Bilirubin Levels in Infancy and Their Associations with Body Weight, Levels of Iron-Related Parameters and Steroid Hormone Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measures
2.3. Anthropometric Data
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
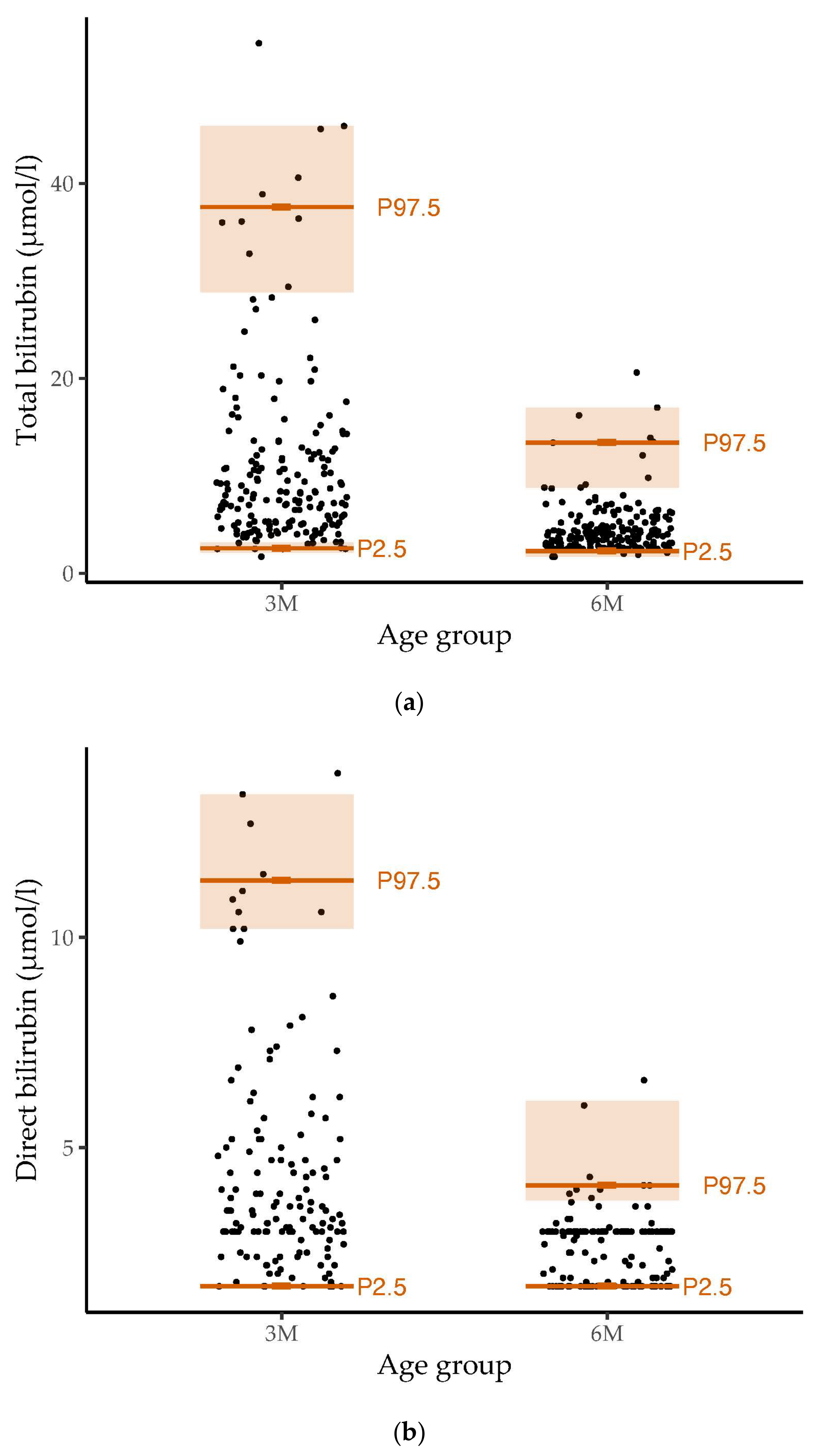
3.2. Reference Values for Bilirubin and Associations with Breastfeeding
3.3. Associations between Body Weight and Bilirubin Levels
3.4. Associations between Concentrations of Ferritin/Transferrin and Bilirubin Levels
3.5. Associations between Concentrations of Selected Sex Hormones and Bilirubin Levels
4. Discussion
4.1. Principal Findings
4.2. Reference Values for Bilirubin and Associations with Breastfeeding
4.3. Associations between Body Weight and Bilirubin Levels
4.4. Associations between Concentrations of Ferritin/Transferrin and Bilirubin Levels
4.5. Associations between Concentrations of Selected Sex Hormones and Bilirubin Levels
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine Green Kinetics to Assess Liver Function: Ready for a Clinical Dynamic Assessment in Major Liver Surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef]
- López-Velázquez, J.A.; Chávez-Tapia, N.C.; Ponciano-Rodríguez, G.; Sánchez-Valle, V.; Caldwell, S.H.; Uribe, M.; Méndez-Sánchez, N. Bilirubin Alone as a Biomarker for Short-Term Mortality in Acute-on-Chronic Liver Failure: An Important Prognostic Indicator. Ann. Hepatol. 2013, 13, 98–104. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The Enzymatic Conversion of Heme to Bilirubin by Microsomal Heme Oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Vítek, L.; Tiribelli, C. Bilirubin: The Yellow Hormone? J. Hepatol. 2021, 75, 1485–1490. [Google Scholar] [CrossRef]
- Chen, W.; Maghzal, G.J.; Ayer, A.; Suarna, C.; Dunn, L.L.; Stocker, R. Absence of the Biliverdin Reductase-a Gene Is Associated with Increased Endogenous Oxidative Stress. Free Radic. Biol. Med. 2018, 115, 156–165. [Google Scholar] [CrossRef]
- Turi, K.N.; McKennan, C.; Gebretsadik, T.; Snyder, B.; Seroogy, C.M.; Lemanske, R.F.; Zoratti, E.; Havstad, S.; Ober, C.; Lynch, S.; et al. Unconjugated Bilirubin Is Associated with Protection from Early-Life Wheeze and Childhood Asthma. J. Allergy Clin. Immunol. 2021, 148, 128–138. [Google Scholar] [CrossRef]
- Adin, C.A.; VanGundy, Z.C.; Papenfuss, T.L.; Xu, F.; Ghanem, M.; Lakey, J.; Hadley, G.A. Physiologic Doses of Bilirubin Contribute to Tolerance of Islet Transplants by Suppressing the Innate Immune Response. Cell Transplant. 2017, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Bloc, S.; Foresti, R.; Almolki, A.; Taillé, C.; Callebert, J.; Conti, M.; Goven, D.; Aubier, M.; Dureuil, B.; et al. Bilirubin Decreases Nos2 Expression via Inhibition of NAD(P)H Oxidase: Implications for Protection against Endotoxic Shock in Rats. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1890–1892. [Google Scholar] [CrossRef]
- Muraca, M.; Fevery, J. Influence of Sex and Sex Steroids on Bilirubin Uridine Diphosphate-Glucuronosyltransferase Activity of Rat Liver. Gastroenterology 1984, 87, 308–313. [Google Scholar] [CrossRef]
- Gordon, D.M.; Neifer, K.L.; Hamoud, A.-R.A.; Hawk, C.F.; Nestor-Kalinoski, A.L.; Miruzzi, S.A.; Morran, M.P.; Adeosun, S.O.; Sarver, J.G.; Erhardt, P.W.; et al. Bilirubin Remodels Murine White Adipose Tissue by Reshaping Mitochondrial Activity and the Coregulator Profile of Peroxisome Proliferator–Activated Receptor α. J. Biol. Chem. 2020, 295, 9804–9822. [Google Scholar] [CrossRef]
- Zou, L.; Yu, H.; He, Y.; Luo, L.; Dong, W.; Zhang, J.; Lei, X.; Wieg, C. Associations between Neonatal Serum Bilirubin and Childhood Obesity in Term Infants. Sci. Rep. 2019, 9, 14575. [Google Scholar] [CrossRef]
- Seyed Khoei, N.; Grindel, A.; Wallner, M.; Mölzer, C.; Doberer, D.; Marculescu, R.; Bulmer, A.; Wagner, K.-H. Mild Hyperbilirubinaemia as an Endogenous Mitigator of Overweight and Obesity: Implications for Improved Metabolic Health. Atherosclerosis 2018, 269, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Hosick, P.A.; Chen, S.; Tukey, R.H.; Hankins, M.W.; Nestor-Kalinoski, A.; Stec, D.E. Mice with Hyperbilirubinemia Due to Gilbert’s Syndrome Polymorphism Are Resistant to Hepatic Steatosis by Decreased Serine 73 Phosphorylation of PPARα. Am. J. Physiol.-Endocrinol. Metab. 2017, 312, E244–E252. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, H.; Zhang, Y.; Cao, M.; Song, L.; Pan, Q.; Bulmer, A.; Adams, D.B.; Dong, X.; Wang, H. Bilirubin Increases Insulin Sensitivity by Regulating Cholesterol Metabolism, Adipokines and PPARγ Levels. Sci. Rep. 2015, 5, 9886. [Google Scholar] [CrossRef] [PubMed]
- Poulain, T.; Baber, R.; Vogel, M.; Pietzner, D.; Kirsten, T.; Jurkutat, A.; Hiemisch, A.; Hilbert, A.; Kratzsch, J.; Thiery, J.; et al. The LIFE Child Study: A Population-Based Perinatal and Pediatric Cohort in Germany. Eur. J. Epidemiol. 2017, 32, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Hesse, M.; Döhnert, M.; Fuchs, M.; Hirsch, C.; Sergeyev, E.; Casprzig, N.; Geserick, M.; Naumann, S.; Koch, C.; et al. The LIFE Child Study: A Life Course Approach to Disease and Health. BMC Public Health 2012, 12, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gaudl, A.; Kratzsch, J.; Bae, Y.J.; Kiess, W.; Thiery, J.; Ceglarek, U. Liquid Chromatography Quadrupole Linear Ion Trap Mass Spectrometry for Quantitative Steroid Hormone Analysis in Plasma, Urine, Saliva and Hair. J. Chromatogr. A 2016, 1464, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gemeinsamer Bundesausschuss (G-BA). Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krankheiten bei Kindern (Kinder-Richtlinie). Available online: https://www.g-ba.de/downloads/62-492-3190/Kinder-RL_2023-05-12_iK-2023-07-13.pdf (accessed on 5 December 2023).
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/(accessed on 17 December 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Robinson, D.; Hayes, A.; Couch, S. broom: Convert Statistical Objects into Tidy Tibbles. R Package Version 1.0.5. 2023. Available online: https://CRAN.R-project.org/package=broom (accessed on 1 May 2024).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Pedersen, T.; Seidel, D. scales: Scale Functions for Visualization. R Package Version 1.3.0. 2023. Available online: https://CRAN.R-project.org/package=scales (accessed on 1 May 2024).
- Badakhshan, S.N.; Ghazizadeh, H.; Mohammadi-Bajgiran, M.; Esmaily, H.; Khorasani, M.Y.; Bohn, M.K.; Pashirzad, M.; Khodabandeh, A.K.; Zadeh, S.G.; Alami-Arani, I.; et al. Age-specific Reference Intervals for Liver Function Tests in Healthy Neonates, Infants, and Young Children in Iran. J. Clin. Lab. Anal. 2023, 37, e24995. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Chen, H.; Gan, Y.; Li, J.; Huang, H. Establishing Reference Intervals of 20 Biochemical Markers for Children in Southwestern Fujian, China Based on the UniCel DxC 800 System. Ann. Clin. Biochem. 2020, 57, 435–443. [Google Scholar] [CrossRef]
- Kolho, K.-L.; Lahtiharju, T.; Merras-Salmio, L.; Pakarinen, M.P.; Knip, M. Infant Liver Biochemistry Is Different than Current Laboratory Accepted Norms. Eur. J. Pediatr. 2023, 182, 5707–5711. [Google Scholar] [CrossRef] [PubMed]
- Humberg, A.; Kammer, J.; Mordmüller, B.; Kremsner, P.G.; Lell, B. Haematological and Biochemical Reference Intervals for Infants and Children in Gabon. Trop. Med. Int. Health TMIH 2011, 16, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Hassan, B.; Zakerihamidi, M. The Correlation between Frequency and Duration of Breastfeeding and the Severity of Neonatal Hyperbilirubinemia. J. Matern. Fetal Neonatal Med. 2018, 31, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, Y.; Huang, M.; He, J.; Qiu, X. Breast Milk Constituents and the Development of Breast Milk Jaundice in Neonates: A Systematic Review. Nutrients 2023, 15, 2261. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Q.; Zheng, L.; Lin, M.; Zheng, X.; Lin, F.; Yang, L.-Y. Multiple Genetic Modifiers of Bilirubin Metabolism Involvement in Significant Neonatal Hyperbilirubinemia in Patients of Chinese Descent. PLoS ONE 2015, 10, e0132034. [Google Scholar] [CrossRef] [PubMed]
- El-Eshmawy, M.M.; Mahsoub, N.; Asar, M.; Elsehely, I. Association Between Total Bilirubin Levels and Cardio-Metabolic Risk Factors Related to Obesity. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kardum, D.; Serdarušić, I.; Biljan, B.; Šantić, K.; Živković, V.; Kos, M. Cord Blood Bilirubin and Prediction of Neonatal Hyperbilirubinemia and Perinatal Infection in Newborns at Risk of Hemolysis. J. Pediatr. (Rio J.) 2021, 97, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Guo, H.; Ma, R.; Yan, Y.; Hu, Y.; Ma, J.; Zhang, X.; Wang, X.; Wang, K.; Mu, L.; et al. Association of Total Bilirubin and Indirect Bilirubin Content with Metabolic Syndrome among Kazakhs in Xinjiang. BMC Endocr. Disord. 2020, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Kipp, Z.A.; Martinez, G.J.; Bates, E.A.; Maharramov, A.B.; Flight, R.M.; Moseley, H.N.B.; Morris, A.J.; Stec, D.E.; Hinds, T.D. Bilirubin Nanoparticle Treatment in Obese Mice Inhibits Hepatic Ceramide Production and Remodels Liver Fat Content. Metabolites 2023, 13, 215. [Google Scholar] [CrossRef]
- Kawamoto, R.; Kikuchi, A.; Akase, T.; Ninomiya, D.; Kasai, Y.; Ohtsuka, N.; Kumagi, T. Total Bilirubin Independently Predicts Incident Metabolic Syndrome among Community-Dwelling Women. Diabetes Metab. Syndr. 2019, 13, 1329–1334. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Luo, Y. Impact of Triglyceride Playing on Stroke Severity Correlated to Bilirubin. Medicine 2020, 99, e21792. [Google Scholar] [CrossRef] [PubMed]
- Bellarosa, C.; Bedogni, G.; Bianco, A.; Cicolini, S.; Caroli, D.; Tiribelli, C.; Sartorio, A. Association of Serum Bilirubin Level with Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease: A Cross-Sectional Study of 1672 Obese Children. J. Clin. Med. 2021, 10, 2812. [Google Scholar] [CrossRef]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D. Bilirubin Binding to PPARα Inhibits Lipid Accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhu, H.; Wang, Y.; Zhang, K.; Chen, Y.; Fang, S.; Xia, F.; Wang, N.; Zhang, W.; Lu, Y. Associations between Different Bilirubin Subtypes and Diabetic Microvascular Complications in Middle-Aged and Elderly Individuals. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820937897. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dong, M.; Deng, Y.; Zhang, L.; Deng, F.; Zhou, J.; Yuan, Z. Relation of Direct, Indirect, and Total Bilirubin to Adverse Long-Term Outcomes Among Patients With Acute Coronary Syndrome. Am. J. Cardiol. 2019, 123, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Terrenato, L.; Bertilaccio, C.; Spinelli, P.; Colombo, B. The Switch from Haemoglobin F to A: The Time Course of Qualitative and Quantitative Variations of Haemoglobins after Birth. Br. J. Haematol. 1981, 47, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bard, H. The Postnatal Decline of Hemoglobin F Synthesis in Normal Full-Term Infants. J. Clin. Investig. 1975, 55, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chau, L.-Y.; Chen, J.-W.; Lin, S.-J. Serum Bilirubin and Ferritin Levels Link Heme Oxygenase-1 Gene Promoter Polymorphism and Susceptibility to Coronary Artery Disease in Diabetic Patients. Diabetes Care 2008, 31, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.; Vogel, M.; Engel, C.; Ceglarek, U.; Harms, K.; Wurst, U.; Lengfeld, H.; Richter, M.; Kiess, W. Does Physiological Distribution of Blood Parameters in Children Depend on Socioeconomic Status? Results of a German Cross-Sectional Study. BMJ Open 2018, 8, e019143. [Google Scholar] [CrossRef]
- Holmlund-Suila, E.M.; Hauta-Alus, H.H.; Enlund-Cerullo, M.; Rosendahl, J.; Valkama, S.M.; Andersson, S.; Mäkitie, O. Iron Status in Early Childhood Is Modified by Diet, Sex and Growth: Secondary Analysis of a Randomized Controlled Vitamin D Trial. Clin. Nutr. Edinb. Scotl. 2022, 41, 279–287. [Google Scholar] [CrossRef]
- Larsson, S.M.; Hillarp, A.; Hellström-Westas, L.; Domellöf, M.; Lundahl, T.; Andersson, O. When Age Really Matters; Ferritin Reference Intervals during Infancy Revisited. Scand. J. Clin. Lab. Investig. 2019, 79, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Acosta, A.; Duque, X.; Trejo-Valdivia, B.; Flores-Huerta, S.; Flores-Hernández, S.; Martínez-Andrade, G.; González-Unzaga, M.; Turnbull, B.; Escalante-Izeta, E.; Klünder-Klünder, M.; et al. Cut-off Points for Serum Ferritin to Identify Low Iron Stores during the First Year of Life in a Cohort of Mexican Infants. Matern. Child. Nutr. 2021, 17, e13205. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-N.; Niu, Q.-M.; Ge, L.; Zhang, N.; Yan, S.-F.; Chen, W.-B.; Chang, Y.-Z.; Zhao, S.-E. Sex Differences in Iron Status and Hepcidin Expression in Rats. Biol. Trace Elem. Res. 2014, 160, 258–267. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, S.; Page, K.E.; Lee, S.-M.; Loguinov, A.; Valore, E.; Hui, S.T.; Jung, G.; Zhou, J.; Lusis, A.J.; Fuqua, B.; et al. Hamp1 mRNA and Plasma Hepcidin Levels Are Influenced by Sex and Strain but Do Not Predict Tissue Iron Levels in Inbred Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G511–G523. [Google Scholar] [CrossRef] [PubMed]
- Vulinović, M.P.; Turčić, P.; Micek, V.; Ljubojević, M. Light and Heavy Ferritin Chain Expression in The Liver and Kidneys of Wistar Rats: Aging, Sex Differences, and Impact of Gonadectomy. Arch. Ind. Hyg. Toxicol. 2022, 73, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zimiao, C.; Dongdong, L.; Shuoping, C.; Peng, Z.; Fan, Z.; Rujun, C.; Xiaohua, G. Correlations Between Iron Status and Body Composition in Patients With Type 2 Diabetes Mellitus. Front. Nutr. 2022, 9, 911860. [Google Scholar] [CrossRef] [PubMed]
- Kanias, T.; Sinchar, D.; Osei-Hwedieh, D.; Baust, J.J.; Jordan, A.; Zimring, J.C.; Waterman, H.R.; de Wolski, K.S.; Acker, J.P.; Gladwin, M.T. Testosterone-Dependent Sex Differences in Red Blood Cell Hemolysis in Storage, Stress and Disease. Transfusion 2016, 56, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Ohlander, S.J.; Varghese, B.; Pastuszak, A.W. Erythrocytosis Following Testosterone Therapy. Sex. Med. Rev. 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Jansen, C.A.; Rutten, V.P.M.G.; Kemp, B.; Engel, B.; Wolthuis-Fillerup, M.; van Reenen, K. Effects of Pre-Transport Diet, Transport Duration and Transport Condition on Immune Cell Subsets, Haptoglobin, Cortisol and Bilirubin in Young Veal Calves. PLoS ONE 2021, 16, e0246959. [Google Scholar] [CrossRef]
- Dvorácsek, E.; Kassai, Z.; Török, I.; Balogh, A. Sexual Steroid Levels and Their Clinical Significance in the Early Neonatal Age. Acta Physiol. Hung. 1990, 76, 151–158. [Google Scholar]
- Soltys, B.J.; Hsia, J.C. Steroid Modulation of Human Serum Albumin Binding Properties. A Spin Label Study. J. Biol. Chem. 1978, 253, 4266–4269. [Google Scholar] [CrossRef] [PubMed]
- Raju, V.S.; McCoubrey, W.K.; Maines, M.D. Regulation of Heme Oxygenase-2 by Glucocorticoids in Neonatal Rat Brain: Characterization of a Functional Glucocorticoid Response Element. Biochim. Biophys. Acta BBA-Gene Struct. Expr. 1997, 1351, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Zeidler, R.; Baber, R.; Vogel, M.; Wirkner, K.; Loeffler, M.; Ceglarek, U.; Kiess, W.; Körner, A.; Thiery, J.; et al. Reference Intervals of Nine Steroid Hormones over the Life-Span Analyzed by LC-MS/MS: Effect of Age, Gender, Puberty, and Oral Contraceptives. J. Steroid Biochem. Mol. Biol. 2019, 193, 105409. [Google Scholar] [CrossRef] [PubMed]
- Poulain, T.; Vogel, M.; Sobek, C.; Hilbert, A.; Körner, A.; Kiess, W. Associations Between Socio-Economic Status and Child Health: Findings of a Large German Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 677. [Google Scholar] [CrossRef] [PubMed]




| 3 Months | 6 Months | |||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||
| N = 99 | N = 107 | N = 97 | N = 108 | |||
| Mean (±SD) | Mean (±SD) | p Value | Mean (±SD) | Mean (±SD) | p Value | |
| Age (years) | 0.24 (0.04) | 0.23 (0.04) | 0.405 | 0.49 (0.04) | 0.49 (0.05) | 0.772 |
| Height (cm) | 59.6 (2.48) | 61.5 (2.84) | <0.001 | 66.2 (2.87) | 68.8 (2.53) | <0.001 |
| Weight (kg) | 5.50 (0.60) | 6.05 (0.75) | <0.001 | 7.07 (0.87) | 7.88 (0.86) | <0.001 |
| BMI (kg/m2) | 15.5 (1.08) | 15.9 (1.32) | 0.004 | 16.1 (1.31) | 16.6 (1.18) | 0.004 |
| PI (kg/m3) | 26.0 (2.12) | 26.0 (2.43) | 0.903 | 24.4 (2.18) | 24.2 (1.79) | 0.508 |
| Total bilirubin (µmol/l) n | 10.7 (7.82) 89 (89.9%) | 10.6 (9.98) 93 (86.9%) | 0.964 | 4.62 (3.08) 89 (91.8%) | 4.34 (2.30) 105 (97.2%) | 0.478 |
| Direct bilirubin (µmol/l) n | 4.35 (2.47) 60 (60.6%) | 4.37 (2.73) 76 (71.0%) | 0.969 | 2.66 (0.84) 69 (71.1%) | 2.61 (0.77) 85 (78.7%) | 0.680 |
| Indirect bilirubin (µmol/l) n | 7.16 (5.77) 50 (50.5%) | 7.47 (8.29) 63 (58.9%) | 0.811 | 4.74 (3.43) 20 (20.6%) | 3.67 (2.50) 28 (25.9%) | 0.242 |
| Hemoglobin (g/dl) n | 11.0 (1.12) 91 (91.9%) | 11.0 (0.95) 101 (94.4%) | 0.984 | 11.7 (1.06) 90 (92.8%) | 11.7 (1.17) 104 (96.3%) | 1.00 |
| Ferritin (ng/mL) n | 239 (134) 23 (23.2%) | 272 (129) 24 (22.2%) | 0.397 | 57.4 (37.3) 24 (24.7%) | 63.3 (44.0) 38 (35.2%) | 0.573 |
| Transferrin (g/l) n | 2.39 (0.34) 20 (20.2%) | 2.41 (0.37) 22 (20.6%) | 0.830 | 2.68 (0.47) 24 (24.7%) | 2.72 (0.37) 38 (35.2%) | 0.707 |
| Testosterone (nmol/l) n | 0.08 (0.06) 34 (35.1%) | 1.04 (1.13) 50 (46.3%) | <0.001 | |||
| Estradiol (pmol/l) n | 51.6 (12.6) 34 (35.1%) | 38.1 (3.38) 50 (46.3%) | <0.001 | |||
| Progesterone (nmol/l) n | 0.34 (0.21) 34 (35.1%) | 0.28 (0.07) 50 (46.3%) | 0.089 | |||
| 17-OH-progesterone (nmol/l) n | 0.90 (0.51) 34 (35.1%) | 0.65 (0.32) 50 (46.3%) | 0.014 | |||
| Androstenedione (nmol/l) n | 0.39 (0.22) 34 (35.1%) | 0.23 (0.15) 50 (46.3%) | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosse-Thie, C.; Vogel, M.; Baber, R.; Ceglarek, U.; Kiess, W. Bilirubin Levels in Infancy and Their Associations with Body Weight, Levels of Iron-Related Parameters and Steroid Hormone Levels. Metabolites 2024, 14, 393. https://doi.org/10.3390/metabo14070393
Grosse-Thie C, Vogel M, Baber R, Ceglarek U, Kiess W. Bilirubin Levels in Infancy and Their Associations with Body Weight, Levels of Iron-Related Parameters and Steroid Hormone Levels. Metabolites. 2024; 14(7):393. https://doi.org/10.3390/metabo14070393
Chicago/Turabian StyleGrosse-Thie, Charlotte, Mandy Vogel, Ronny Baber, Uta Ceglarek, and Wieland Kiess. 2024. "Bilirubin Levels in Infancy and Their Associations with Body Weight, Levels of Iron-Related Parameters and Steroid Hormone Levels" Metabolites 14, no. 7: 393. https://doi.org/10.3390/metabo14070393





