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. BioMed Pharmacother. 2018, 97, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Portolés, C.; Feliu, J.; Reglero, G.; Ramírez de Molina, A. ABCA1 overexpression worsens colorectal cancer prognosis by facilitating tumour growth and caveolin-1-dependent invasiveness, and these effects can be ameliorated using the BET inhibitor apabetalone. Mol. Oncol. 2018, 12, 1735–1752. [Google Scholar] [CrossRef] [PubMed]
- Georgila, K.; Vyrla, D.; Drakos, E. Apolipoprotein A-I (ApoA-I), Immunity, Inflammation and Cancer. Cancers 2019, 11, 1097. [Google Scholar] [CrossRef]
- Ohmoto, T.; Nishitsuji, K.; Yoshitani, N.; Mizuguchi, M.; Yanagisawa, Y.; Saito, H.; Sakashita, N. K604, a specific acylCoA:cholesterol acyltransferase 1 inhibitor, suppresses proliferation of U251MG glioblastoma cells. Mol. Med. Rep. 2015, 12, 6037–6042. [Google Scholar] [CrossRef]
- LaPensee, C.R.; Mann, J.E.; Rainey, W.E.; Crudo, V.; Hunt, S.W.; Hammer, G.D. ATR-101, a Selective and Potent Inhibitor of Acyl-CoA Acyltransferase 1, Induces Apoptosis in H295R Adrenocortical Cells and in the Adrenal Cortex of Dogs. Endocrinology 2016, 157, 1775–1788. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Ratre, Y.K.; Soni, V.K.; Shukla, D.; Sonkar, S.C.; Kumar, A.; Vishvakarma, N.K. Orchestral role of lipid metabolic reprogramming in T-cell malignancy. Front. Oncol. 2023, 13, 1122789. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Cao, J.; van der Kroft, G.; van Dijk, D.P.; Aberle, M.R.; Grgic, A.; Neumann, U.P.; Wiltberger, G.; Balluff, B.; Schaap, F.G.; et al. Inflammation-associated intramyocellular lipid alterations in human pancreatic cancer cachexia. J. Cachexia Sarcopenia Muscle, 2024. [Google Scholar] [CrossRef]
- Sieminska, J.; Miniewska, K.; Mroz, R.; Sierko, E.; Naumnik, W.; Kisluk, J.; Michalska-Falkowska, A.; Reszec, J.; Kozlowski, M.; Nowicki, L.; et al. First insight about the ability of specific glycerophospholipids to discriminate non-small cell lung cancer subtypes. Front. Mol. Biosci. 2024, 11, 1379631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, Y.; Rong, J.; Xu, L.; Huang, X.; Zhu, J.; Chen, Z.; Mao, W. Plasma-based lipidomics reveals potential diagnostic biomarkers for esophageal squamous cell carcinoma: A retrospective study. PeerJ 2024, 12, e17272. [Google Scholar] [CrossRef]


| Categories | Classes | Example |
|---|---|---|
| Fatty acyls, FA | Fatty acids: straight-chain fatty acids, Eicosanoids, fatty alcohols, fatty esters, fatty amides | 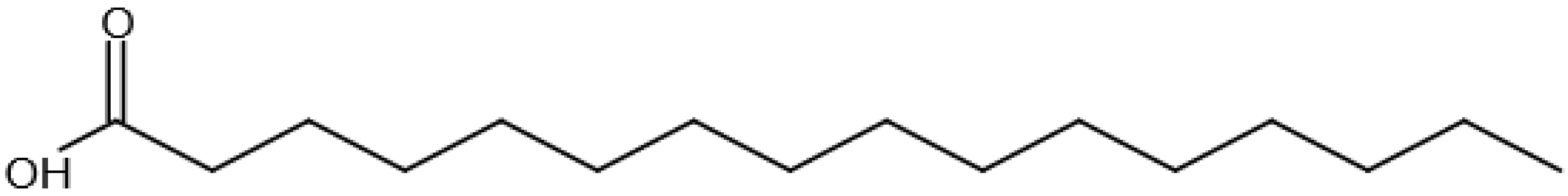 Hexadecanoic acid Hexadecanoic acid |
| Prenol lipids, PR | Isoprenoids, Quinones and hydroquinones, Polyprenols |  2E,6E-Farnesol 2E,6E-Farnesol |
| Glycerolipids, GL | Monoradylglycerols: monoacyl glycerols, Diradylglycerols: diacyl glycerols, Triradylglycerols: triacyl glycerols | 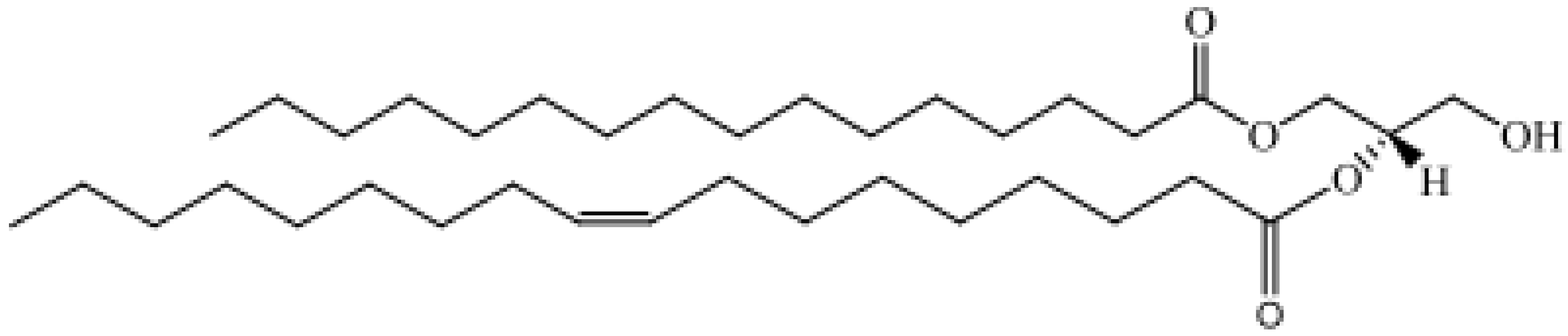 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero |
| Glycerophospholipids, GP | Glycerophosphocholines, glycerophosphoenolamines, glycerophosphoserines, glycerophospholycerols, glycerophosphoglycerophosphates, glycerophosphoinositols, glycerophosphoglycerophosphoglycerols |  1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine 1-Hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycero-3-phosphocholine |
| Sphingolipids, SP | Sphingoid bases, ceramides, phosphosphingolipids, neutral glycosphingolipids, acidic glycosphingolipid | 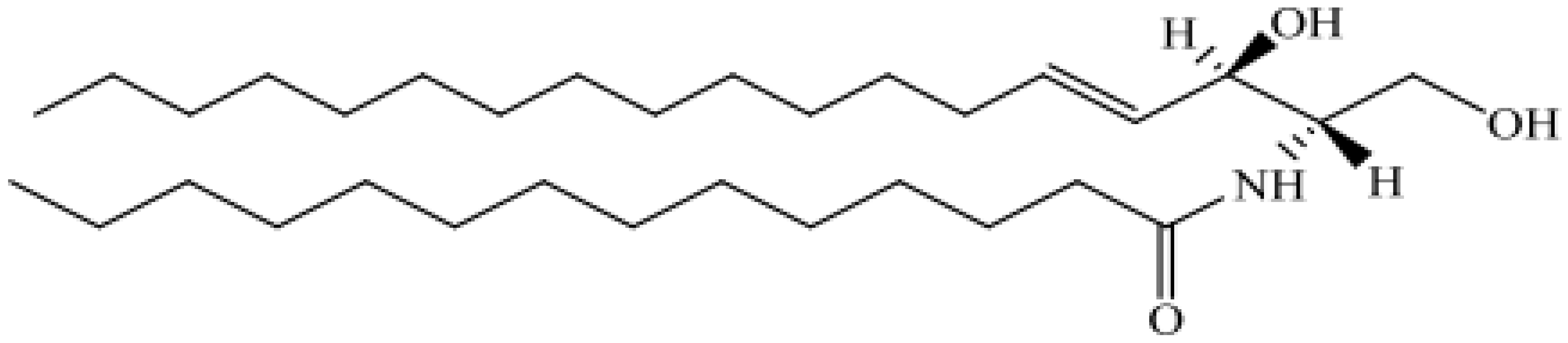 N-(Tetradecanoyl)-sphing-4-enine N-(Tetradecanoyl)-sphing-4-enine |
| Saccharolipids, SL | Acylaminosugars, Acylaminosugar glycans, Acyltrehaloses, Acyltrehalose glycans |  UDP-3-O-(3R-Hydroxyl-tetradecanoyl)-αD -N-acetylglucosamine UDP-3-O-(3R-Hydroxyl-tetradecanoyl)-αD -N-acetylglucosamine |
| Sterol lipids, ST | Sterols, cholesterol and derivatives, steroids, bile acids and derivatives |  Cholest-5-en-3β-ol Cholest-5-en-3β-ol |
| Polyketides, PK | Macrolide polyketides, Aromatic polyketides, Nonribosomal peptide/polyketides hybrids | 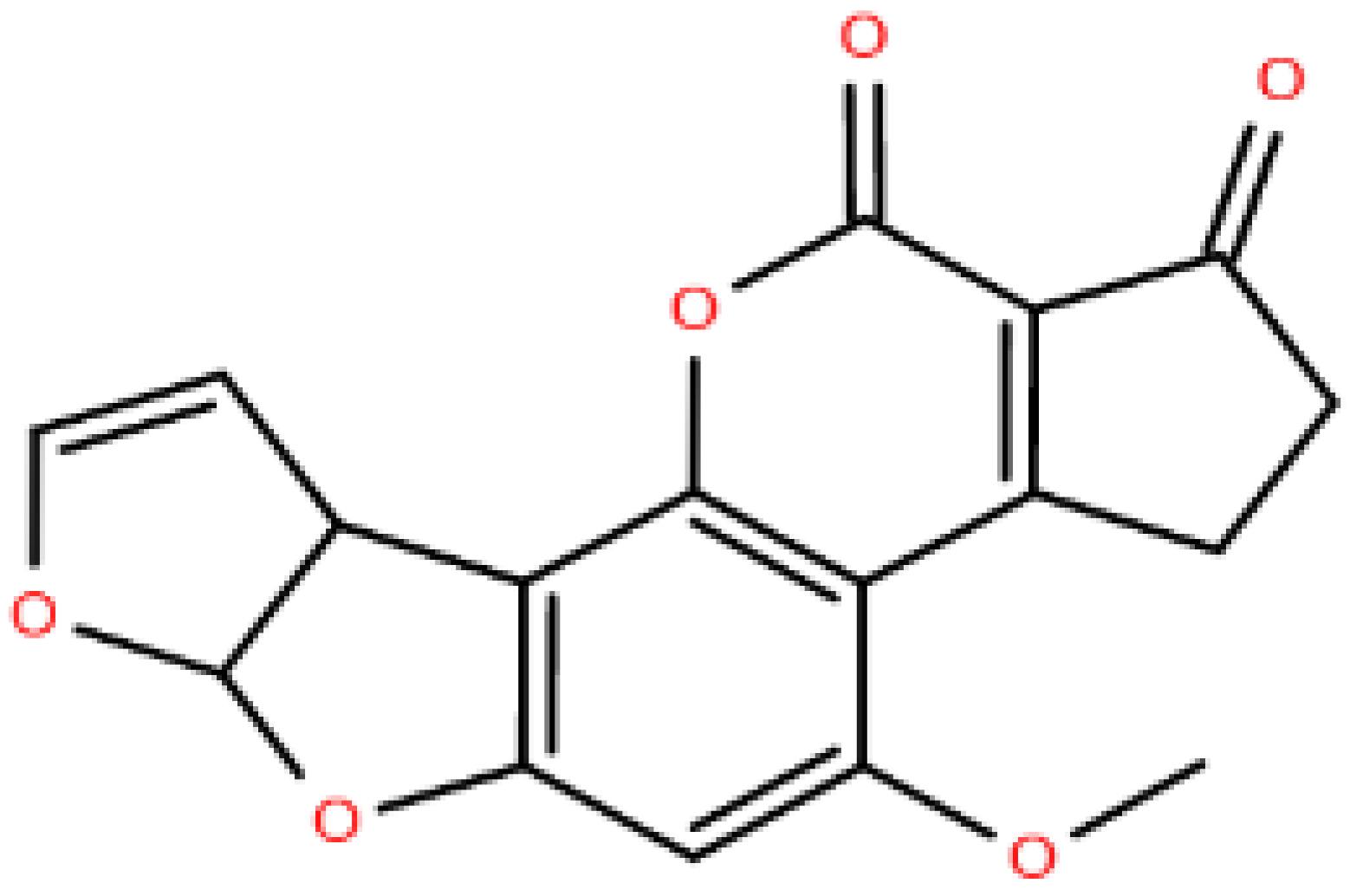 Aflatoxin B1 Aflatoxin B1 |
| Membrane components | Glycerophospholipids (e.g., PC, PE, PI, PS, PG, PA, etc.), sphingolipids (e.g., sphingomyelin, cardiolipin, cerebroside, sulfatide, gangliosides, etc.), glycolipids, sterol lipids (e.g., cholesterol, etc.) |
| Energy storage and metabolism | Glycerolipids (e.g., NEFA, TAG, diacylglycerol, monoacylglycerol, acyl CoA, acylcarnitine, etc.) |
| Signaling | Glycerolipids (e.g., diacylglycerol, monoacylglycerol, acyl CoA, acylcarnitine, NEFA, oxidized fatty acid), sphingolipids (e.g., Cer, sphingosine, sphingosine-1-phosphate), psychosine, N-acylethanolamine, Lyso-lipids, etc. |
| Other functions | Plasmalogen (antioxidant) Acylcarnitine (lipid transport) CL (mitochondrial respiration), PS (cofactor, substrate of PE synthesis) |
| Cancer | Lipids Modified | Technique |
|---|---|---|
| Ovarian | PPE(16:0, 18:1), LPC15:0 LPA18:2, PPE(18:0, 22:6) | LC/ESI/MS/MS |
| Liver | LPC16:0 LPC18:0 PS16:0, PC18:0 | UPLC-MS |
| Liver | 18:2n-6, 18:1n-9, 20:4n, 16:0 | GC-MS |
| Lung | SM(16:0, 16:1), LPC18:1 LPC20:4 LPC20:3 LPC22:6 | FTICR-MS |
| Lung | LPE18:1 CE18:2 ePE40:4, SM22:0 | ESI-MS |
| Prostate | ePC38:5 PC40:3, PC42:4 | Q-TOF-MS |
| Breast | Palmitic acid, 16:0, Stearic acid 18:0, Linoleic acid, 18:2, Alpha-Linolenic acid, 18:3, Eicosapentaenoic acid 20:5, Docosapentaenoic acid 22:5 | GC-MS |
| Colorectal | Capric acid, 10:0 | GC-MS |
| Tumor | Lipid Class | Lipid Type | Sample | Up | Down | Ref. |
|---|---|---|---|---|---|---|
| Lung cancer (adenocarcinomas) | GPLs | PI arachidonate containing phospholipids | Tissue | X | [47] | |
| Lung cancer (adenocarcinomas) | GPLs | PC 32:0, PC 32:1, PGs | Tissue | X | [47] | |
| Lung cancer (adenocarcinomas) | FAs | free arachidonic | Tissue | X | [47] | |
| Lung cancer (NSCLC) | GPLs | PC 34:1, PC 36:2, PC 36:3 | Tissue | X | [48] | |
| Lung cancer (NSCLC) | GPLs, SLs | PC 32:0, ST-OH 42:1 | Tissue | X | [48] | |
| Lung cancer (NSCLC) | FAs | EPA | Tissue | X | X | [49] |
| Lung cancer (Different cancer type) | GPLs | SM 16:0/1, LPC 18:1, LPC 20:4, LPC 20:3, LPC 22:6 | Serum | X | [50] | |
| Lung cancer (NSCLC) | GPLs | PI 38:3, PI 40:3, PI 38:2 | Tissue | X | [51] | |
| Lung cancer (NSCLC) | SLs | SM 40:1, SM 42:1, SM 36:1 | Tissue | X | [51] | |
| Breast cancer NS | GPLs, SLs | PC, PE, PI, SMs | Tissue | X | [52] | |
| Breast cancer (luminal, HER2+ and triple-negative) | GPLs | PC 34:1 | Tissue | X | [53] | |
| Breast cancer (MDA-MB 231 model) | Palmitoyl carnitines, stearoyl carnitine GPLs, SLs | PC16:0/16:0,PC16:0/18:1, PC18:1/18:1,PC18:0/18:1 PC16:0/22:1 SMd18:1/16:0 | Tissue | X | [54] | |
| Breast cancer NS | GPLs | PI18:0/20:4 | Urine | X | [55] | |
| Breast cancer NS | GPLs | PS (18:1/18:1 18:2/18:0) | Urine | X | [55] | |
| Breast cancer NS | GPLs | PI 18:0/18:1, PI 18:0/20:3 | Tissue | X | [56] | |
| Breast cancer (mammary epithelial and breast cancer) | GPLs | PCs, PI 22:5/18:0, PI 18:0/18:1 | Cell line | X | X | [57] |
| Breast cancer NS | GPLs | PS 18:0/20:4, PI 18:0/20:4, PC 18:0/20:4 | Cell line | X | [58] | |
| Breast cancer NS | GPLs | PIs, Pes, PCs, LPCs | Tissue | X | X | [59] |
| Breast cancer NS | GPLs | TGs containing C18:1 fatty acyl chain | Serum | X | [60] | |
| Breast cancer NS | FAs | Linoleic acid (C18:2) | Serum | X | [61] | |
| Prostate cancer NS | GPLs | PS 18:0/18:1, PS 16:0/22:6 | Urine | X | [62] | |
| Prostate cancer NS | GPLs | PS 18:1/18:0, PS 18:0/20:5 | Urine | X | [62] | |
| Prostate cancer NS | GPLs | PI 18:0/18:1, PI 18:0/20:3, PI 18:0/20:2 | Tissue | X | [63] | |
| Localized prostate cancer | GPLs | LPC 16:0/OH, SM d18:1/16:0 | Tissue | X | [64] | |
| Localized prostate cancer | GPLs | LPC 16:0/OH | Tissue | X | [64] | |
| Localized prostate cancer | GPLs | PC 40:3, PC 42:4 | Serum | X | [65] | |
| Prostate cancer NS | GPLs | PC 39:6 | Serum | X | [66] | |
| Prostate cancer NS | FAs | FA 22:3 | Serum | X | [66] | |
| Colorectal cancer NS | GPLs | LPC 18:1, LPC 18:2 | Plasma | X | [67] | |
| Colorectal cancer (pT3 stage, various grades (G2, G3)) | GPLs | PC/PE ratio | Cell lines | X | [68] | |
| Colorectal cancer NS | GPLs | PC 16:0/16:1 | Tissue | X | [69] | |
| Colorectal cancer NS | GPLs | PC 16:0/18:1, LPC 16:0, LPC 18:1 | Tissue | X | [70] | |
| Colorectal cancer liver metastasis | GPLs | PE 38:6, PE 40:4 | Tissue | X | [71] | |
| Colorectal cancer NS | FAs | n-3 PUFAs | Red Blood cell | X | [72] | |
| Colorectal cancer NS | FAs | n-6-PUFA/n-3-PUFA | Red Blood cell | X | [72] | |
| Ovarian cancer NS | GPLs | LPC | Plasma | X | [73] | |
| Ovarian cancer NS | GPLs | PC, TG | Plasma | X | [73] | |
| Epithelial ovarian cancer | GPLs | TGs 50:2 50:152:2 54:4 54:3 | Cell lines | X | [74] | |
| Ovarian cancer NS | GPLs | PC 32:3, PC 34:1, PC 36:2 | Tissue | X | [75] | |
| Ovarian cancer NS | GPLs | LPA 16:0, LPA 20:4 | Plasma | X | [76] | |
| Ovarian cancer and other gynecological cancers | GPLs | LPA | Serum/plasma | X | [77] | |
| Ovarian cancer and other gynecological cancers | GPLs | LPA 16:0, LPA 18:2, LPA18:1, LPA18:0, LPI 16:0, LPI 18:0, LPI 20:4 | Plasma | X | [78] | |
| Benign and malignant ovarian cancer | GPLs | LPA | Plasma | X | [79] | |
| Benign and malignant ovarian cancer | GPLs | LPA | Plasma | X | [80] | |
| Benign and malignant ovarian cancer | GPLs | Plasmalogen phospatidylethanol, PC, plasmalogen PC, SM and LPC | X | [81] | ||
| Metastatic pancreatic cancer | SLs | Ceramides species (C16:0 and C24:1) | Tissue/Plasma | X | [82] | |
| Metastatic pancreatic cancer | SLs | C18:0 C20:0 C22:0 C24:0 C24:1 | Tissue/Plasma | X | [82] | |
| Metastatic pancreatic cancer | SLs | C16:0 C20:0 C22:0 C24:0 C24:1 | Tissue/Plasma | X | [82] | |
| Pancreatic cancer PANC-1 cells | GPLs | LPA | Cell lines | X | [83] | |
| Pancreatic cancer NS | FAs | MUFA | Plasma | X | [84] | |
| Gastric cancer NS | GPLs | PC16:0/18:1 | Tissue | X | [85] | |
| Gastric cancer NS | GPLs | LPC 16:0 | Tissue | X | [85] | |
| Bladder Cancer NS | GPLs | PS 18:0/18:1 | Tissue | X | [86] | |
| Bladder Cancer | GPLs | PI 18:0/20:4 | Tissue | X | [86] | |
| Bladder Cancer (Model of human invasive bladder cancer) | GPLs | PS 18:0/18:1 | Tissue | X | [87] | |
| Bladder Cancer (Model of human invasive bladder cancer) | GPLs | PG 18:1/18:1 | Tissue | X | [87] | |
| Bladder Cancer (Model of human invasive bladder cancer) | GPLs | PI 16:0/18:1 | Tissue | X | [87] | |
| Bladder Cancer (Model of human invasive bladder cancer) | GPLs | PI 18:0/18:1 | Tissue | X | [87] | |
| Bladder Cancer (Model of human invasive bladder cancer) | GPLs | PS 18:1/18:1 | Tissue | X | [87] | |
| Esophageal cancer (ESCC) | GPLs | Octanoylcarnitine, LPC 16:1, Decanoylcarnitine | Plasma | X | [88] | |
| Esophageal cancer (OSCC) | GPLs | PC 16:0/16:1 | Tissue | X | [89] | |
| Esophageal cancer (OSCC) | GPLs | PC 18:1/20:4 | Tissue | X | [89] | |
| Esophageal cancer (ESCC) | GPLs | PS, PA, PC, PI, PE | Plasma | X | [90] | |
| Kidney cancer NS | GLs SLs | PE (P-16:0e/0:0), ganglioside GM3, (d18:1/22:1), sphinganine C17, SMd18:0/16:1(9Z) | Serum | X | [91] | |
| Kidney cancer NS | GPLs STLs Gls | PC, Plasmalogens, cholesterol esters, TGs | Tissue | X | [92] | |
| Kidney cancer NS | GPLs, FAs | PE, Unsaturated FAs | Tissue | X | [92] | |
| Kidney cancer NS | GPLs | PL, PE 36:1, PC 38:4, PC 36:2, PC 32:0 | Tissue | X | [93] | |
| Kidney cancer NS | GPLs | PE 34:2, PE 36:4, PE 38:4, PC 34:1 PC34:2 PC 36:4 PI36:4 | Tissue | X | [93] | |
| Kidney cancer NS | GPLs | PI 18:0/20:4, PI22:4/18:0 PS18:0/18:1 PG18:1/18:1 | Tissue | X | [94] | |
| Kidney cancer (Human papillary renal carcinoma) | FAs | FA12:0 | Tissue | X | [94] | |
| Thyroid cancer (Thyroid papillary cancer) | GPLs | PC 16:0/18:1 PC 16:0/18:2 | Tissue | X | [95] | |
| Thyroid cancer (Thyroid papillary cancer) | SLs | SMd18:0/16:1 | Tissue | X | [95] | |
| Malignant and benignant thyroid cancer | GPLs | PC 34:1, PC 36:1, PC 32:0 | Tissue/Serum | X | [96] | |
| Malignant and benignant thyroid cancer | GPLs | PA 36:62, PA 36:3, PA 38:4, PA 38:5, PA 40:5 | Tissue/Serum | X | [96] |
| Target | Agent Name | Type of Cancer/Phase of Development | Comments and Benefits | Reference/Clinical Trial |
|---|---|---|---|---|
| Fatty acid synthase (FASN) inhibitor | Orlistat | Prostate, breast, ovarian, colon cancer and other solid tumors/Approved | Antiobesity lipase and FASN inhibitor; not yet assessed clinically for cancer. An anti-obesity drug approved by the FDA and an irreversible inhibitor of FASN | [98] |
| TVB-3166/TVB-3664 | Oral squamous cell carcinoma, colorectal, breast cancer/Preclinical | A reversible and selective FASN inhibitor | [99,100] | |
| Conjugated Linoleic Acid | Breast Cancer/Phase I | Reduce FASN gene expression and spot 14 | NCT00908791 | |
| Omeprazole | Triple-negative breast cancer/Phase II | A proton pump inhibitors that can inhibit FASN | NCT02595372 | |
| IPI-9119 | Castration-resistant prostate cancer/Preclinical | Selectively inhibit FASN and suppress expression of both full-length of androgen receptor (AR) and AR variant V7 | [101] | |
| TVB-2640 | Solid Malignant Tumor/Phase II | Trials in breast cancer +/− trastuzumab/docetaxel. A potent and reversible FASN inhibitor | NCT03032484 NCT03179904 | |
| Acetyl-CoA Carboxylase (ACC) | TOFA | Lung cancer and colon carcinoma/Preclinical | Induce apoptosis as an allosteric inhibitor of ACC-alpha | [102] |
| Soraphen A | Prostate cancer/Preclinical | Inhibit fatty acid synthesis and stimulate fatty acid oxidation | [103] | |
| ND-646 | NSCLC/Preclinical | Inhibit fatty acid synthesis and tumor growth as an allosteric inhibitor of the ACC | [104] | |
| 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) | Statins, e.g., lovastatin, atorvastatin, rosuvastatin and simvastatin | Various leukemia and solid tumors/Phase II | Inhibitors of HMGCR. Association studies between statin use and outcomes in many cancers, some randomized trials | NCT03358017 NCT03324425 NCT02569645 NCT03275376 |
| CD36 | ABT-510 | Prostate cancer/Phase II | Trial in metastatic melanoma. Reduced fatty acid uptake and the abundance of oncogenic signaling lipids | [105] |
| SREBPs | Fatostatin | Prostate cancer/Preclinical | A non-sterol diarylthiazole derivative which has antimitotic properties and perturbs nuclear translocation of SREBP and androgen receptor signaling | [106] |
| Betulin | BRAFV600E-mutant melanoma/Prelinical | Increase membrane lipid poly-unsaturation and lipid peroxidation; sensitize therapy-resistant melanoma cells to MAPK-targeting therapy | [107] | |
| PF-429242 | HCC/Preclinical | Reversible site 1 protease inhibitors, which inhibit endogenous SREBP processing | [108] | |
| AMPK | MT 63–78 | Prostate cancer/Preclinical | MT 63–78 is a specific and potent direct AMPK activator and also induces cell mitotic arrest and apoptosis | [109] |
| 5-aminoimidazole 4- carboxamide riboside (AICAR) | Prostate cancer/Preclinical | Preclinical studies in cancer and an AMPK activator which inhibits cell growth | [109] | |
| LXR | LXR-623 | Phase I for atherosclerosis | Preclinical studies in cancer | [110] |
| GW3965; LXR623 | Melanoma and glioblastoma/Preclinical | LXR agonists which suppress mitochondrial respiration and decrease cholesterol levels by enhancing the excretion and decreasing the resorption of cholesterol | [111] | |
| ATGL | atglistatin | Specific for murine ATGL | ||
| MAGL | JZL184 | Cancer-associated bone disease/Preclinical | Potent and reversible MAGL inhibitors | [112] |
| SCD | A939572 | Glioblastoma and renal cell carcinoma/Preclinical | Inhibit tumor growth both in vitro and in vivo; overcoming chemotherapy agent resistance | [113] |
| CVT-11127 or CVT- 12012 | Lung cancer/Preclinical | A small molecule SCD inhibitor which modulates cancer cell metabolism, proliferation and survival | [114] | |
| MF-438 | Lung cancer/Preclinical | Induce lung cancer stem cell apoptosis | [115] | |
| CPT1 | Etomoxir | Glioma/Preclinical | A CPT1 inhibitor which inhibits proliferative activity | [116] |
| Perhexiline | Breast and gastrointestinal cancer/Preclinical | A CPT1 inhibitor which blocks FFA utilization, OxPhos and proliferation | [117] | |
| PLD | ST1326 | Preclinical | ||
| FIPI | Preclinical | Targets immune infiltration into tumors | ||
| VU0155072–2 | Preclinical | Targets immune infiltration into tumors | ||
| FABP | EI-05 | Preclinical | ||
| SBFI-102, 103 | Preclinical | |||
| Choline kinase | HC-3, JCR and MN derivatives | Preclinical | ||
| Triterpene quinone methides | Preclinical | Derived from natural products | ||
| ICL-CCIC-0019 | Preclinical | |||
| EB-3D, EB-3P | Preclinical | |||
| Farnesyltransferase | tipifarnib | Phase I/II | Phase II studies in metastatic BC and squamous cell carcinoma; Phase I trial in glioblastoma | |
| lonafarnib | Phase I/Ib | Largely combination studies in glioblastoma, BC | ||
| BMS-214662 | Phase I | Single agent or in combination with paclitaxel in advanced solid tumors | ||
| Palmitoylation | 2-bromopalmitate | Preclinical | ||
| Acid sphingomyelinase | Fluphenazine | Preclinical | ||
| Mammalian target of rapamycin (mTOR) | Rapamycin | Breast cancer/Preclinical | Inhibited S6 phosphorylation and cell proliferation, and resulted in lower levels of apoptosis induction | [118] |
| Everolimus | Castrated Resistant Prostate Cancer. Locally Advanced Cervical Cancer/Phase III | Directly inhibit mTORC1 and indirectly inhibit mTORC2 | NCT03580239 | |
| PF-05212384 | Advanced Cancer; Advanced squamous cell lung, pancreatic, head and neck, and other solid tumors/Phase I | Intravenous PI3K/mTOR inhibitor | NCT01347866 NCT03065062 | |
| PF-04691502 | Breast Neoplasms/Phase II | Inhibit PI3K and mTOR kinase | NCT01658176 | |
| Vistusertib/AZD2014 | Endometrial, triple negative breast cancer, ovarian, primary peritoneal, or fallopian tube cancer/Phase II | mTORC1/2 Inhibitor | NCT02208375 | |
| Protein kinase B (PKB), Akt | MK-2206 | Advanced or metastatic solid tumors or breast cancer; prostate cancer/Phase I, II | Inhibit Akt phosphorylation, cell proliferation and apoptosis in a dose-dependent manner | NCT01245205 NCT01277757 NCT01251861 |
| Capivasertib/AZD5363 | Breast cancer, prostate cancer and advanced solid tumors/Phase I, II | A novel pan-AKT kinase catalytic inhibitor | NCT03310541 NCT02525068 | |
| GSK2141795 | Endometrial cancer/Phase I | AKT inhibitor | NCT01935973 | |
| ApoA-I | Apabetalone (RVX-208) | Colorectal cancer/Preclinical | A BET inhibitor which is a stimulator of ApoA-I and regulates the reverse cholesterol transport | [119] |
| ApoA-1 mimetic peptides | Preclinical | Mimetic peptides which are synthesized on the basis of aamphipathic helical repeating structure of ApoA-I | [120] | |
| ACAT | K604 | Glioblastoma/Preclinical | A selective ACAT1 inhibitor, which suppresses proliferation of glioblastoma cells | [121] |
| ATR-101 | Advanced adrenocortical carcinoma/Phase I | An oral selective ACAT inhibitor | NCT01898715 [122] |
| T-Cell Subset | Metabolic Events | Molecular Players | |
|---|---|---|---|
| Naïve T cell | Majorly rely on OXPHOS and FAO | Enzymes of OXPHOS and FAO | |
| Ag- stimulated T cells | Aerobic glycolysis, FA synthesis, uptake, and accumulation | mTOR, PI3K, c-myc, HIF, GLUTs, FASN, SREBP, and PPARg | |
| CD8+ cytotoxic T cells | Mainly rely on FA and lipid synthesis | FASN, ACC1, HMGCR, and SREBP | |
| CD4+ helper T cells | FA and lipid synthesis and FA uptake | ASN, SREBPs, CD36, FABPs, GPR43, GLP84, and LXR | |
| Th1 | Relatively high FA uptake dominates over FA synthesis | CD36, FABPs, FASN, and LCFA favor Th1 activation | |
| Th2 | Relatively low FA uptake, however, dominates over FA synthesis | CD36, FABPs, FASN, and PUFA favor Th2 activation | |
| Th3 | Profound FA synthesis and aerobic glycolysis | FASN, ACC1, PDHK, LXR, and 2-HG favor Th17 activation | |
| Regulatory T cells (Treg) | Exogenous FA uptake dominates over FA synthesis, OXPHOS, and FAO | Foxp3, FABP5, CPT1, downregulation of ACC1, SREBPs, and SCAP | |
| Memory T cells (Tm) | OXPHOS and FAO, FA uptake, and downregulation of FA synthesis | Enzymes of OXPHOS and FAO, FABP4/5, AMPK, and downregulation of ACC1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassar, A.F.; Nie, X.; Zhang, T.; Yeung, J.; Norris, P.; He, J.; Ogura, H.; Babar, M.U.; Muldoon, A.; Libreros, S.; et al. Is Lipid Metabolism of Value in Cancer Research and Treatment? Part I- Lipid Metabolism in Cancer. Metabolites 2024, 14, 312. https://doi.org/10.3390/metabo14060312
Nassar AF, Nie X, Zhang T, Yeung J, Norris P, He J, Ogura H, Babar MU, Muldoon A, Libreros S, et al. Is Lipid Metabolism of Value in Cancer Research and Treatment? Part I- Lipid Metabolism in Cancer. Metabolites. 2024; 14(6):312. https://doi.org/10.3390/metabo14060312
Chicago/Turabian StyleNassar, Ala F., Xinxin Nie, Tianxiang Zhang, Jacky Yeung, Paul Norris, Jianwei He, Hideki Ogura, Muhammad Usman Babar, Anne Muldoon, Stephania Libreros, and et al. 2024. "Is Lipid Metabolism of Value in Cancer Research and Treatment? Part I- Lipid Metabolism in Cancer" Metabolites 14, no. 6: 312. https://doi.org/10.3390/metabo14060312






