From Hypothalamic Obesity to Metabolic Dysfunction-Associated Steatotic Liver Disease: Physiology Meets the Clinics via Metabolomics
Abstract
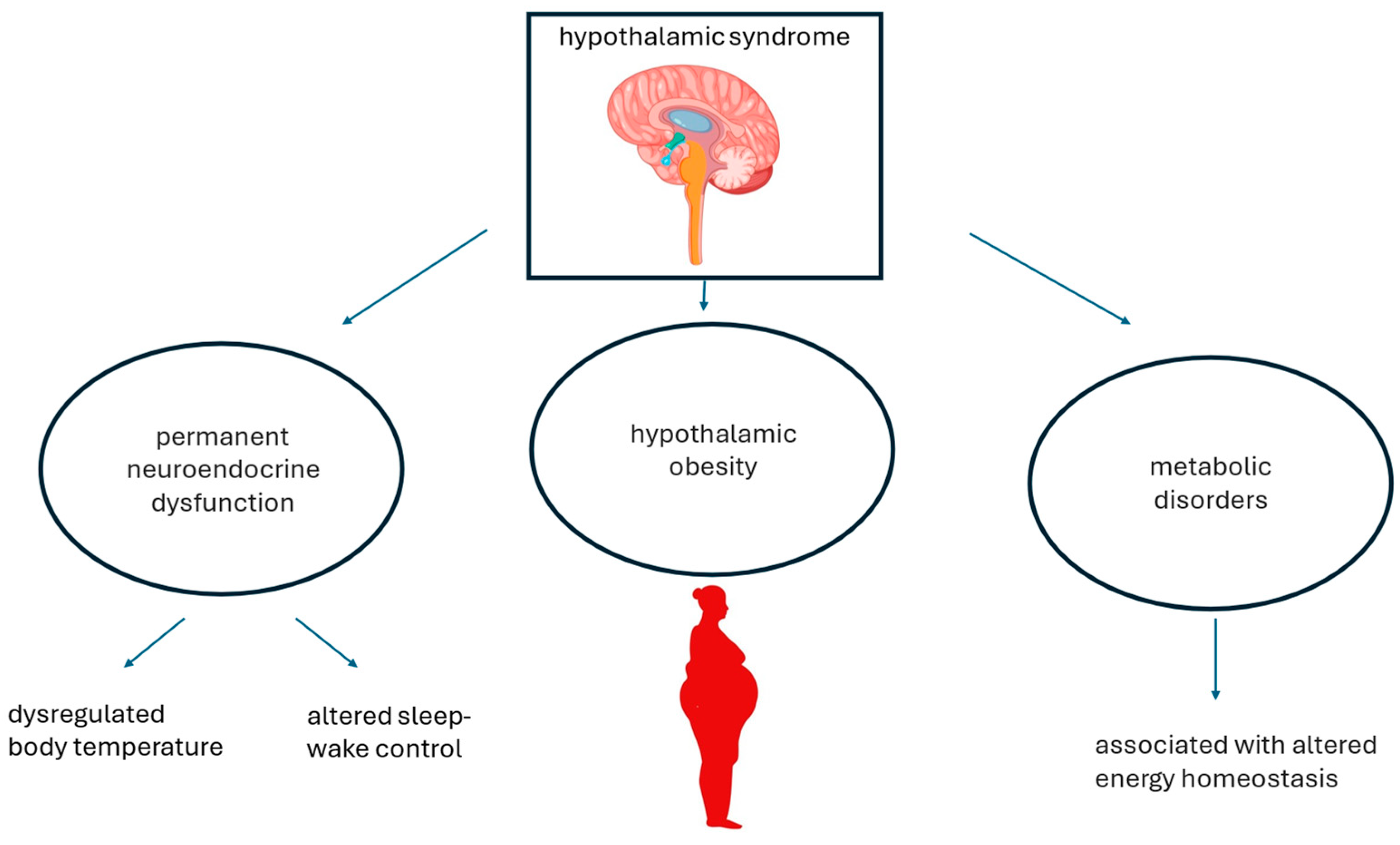
1. Introduction
2. Historical Background
3. Metabolic Dysfunction-Associated Steatotic Liver Disease
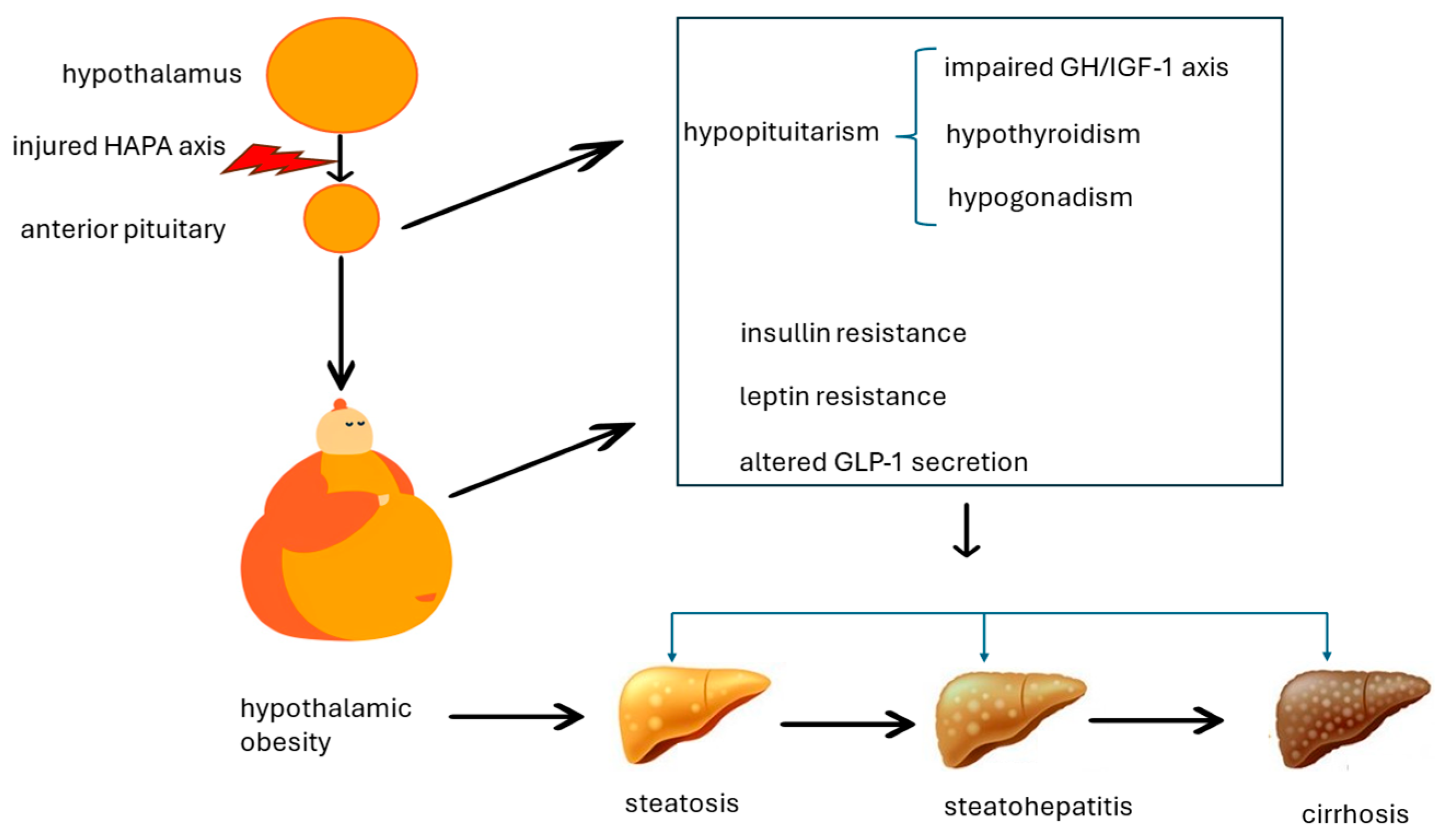
4. Putative Pathomechanisms Associating Hypothalamic Obesity and Metabolic Dysfunction-Associated Steatotic Liver Disease
4.1. Impaired Growth Hormone/Insulin-like Growth Factor-1 Axis
4.2. Hypogonadism
4.3. Hypothyroidism
4.4. Leptin Resistance and Insulin Resistance
4.5. Altered GLP-1 Secretion
5. Progress in Metabolomics
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AR(s) | androgen receptor(s) |
| FFA(s) | free fatty acid(s) |
| GH | growth hormone |
| GLP-1 | glucagon-like peptide 1 |
| IGF-1 | insulin-like growth factor-1 |
| HAPA | hypothalamic-anterior pituitary axis |
| HO | hypothalamic obesity |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MASH | metabolic dysfunction-associated steatohepatitis |
| Pcyt2 | cytidine triphosphate:phosphoethanolamine cytidylyltransferase |
| SUA | uric acid |
| T2D | type 2 diabetes |
References
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, N.; Lebensztejn, D.M. Non-alcoholic fatty liver disease and lipotoxicity. Clin. Exp. Hepatol. 2021, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brobeck, J.R. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol. Rev. 1946, 26, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hirose, H.; Ohneda, M.; Johnson, J.H.; McGarry, J.D.; Unger, R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: Impairment in adipocyte beta-cell relationships. Proc. Natl. Acad. Sci. USA 1994, 91, 10878–10882. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M. Lipotoxicity. Kidney Int. 2006, 70, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.I.; Virtue, S.; Vidal-Puig, A. Obesity as a clinical and public health problem: Is there a need for a new definition based on lipotoxicity effects? Biochim. Biophys. Acta 2010, 1801, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Feldstein, A.; Lindor, K.D.; Angulo, P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology 2004, 39, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Carani, C.; Carulli, N.; Loria, P. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J. Hepatol. 2006, 44, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Loria, P.; Carulli, L.; Bertolotti, M.; Lonardo, A. Endocrine and liver interaction: The role of endocrine pathways in NASH. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 236–247. [Google Scholar] [CrossRef]
- Lee, M.; Korner, J. Review of physiology, clinical manifestations, and management of hypothalamic obesity in humans. Pituitary 2009, 12, 87–95. [Google Scholar] [CrossRef]
- Roth, C.L.; McCormack, S.E. Acquired hypothalamic obesity: A clinical overview and update. Diabetes Obes. Metab. 2024, 26 (Suppl. S2), 34–45. [Google Scholar] [CrossRef]
- Hochberg, I.; Hochberg, Z. Hypothalamic obesity. Endocr. Dev. 2010, 17, 185–196. [Google Scholar] [CrossRef]
- Romigi, A.; Feola, T.; Cappellano, S.; De Angelis, M.; Pio, G.; Caccamo, M.; Testa, F.; Vitrani, G.; Centonze, D.; Colonnese, C.; et al. Sleep Disorders in Patients With Craniopharyngioma: A Physiopathological and Practical Update. Front. Neurol. 2022, 12, 817257. [Google Scholar] [CrossRef]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Gores, G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin. Liver Dis. 2008, 28, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in some common endocrine diseases: Prevalence, pathophysiology, and principles of diagnosis and management. Int. J. Mol. Sci. 2019, 20, 2841. [Google Scholar] [CrossRef] [PubMed]
- Liebe, R.; Esposito, I.; Bock, H.H.; Vom Dahl, S.; Stindt, J.; Baumann, U.; Luedde, T.; Keitel, V. Diagnosis and management of secondary causes of steatohepatitis. J. Hepatol. 2021, 74, 1455–1471. [Google Scholar] [CrossRef]
- Meienberg, F.; Yee, M.; Johnston, D.; Cox, J.; Robinson, S.; Bell, J.D.; Thomas, E.L.; Taylor-Robinson, S.D.; Godsland, I. Liver fat in adults with GH deficiency: Comparison to matched controls and the effect of GH replacement. Clin. Endocrinol. 2016, 85, 76–84. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Zhao, Y.; Nie, M.; Ji, W.; Mao, J.; Wu, X. Metabolic effects of recombinant human growth hormone replacement therapy on juvenile patients after craniopharyngioma tesection. Int. J. Endocrinol. 2022, 2022, 7154907. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Barkan, A.L. Growth hormone therapy in adults with growth hormone deficiency: A critical assessment of the literature. Pituitary 2020, 23, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, H. Sex hormones and their receptors regulate liver energy homeostasis. Int. J. Endocrinol. 2015, 2015, 294278. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Brown, W.C.; Cai, Q.; Krust, A.; Chambon, P.; McGuinness, O.P.; Stafford, J.M. Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 2013, 62, 424–434. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Hu, F. Endocrine disorder in patients with craniopharyngioma. Front. Neurol. 2021, 12, 737743. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Targher, G. Pathogenesis of hypothyroidism-induced NAFLD: Evidence for a distinct disease entity? Dig. Liver Dis. 2019, 51, 462–470. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, randomized, controlled trial of Resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Li, M.D. Leptin and beyond: An odyssey to the central control of body weight. Yale J. Biol. Med. 2011, 84, 1–7. [Google Scholar] [PubMed]
- Guran, T.; Turan, S.; Bereket, A.; Akcay, T.; Unluguzel, G.; Bas, F.; Gunoz, H.; Saka, N.; Bundak, R.; Darendeliler, F.; et al. The role of leptin, soluble leptin receptor, resistin, and insulin secretory dynamics in the pathogenesis of hypothalamic obesity in children. Eur. J. Pediatr. 2009, 168, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cortegana, C.; García-Galey, A.; Tami, M.; Del Pino, P.; Carmona, I.; López, S.; Alba, G.; Sánchez-Margalet, V. Role of leptin in non-alcoholic fatty liver disease. Biomedicines 2021, 9, 762. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R.; Tan, T.M. Glucagon resistance and metabolic-associated steatotic liver disease: A review of the evidence. J. Endocrinol. 2024, 261, e230365. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Epifani, R.; Imbriani, S.; Tortorella, G.; Aprea, C.; Galiero, R.; Rinaldi, L.; Marfella, R.; Sasso, F.C. GLP-1 receptor agonists in non-alcoholic fatty liver disease: Current evidence and future perspectives. Int. J. Mol. Sci. 2023, 24, 1703. [Google Scholar] [CrossRef]
- Chai-Udom, R.; Aroonparkmongkol, S.; Sahakitrungruang, T. Metabolic features and changes in glucose-induced serum glucagon-like peptide-1 levels in children with hypothalamic obesity. J. Pediatr. Endocrinol. Metab. 2020, 33, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, W.; Lyu, X.; Xu, H.; Zhu, H.; Pan, H.; Wang, L.; Yang, H.; Gong, F. The distinct hepatic metabolic profile and relation with impaired liver function in congenital isolated growth hormone-deficient rats. Endocr. Connect. 2024, 13, e230462. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Fang, X. Proteomic and metabolomic analysis of GH deficiency-induced NAFLD in hypopituitarism: Insights into oxidative stress. Front. Endocrinol. 2024, 15, 1371444. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.; Wu, D.; Xie, Y.; Wu, G.; Wu, W.; Wang, H.; Liu, X.; Fan, L.; Xiang, B.; et al. Serum metabolomic and lipidomic profiling reveals the signature for postoperative obesity among adult-onset craniopharyngioma. Metabolites 2024, 14, 338. [Google Scholar] [CrossRef]
- Duncan, R.E. Deficiency of phosphatidylethanolamine synthesis: Consequences for skeletal muscle. Function 2023, 4, zqad044. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Hakimuddin, F.; Bonen, A.; Bakovic, M. The development of a metabolic disease phenotype in CTP:phosphoethanolamine cytidylyltransferase-deficient mice. J. Biol. Chem. 2009, 284, 25704–25713. [Google Scholar] [CrossRef] [PubMed]
- Mourad, S.; Abdualkader, A.M.; Li, X.; Jani, S.; Ceddia, R.B.; Al Batran, R. A high-fat diet supplemented with medium-chain triglycerides ameliorates hepatic steatosis by reducing ceramide and diacylglycerol accumulation in mice. Exp. Physiol. 2024, 109, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, G.; Jamnik, A.; Pajed, L.; Kolleritsch, S.; Hois, V.; Bagaric, A.; Prem, D.; Tilp, A.; Kolb, D.; Wolinski, H.; et al. Carboxylesterase 2a deletion provokes hepatic steatosis and insulin resistance in mice involving impaired diacylglycerol and lysophosphatidylcholine catabolism. Mol. Metab. 2023, 72, 101725. [Google Scholar] [CrossRef] [PubMed]
- Preuss, C.; Jelenik, T.; Bódis, K.; Müssig, K.; Burkart, V.; Szendroedi, J.; Roden, M.; Markgraf, D.F. A new targeted lipidomics approach reveals lipid droplets in liver, muscle and heart as a repository for diacylglycerol and ceramide species in non-alcoholic fatty liver. Cells 2019, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Loria, P.; Leonardi, F.; Borsatti, A.; Neri, P.; Pulvirenti, M.; Verrone, A.M.; Bagni, A.; Bertolotti, M.; Ganazzi, D.; et al. Policentrica Steatosi Epatica Non Alcolica. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig. Liver Dis. 2002, 34, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, T.; Manji, L.; Liu, Y.; Chang, Q.; Zhao, Y.; Ding, Y.; Xia, Y. Association between serum uric acid and non-alcoholic fatty liver disease: An updated systematic review and meta-analysis. Clin. Epidemiol. 2023, 15, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016, 46, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Qiu, K.; Zheng, W.; Kong, W.; Zeng, T. Uric acid may serve as the sixth cardiometabolic criterion for defining MASLD. J. Hepatol. 2024, 80, e152–e153. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cui, W.; Mu, D.; Li, S.; Li, N.; Zhou, W.; Hu, Y. Nomogram for predicting 5-year metabolic dysfunction-associated steatotic liver disease risk: Retrospective cohort study. Endocr. Connect. 2024, 13, e240186. [Google Scholar] [CrossRef]
- Al-Shargi, A.; El Kholy, A.A.; Adel, A.; Hassany, M.; Shaheen, S.M. Allopurinol versus Febuxostat: A new approach for the management of hepatic steatosis in metabolic dysfunction-associated steatotic liver disease. Biomedicines 2023, 11, 3074. [Google Scholar] [CrossRef]
- He, R.; Gao, S.; Yao, H.; Zhao, Z.; Tong, J.; Zhang, H. Mechanism of metabolic response to hepatectomy by integrated analysis of gut microbiota, metabolomics, and proteomics. Microbiol. Spectr. 2023, 11, e0206722. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Liu, L.; Guo, F.; Li, S.; Huang, L.; Sun, C.; Feng, R. Serum metabolomics of NAFLD plus T2DM based on liquid chromatography-mass spectrometry. Clin. Biochem. 2016, 49, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Demirel, M.; Köktaşoğlu, F.; Özkan, E.; Dulun Ağaç, H.; Gül, A.Z.; Sharifov, R.; Sarıkaya, U.; Başaranoğlu, M.; Selek, Ş. Mass spectrometry-based untargeted metabolomics study of non-obese individuals with non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2023, 58, 1344–1350. [Google Scholar] [CrossRef]
- National Library of Medicine. PubChem Compound. Available online: https://www.ncbi.nlm.nih.gov/pccompound/ (accessed on 24 June 2024).
- Lonardo, A.; Byrne, C.D.; Targher, G. Precision medicine approaches in metabolic disorders and target organ damage: Where are we now, and where are we going? Metab. Target. Organ. Damage 2021, 1, 3. [Google Scholar] [CrossRef]
| Genetic | Prader–Willy Syndrome |
|---|---|
| Intracranial neoplasm | Craniopharingyoma, pituitary macroadenoma, glioma, meningioma, teratoma, germ cell tumor, chordoma, hystiocytosis X, hamartoma, metastasis |
| Iatrogenic | Surgery, radiation therapy, subthalamic implants |
| Trauma | Head trauma |
| Inflammatory conditions | Sarcodosis, arachnoditis, encephalitis, tuberculosis |
| Vascular | Aneurysm of the internal carotid artery |
| Others | Hypodipsia–hypernatremia syndrome |
| Species | Findings |
|---|---|
| Rats | In rats, there is an unbalanced ratio of palmitoyl acid, dehydrocholic acid, and 7-ketolithocholic acid, all of which are increased, while lysophosphatidylcholine and fatty acid esters of hydroxy fatty acids are decreased [37]. Additionally, mitochondrial dysfunction, oxidative stress, lipid peroxidation, and reduced glutathione are all increased [38]. |
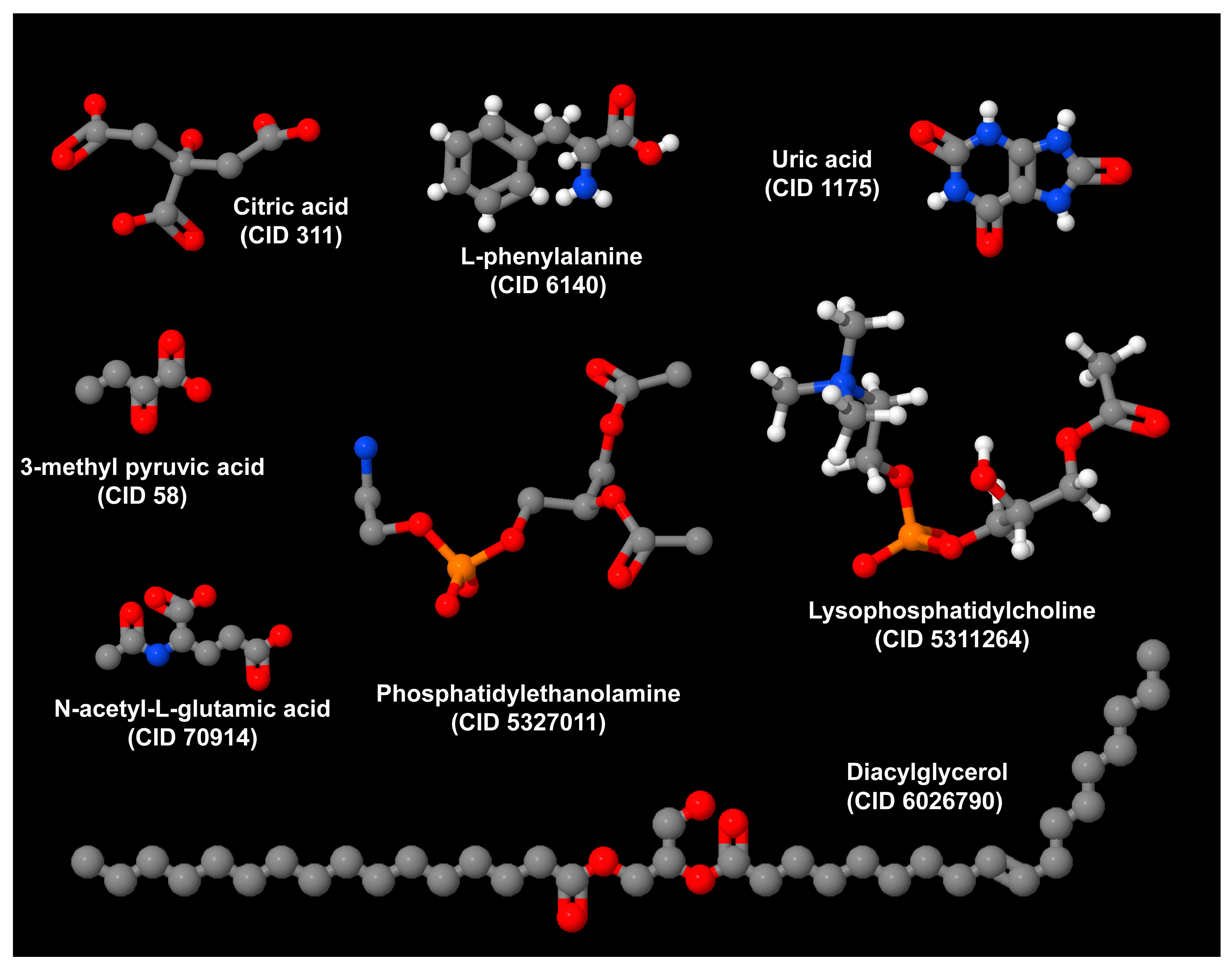
| Humans | In humans, phosphatidylethanolamine (16:0e/22:4), ceramides [(d16:0/16:0) and (d18:1/16:0)], and diacylglycerol (18:2/20:4) predict HO among patients with craniopharyngioma treated with surgery [39]. Additional predictors include citric acid, serum uric acid, N-acetylglutamic acid, 3-methyl pyruvic acid, and L-phenylalanine [39]. Phosphatidylethanolamine, ceramides, and diacylglycerol play a key role in the pathogenesis of MASLD. |
| Method | Findings | Comment | Reference |
|---|---|---|---|
| Ten Lewis dwarf homozygous (dw/dw) rats, and ten Lewis dwarf heterozygous (dw/+) rats were analyzed. | dw/dw rats exhibited more pronounced hepatic steatosis accompanied by higher serum transaminase values. Among dw/dw rats, compared with dw/+ rats, levels of LPC 16:2, LPC 18:3, LPC 22:6, and FAHFA18:1 were significantly decreased, while levels of palmitoyl acid, dehydrocholic acid, and 7-ketolithocholic acid were significantly increased | Distinctive hepatic metabolic profiles are associated with liver steatosis and elevated transaminases in Lewis dw/dw rats with congenital IGHD. | [37] |
| Serum untargeted metabolomics was evaluated in male rats in which HP was induced by hypophysectomy, followed by rhGH HRT replacement therapy. | Among rats with HP, biomarkers of mitochondrial dysfunction and oxidative stress were significantly increased compared with age-matched controls. Additionally, hypophysectomy was associated with severe hepatic steatosis, lipid peroxidation, and reduced levels of GSH, which were subsequently modulated by rhGH HRT. Proteomic analysis identified cytochrome P450s, mitochondrial translation elongation, and PPARA-activating genes as the major distinguishing pathways in hypophysectomized rats. Downregulation of JAK2-STAT5B and upregulation of mTOR signaling pathway. | This study demonstrates an imbalance in oxidative stress resulting from abnormal fatty acid oxidation and NADPH regeneration in a rat model of MASLD associated with hypophysectomy. | [38] |
| Serum metabolomics and lipidomics were compared across three BMI categories in 120 postoperative Chinese adult patients with CP. | CA and SUA had predictive potential for postoperative obesity and overweight in patients with CP, while N-acetylglutamic acid, 3-methyl pyruvic acid, and L-phe precisely predicted the occurrence of postoperative obesity in patients with CP. PE (16:0e/22:4), Cer (d16:0/16:0), DG (36:2e), Cer (d18:1/16:0), and DG (18:2/20:4) were identified as potential predictors for postoperative obesity in patients with CP. | In patients with CP, the leading cause of HO, metabolomics and lipidomics offer a promising tool for discriminating the occurrence of postoperative obesity, with lipidomics exhibiting higher sensitivity. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonardo, A.; Weiskirchen, R. From Hypothalamic Obesity to Metabolic Dysfunction-Associated Steatotic Liver Disease: Physiology Meets the Clinics via Metabolomics. Metabolites 2024, 14, 408. https://doi.org/10.3390/metabo14080408
Lonardo A, Weiskirchen R. From Hypothalamic Obesity to Metabolic Dysfunction-Associated Steatotic Liver Disease: Physiology Meets the Clinics via Metabolomics. Metabolites. 2024; 14(8):408. https://doi.org/10.3390/metabo14080408
Chicago/Turabian StyleLonardo, Amedeo, and Ralf Weiskirchen. 2024. "From Hypothalamic Obesity to Metabolic Dysfunction-Associated Steatotic Liver Disease: Physiology Meets the Clinics via Metabolomics" Metabolites 14, no. 8: 408. https://doi.org/10.3390/metabo14080408
APA StyleLonardo, A., & Weiskirchen, R. (2024). From Hypothalamic Obesity to Metabolic Dysfunction-Associated Steatotic Liver Disease: Physiology Meets the Clinics via Metabolomics. Metabolites, 14(8), 408. https://doi.org/10.3390/metabo14080408






