Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training
Abstract
1. Introduction
2. Materials and Methods
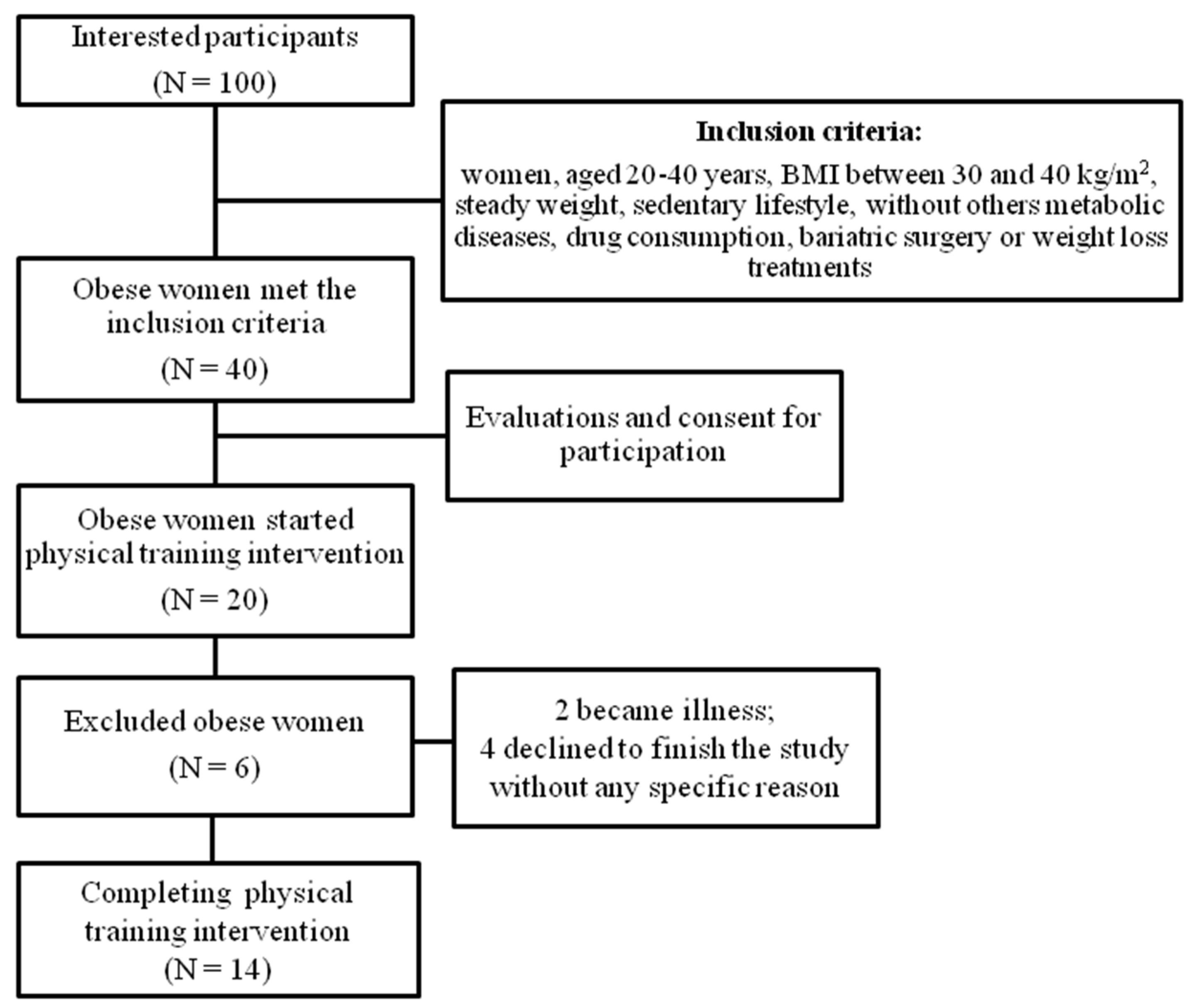
2.1. Ethical Aspects, Participants, and Study Design
2.2. Body Composition and Anthropometric Data Wall-Mounted Stadiometer
2.3. Plasma Collection and Biochemical Quantification
2.4. Physical Performance Test
2.5. Physical Training Intervention
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Riccò, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. IARC working group on Energy Balance and Obesity. Energy balance and obesity: What are the main drivers? Cancer Causes Control. 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Panuganti, K.K.; Nguyen, M.; Kshirsagar, R.K. Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sturm, R.; An, R. Obesity and economic environments. CA Cancer J. Clin. 2014, 64, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017, 28, 379–405. [Google Scholar]
- Kesherwani, V.; Chavali, V.; Hackfort, B.T.; Tyagi, S.C.; Mishra, P.K. Exercise ameliorates high fat diet induced cardiac dysfunction by increasing interleukin 10. Front. Physiol. 2015, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Di Tullio, M.R. Effect of obesity and overweight on left ventricular diastolic function: A community-based study in an elderly cohort. J. Am. Coll. Cardiol. 2011, 57, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Sun, D.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Changes in Gut Microbiota-Related Metabolites and Long-term Successful Weight Loss in Response to Weight-Loss Diets: The POUNDS Lost Trial. Diabetes Care 2018, 41, 413–419. [Google Scholar] [CrossRef]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J.; Allenspach, M.; von Eckardstein, A.; Müller, D. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J. Nutr. 2016, 146, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef] [PubMed]
- Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013, 17, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Bikman, B.T.; Summers, S.A. Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 2011, 121, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Hussain, M.M. Sphingolipids and Lipoproteins in Health and Metabolic Disorders. Trends Endocrinol. Metab. 2017, 28, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Meng, J.; Yu, H. Trimethylamine N-oxide Supplementation Abolishes the Cardioprotective Effects of Voluntary Exercise in Mice Fed a Western Diet. Front. Physiol. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Latino, F.; Cataldi, S.; Carvutto, R.; De Candia, M.; D’Elia, F.; Patti, A.; Bonavolontà, V.; Fischetti, F. The Importance of Lipidomic Approach for Mapping and Exploring the Molecular Networks Underlying Physical Exercise: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8734. [Google Scholar] [CrossRef]
- He, M.; Hu, S.; Wang, J.; Wang, J.; Găman, M.A.; Hariri, Z.; Tian, Y. Effect of resistance training on lipid profile in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obs. Obstet. Gynecol. Reprod. Biol. 2023, 288, 18–28. [Google Scholar] [CrossRef]
- San Martin, R.; Brandao, C.F.C.; Junqueira-Franco, M.V.M.; Junqueira, G.P.; de Freitas, E.C.; de Carvalho, F.G.; Rodrigues, C.H.P.; Aguesse, A.; Billon-Crossouard, S.; Krempf, M.; et al. Untargeted lipidomic analysis of plasma from obese women submitted to combined physical exercise. Sci. Rep. 2022, 12, 11541. [Google Scholar] [CrossRef]
- Argyridou, S.; Bernieh, D.; Henson, J.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Suzuki, T.; Yates, T. Associations between physical activity and trimethylamine N-oxide in those at risk of type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001359. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Lee, J.K. Effect of Different Intensities of Aerobic Exercise Combined with Resistance Exercise on Body Fat, Lipid Profiles, and Adipokines in Middle-Aged Women with Obesity. Int. J. Environ. Res. Public Health 2023, 20, 3991. [Google Scholar] [CrossRef]
- Mezghani, N.; Ammar, A.; Boukhris, O.; Abid, R.; Hadadi, A.; Alzahrani, T.M.; Trabelsi, O.; Boujelbane, M.A.; Masmoudi, L.; Ouergui, I.; et al. The Impact of Exercise Training Intensity on Physiological Adaptations and Insulin Resistance in Women with Abdominal Obesity. Healthcare 2022, 10, 2533. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.D.S.; Rodrigues, K.P.; de Almeida, M.L.; Sobrinho, A.C.D.S.; Noronha, N.Y.; Benjamim, C.J.R.; Silva, S.D.; Rodrigues, J.A.L.; Júnior, C.R.B. Comparing Fourteen Weeks of Multicomponent Training Versus Combined Training in Physically Inactive Older Women: A Randomized Trial. Int. J. Environ. Res. Public Health 2023, 20, 2699. [Google Scholar] [CrossRef]
- Yumi Noronha, N.; da Silva Rodrigues, G.; Harumi Yonehara Noma, I.; Fernanda Cunha Brandao, C.; Pereira Rodrigues, K.; Colello Bruno, A.; Sae-Lee, C.; Moriguchi Watanabe, L.; Augusta de Souza Pinhel, M.; Mello Schineider, I.; et al. 14-weeks combined exercise epigenetically modulated 118 genes of menopausal women with prediabetes. Front. Endocrinol. 2022, 13, 895489. [Google Scholar] [CrossRef]
- da Silva Rodrigues, G.; Noronha, N.Y.; Almeida, M.L.; Sobrinho, A.C.D.S.; Watanabe, L.M.; Pinhel, M.A.S.; de Lima, J.G.R.; Zhang, R.; Nonino, C.B.; Alves, C.R.R.; et al. Exercise training modifies the whole blood DNA methylation profile in middle-aged and older women. J. Appl. Physiol. 2023, 134, 610–621. [Google Scholar] [CrossRef]
- Resende, C.M.; Camelo Júnior, J.S.; Vieira, M.N.; Ferriolli, E.; Pfrimer, K.; Perdoná, G.S.; Monteiro, J.P. Body composition measures of obese adolescents by the deuterium oxide dilution method and by bioelectrical impedance. Braz. J. Med. Biol. Res. 2011, 44, 1164–1170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trenteseaux, C.; Gaston, A.T.; Aguesse, A.; Poupeau, G.; de Coppet, P.; Andriantsitohaina, R.; Laschet, J.; Amarger, V.; Krempf, M.; Nobecourt-Dupuy, E.; et al. Perinatal Hypercholesterolemia Exacerbates Atherosclerosis Lesions in Offspring by Altering Metabolism of Trimethylamine-N-Oxide and Bile Acids. Arter. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Croyal, M.; Kaabia, Z.; León, L.; Ramin-Mangata, S.; Baty, T.; Fall, F.; Billon-Crossouard, S.; Aguesse, A.; Hollstein, T.; Sullivan, D.R.; et al. Fenofibrate decreases plasma ceramide in type 2 diabetes patients: A novel marker of CVD? Diabetes Metab. 2018, 44, 143–149. [Google Scholar] [CrossRef]
- Singh, S.J.; Morgan, M.D.; Scott, S.; Walters, D.; Hardman, A.E. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992, 47, 1019–1024. [Google Scholar] [CrossRef]
- Brandao, C.F.C.; Nonino, C.B.; de Carvalho, F.G.; Nicoletti, C.F.; Noronha, N.Y.; San Martin, R.; de Freitas, E.C.; Junqueira-Franco, M.V.M.; Marchini, J.S. The effects of short-term combined exercise training on telomere length in obese women: A prospective, interventional study. Sports Med. Open 2020, 6, 5. [Google Scholar] [CrossRef]
- Heyward, V.H. Advanced fitness assessment and exercise prescription. Med. Sci. Sports Exerc. 1992, 24, 278. [Google Scholar] [CrossRef]
- Brandao, C.F.C.; de Carvalho, F.G.; Souza, A.O.; Junqueira-Franco, M.V.M.; Batitucci, G.; Couto-Lima, C.A.; Fett, C.A.; Papoti, M.; Freitas, E.C.; Alberici, L.C.; et al. Physical training, UCP1 expression, mitochondrial density, and coupling in adipose tissue from women with obesity. Scand. J. Med. Sci. Sports 2019, 29, 1699–1706. [Google Scholar] [CrossRef]
- Foster, C. Monitoring training in athletes with reference to overtraining syndrome. Med. Sci. Sports Exerc. 1998, 30, 1164–1168. [Google Scholar] [CrossRef]
- Szwarcwald, C.L.; Malta, D.C.; Pereira, C.A.; Figueiredo, A.W.; Almeida, W.D.S.; Machado, I.E.; Bacal, N.S.; Silva, A.G.D.; Silva Júnior, J.B.D.; Rosenfeld, L.G. Reference values for laboratory tests of cholesterol, glycosylated hemoglobin and creatinine of the Brazilian adult population. Rev. Bras. Epidemiol. 2019, 22 (Suppl. 02), e190002. [Google Scholar]
- Ferreira, F.C.; Bertucci, D.R.; Barbosa, M.R.; Nunes, J.E.; Botero, J.P.; Rodrigues, M.F.; Shiguemoto, G.E.; Santoro, V.; Verzola, A.C.; Nonaka, R.O.; et al. Circuit resistance training in women with normal weight obesity syndrome: Body composition, cardiometabolic and echocardiographic parameters, and cardiovascular and skeletal muscle fitness. J. Sports Med. Phys. Fitness 2017, 57, 1033–1044. [Google Scholar] [CrossRef]
- Tan, S.; Wang, J.; Cao, L.; Guo, Z.; Wang, Y. Positive effect of exercise training at maximal fat oxidation intensity on body composition and lipid metabolism in overweight middle-aged women. Clin. Physiol. Funct. Imaging 2016, 36, 225–230. [Google Scholar] [CrossRef]
- Kennedy, A.B.; Lavie, C.J.; Blair, S.N. Fitness or Fatness: Which Is More Important? JAMA 2018, 319, 231–232. [Google Scholar] [CrossRef]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term High-Intensity Interval Training on Body Composition and Blood Glucose in Overweight and Obese Young Women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef]
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.R.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, L.; Sawicka, A.K.; Szarmach, A.; Winklewski, P.J.; Olek, R.A.; Gabbianelli, R. A Pilot Study on the Effects of l-Carnitine and Trimethylamine-N-Oxide on Platelet Mitochondrial DNA Methylation and CVD Biomarkers in Aged Women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef] [PubMed]
- Vittori, L.N.; Romasco, J.; Tarozzi, A.; Latessa, P.M. Urinary Markers and Chronic Effect of Physical Exercise. Methods Mol. Biol. 2021, 2292, 193–200. [Google Scholar] [PubMed]
- Trøseid, M.; Hov, J.R.; Nestvold, T.K.; Thoresen, H.; Berge, R.K.; Svardal, A.; Lappegård, K.T. Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.L.; Malin, S.K.; Wang, Z.; Brown, J.M.; Hazen, S.L.; Kirwan, J.P. Effects of Lifestyle Intervention on Plasma Trimethylamine N-Oxide in Obese Adults. Nutrients 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. (1)H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, J.; He, Q.; Geng, Z.; Deng, Z.; Qiao, D. Applying (1)H NMR Spectroscopy to Detect Changes in the Urinary Metabolite Levels of Chinese Half-Pipe Snowboarders after Different Exercises. J. Anal. Methods Chem. 2015, 2015, 315217. [Google Scholar] [CrossRef] [PubMed]
- Enea, C.; Seguin, F.; Petitpas-Mulliez, J.; Boildieu, N.; Boisseau, N.; Delpech, N.; Diaz, V.; Eugène, M.; Dugué, B. (1)H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010, 396, 1167–1176. [Google Scholar] [CrossRef]
- Ufnal, M.; Zadlo, A.; Ostaszewski, R. TMAO: A small molecule of great expectations. Nutrition 2015, 31, 1317–1323. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Fan, Y.; Levison, B.; Hazen, J.E.; Donahue, L.M.; Wu, Y.; Hazen, S.L. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: Refining the gut hypothesis. J. Am. Coll. Cardiol. 2014, 64, 1908–1914. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Denou, E.; Marcinko, K.; Surette, M.G.; Steinberg, G.R.; Schertzer, J.D. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E982–E993. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef]
- Bajpeyi, S.; Myrland, C.K.; Covington, J.D.; Obanda, D.; Cefalu, W.T.; Smith, S.R.; Rustan, A.C.; Ravussin, E. Lipid in skeletal muscle myotubes is associated to the donors’ insulin sensitivity and physical activity phenotypes. Obesity 2014, 22, 426–434. [Google Scholar] [CrossRef]
- Weir, J.M.; Wong, G.; Barlow, C.K.; Greeve, M.A.; Kowalczyk, A.; Almasy, L.; Comuzzie, A.G.; Mahaney, M.C.; Jowett, J.B.; Shaw, J.; et al. Plasma lipid profiling in a large population-based cohort. J. Lipid Res. 2013, 54, 2898–2908. [Google Scholar] [CrossRef]
- Mamtani, M.; Meikle, P.J.; Kulkarni, H.; Weir, J.M.; Barlow, C.K.; Jowett, J.B.; Bellis, C.; Dyer, T.D.; Almasy, L.; Mahaney, M.C.; et al. Plasma dihydroceramide species associate with waist circumference in Mexican American families. Obesity 2014, 22, 950–956. [Google Scholar] [CrossRef]
- Ramírez, S.; Martins, L.; Jacas, J.; Carrasco, P.; Pozo, M.; Clotet, J.; Serra, D.; Hegardt, F.G.; Diéguez, C.; López, M.; et al. Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 2013, 62, 2329–2337. [Google Scholar] [CrossRef]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef]
- Solomon, T.P.; Sistrun, S.N.; Krishnan, R.K.; Del Aguila, L.F.; Marchetti, C.M.; O’Carroll, S.M.; O’Leary, V.B.; Kirwan, J.P. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J. Appl. Physiol. 2008, 104, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Edreira, M.M.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.; et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef]
- Baranowski, M.; Charmas, M.; Długołęcka, B.; Górski, J. Exercise increases plasma levels of sphingoid base-1 phosphates in humans. Acta Physiol. 2011, 203, 373–380. [Google Scholar] [CrossRef]
- Książek, M.; Charmas, M.; Klusiewicz, A.; Zabielski, P.; Długołęcka, B.; Chabowski, A.; Baranowski, M. Endurance training selectively increases high-density lipoprotein-bound sphingosine-1-phosphate in the plasma. Scand. J. Med. Sci. Sports 2018, 28, 57–64. [Google Scholar] [CrossRef]
- Hoofnagle, A.N.; Vaisar, T.; Mitra, P.; Chait, A. HDL lipids and insulin resistance. Curr. Diab. Rep. 2010, 10, 78–86. [Google Scholar] [CrossRef]
- Ooi, E.M.; Watts, G.F.; Chan, D.C.; Chen, M.M.; Nestel, P.J.; Sviridov, D.; Barrett, P.H. Dose-dependent effect of rosuvastatin on VLDL-apolipoprotein C-III kinetics in the metabolic syndrome. Diabetes Care 2008, 31, 1656–1661. [Google Scholar] [CrossRef]
- Kang, S.C.; Kim, B.R.; Lee, S.Y.; Park, T.S. Sphingolipid metabolism and obesity-induced inflammation. Front. Endocrinol. 2013, 4, 67. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum sphingolipids: Relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef] [PubMed]
| Variables | Pre | Post | p-Value | d-Cohen |
|---|---|---|---|---|
| BMI (kg/m2) * | 32 ± 2 | 33 ± 2 | 0.169 | −0.389 |
| Weight (kg) * | 86 ± 8 | 87 ± 9 | 0.150 | −0.408 |
| %FM | 47 ± 3 | 45 ± 5 | 0.312 | 0.281 |
| %FFM | 53 ± 3 | 55 ± 5 | 0.312 | −0.281 |
| Waist circumference * | 93 ± 2 | 91 ± 2 | 0.027 | 0.664 |
| Hip circumference | 118 ± 7 | 117 ± 7 | 0.702 | 0.105 |
| Waist/Hip rate | 0.79 ± 0.08 | 0.77 ± 0.06 | 0.001 | 1.104 |
| VO2max (ml/kg/min) * | 35 ± 3 | 38 ± 3 | 0.002 | −1.015 |
| SpeedLT1 (km/h) | 5 ± 1 | 6 ± 1 | 0.001 | −1.134 |
| SpeedLT2 (km/h) | 6 ± 1 | 7 ± 1 | <0.001 | −1.483 |
| Variables | Pre | Post | p-Value | d-Cohen | Reference Value |
|---|---|---|---|---|---|
| Creatinine (mg/dL) | 0.83 ± 0.9 | 0.84 ± 0.9 | 0.850 | −0.051 | 0.6 and 1.2 |
| Cholesterol (mg/dL) | 177.1 ± 17.5 | 166.8 ± 18.2 | 0.049 | 0.581 | <190 |
| HDL-c (mg/dL) | 29.8 ± 6.2 | 31.9 ± 10.2 | 0.281 | −0.300 | >40 |
| LDL-c (mg/dL) | 126.1 ± 20.6 | 115.4 ± 18.9 | 0.095 | 0.481 | <130 |
| Triglycerides (mg/dL) | 110.8 ± 56.4 | 98.1 ± 49.2 | 0.143 | 0.416 | <150 |
| TMAO (µmol) | 8.5 ± 6.2 | 5.1 ± 2.8 | 0.017 | 0.730 | - |
| Choline (µmol) | 1.7 ± 0.3 | 1.9 ± 0.4 | 0.235 | −0.333 | - |
| Betaine (µmol) | 31.7 ± 7.5 | 32.3 ± 11.1 | 0.768 | −0.080 | - |
| Carnitine (µmol) | 41.3 ± 7.5 | 41.0 ± 7.2 | 0.944 | 0.054 | - |
| Lipids | Pre (nmol/L) | Post (nmol/L) | p-Value | d-Cohen |
|---|---|---|---|---|
| S1P d18:1 | 366.27 ± 82.77 | 471.214 ± 75.87 | 0.003 | −0.974 |
| CER 16:0 | 201.88 ± 36.83 | 160.10 ± 50.19 | 0.028 | 0.659 |
| CER 18:0 | 133.04 ± 60.77 | 104.79 ± 42.11 | 0.044 | 0.597 |
| CER 20:0 | 14.87 ± 6.97 | 13.58 ± 4.62 | 0.498 | 0.194 |
| CER 22:0 | 1909.23 ± 411.61 | 1421.06 ± 375.39 | 0.015 | 0.753 |
| CER 24:0 | 265.79 ± 45.14 | 214.64 ± 67.71 | 0.072 | 0.523 |
| CER 18:1 | 9.50 ± 2.38 | 7.81 ± 2.24 | 0.112 | 0.455 |
| CER 20:1 | 7.83 ± 2.18 | 6.38 ±2.74 | 0.120 | 0.444 |
| CER 22:1 | 7.18 ± 3.46 | 6.65 ± 2.45 | 0.588 | 0.148 |
| CER 24:1 | 1098.98 ± 340.48 | 956.50 ± 370.26 | 0.329 | 0.271 |
| SM 16:0 | 54.64 ± 5.47 | 47.96 ± 9.05 | 0.024 | 0.681 |
| SM 18:0 | 9.35 ± 2.64 | 7.37 ± 2.35 | 0.025 | 0.675 |
| SM 20:0 | 18.34 ± 3.09 | 16.22 ± 3.90 | 0.059 | 0.553 |
| SM 22:0 | 19.77 ± 2.95 | 17.01 ± 4.45 | 0.025 | 0.677 |
| SM 24:0 | 20.44 ± 2.88 | 15.16 ± 5.63 | 0.003 | 0.963 |
| SM 18:1 | 3.35 ± 0.81 | 2.80 ± 0.85 | 0.043 | 0.598 |
| SM 20:1 | 1.19 ± 0.22 | 0.99 ± 0.282 | 0.047 | 0.586 |
| SM 22:1 | 19.66 ± 2.99 | 18.50 ± 4.66 | 0.333 | 0.268 |
| SM 24:1 | 34.49 ± 6.74 | 33.36 ± 9.48 | 0.682 | 0.112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandao, C.F.C.; Krempf, M.; Giolo de Carvalho, F.; Aguesse, A.; Junqueira-Franco, M.V.M.; Batitucci, G.; de Freitas, E.C.; Noronha, N.Y.; Rodrigues, G.d.S.; Junqueira, G.P.; et al. Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training. Metabolites 2024, 14, 398. https://doi.org/10.3390/metabo14080398
Brandao CFC, Krempf M, Giolo de Carvalho F, Aguesse A, Junqueira-Franco MVM, Batitucci G, de Freitas EC, Noronha NY, Rodrigues GdS, Junqueira GP, et al. Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training. Metabolites. 2024; 14(8):398. https://doi.org/10.3390/metabo14080398
Chicago/Turabian StyleBrandao, Camila Fernanda Cunha, Michel Krempf, Flávia Giolo de Carvalho, Audrey Aguesse, Márcia Varella Morandi Junqueira-Franco, Gabriela Batitucci, Ellen Cristini de Freitas, Natalia Yumi Noronha, Guilherme da Silva Rodrigues, Gizela Pedroso Junqueira, and et al. 2024. "Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training" Metabolites 14, no. 8: 398. https://doi.org/10.3390/metabo14080398
APA StyleBrandao, C. F. C., Krempf, M., Giolo de Carvalho, F., Aguesse, A., Junqueira-Franco, M. V. M., Batitucci, G., de Freitas, E. C., Noronha, N. Y., Rodrigues, G. d. S., Junqueira, G. P., Borba, D. A., Billon-Crossouard, S., Croyal, M., & Marchini, J. S. (2024). Sphingolipid and Trimethylamine-N-Oxide (TMAO) Levels in Women with Obesity after Combined Physical Training. Metabolites, 14(8), 398. https://doi.org/10.3390/metabo14080398










