Metabolic Pathways in Hydrocephalus: Profiling with Proteomics and Advanced Imaging
Abstract
1. Introduction
2. Causes of Neonatal Hydrocephalus
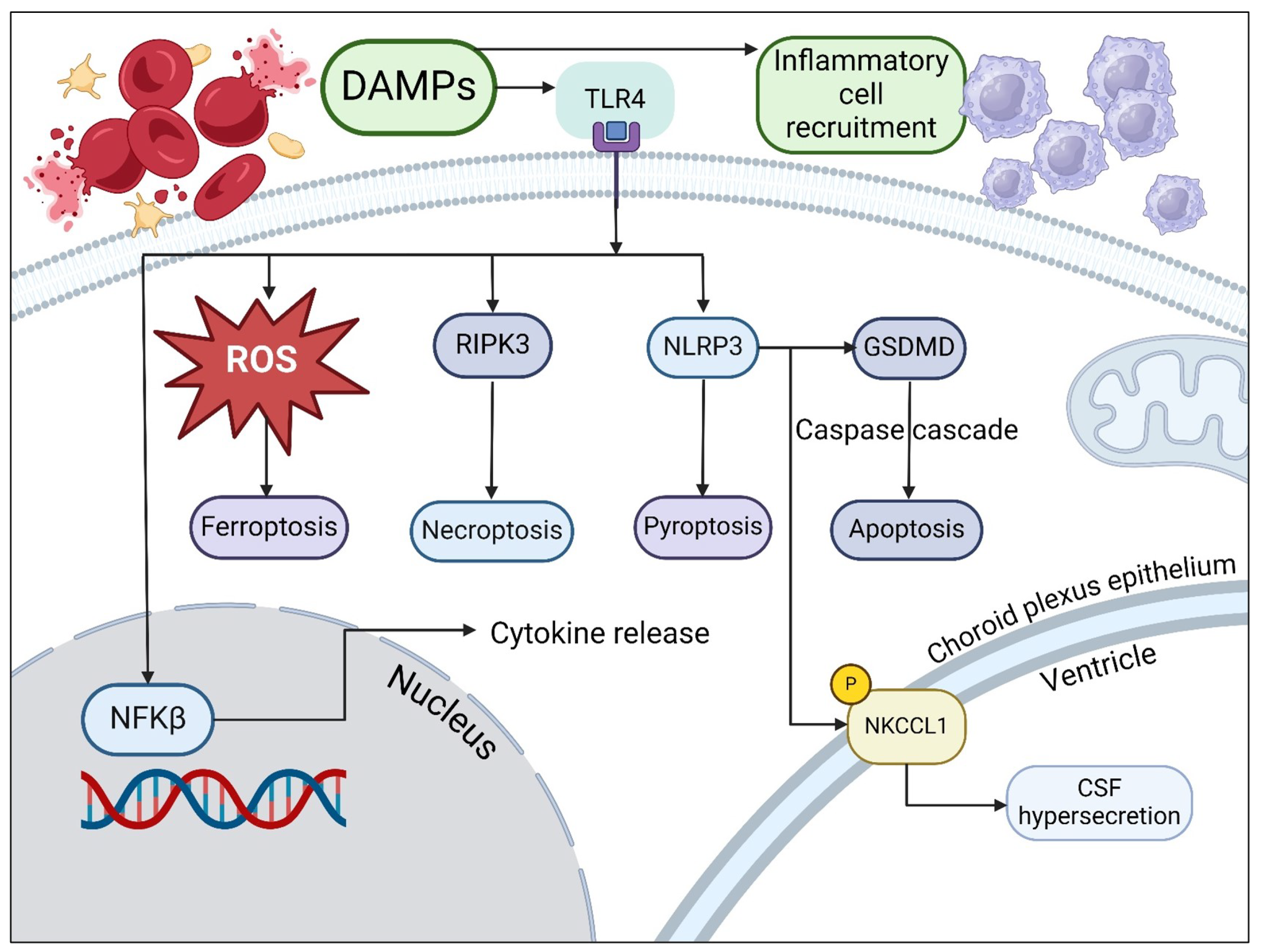
3. Causes of Metabolic Dysfunction in Hemorrhagic Hydrocephalus
4. Neurometabolic Dysfunction
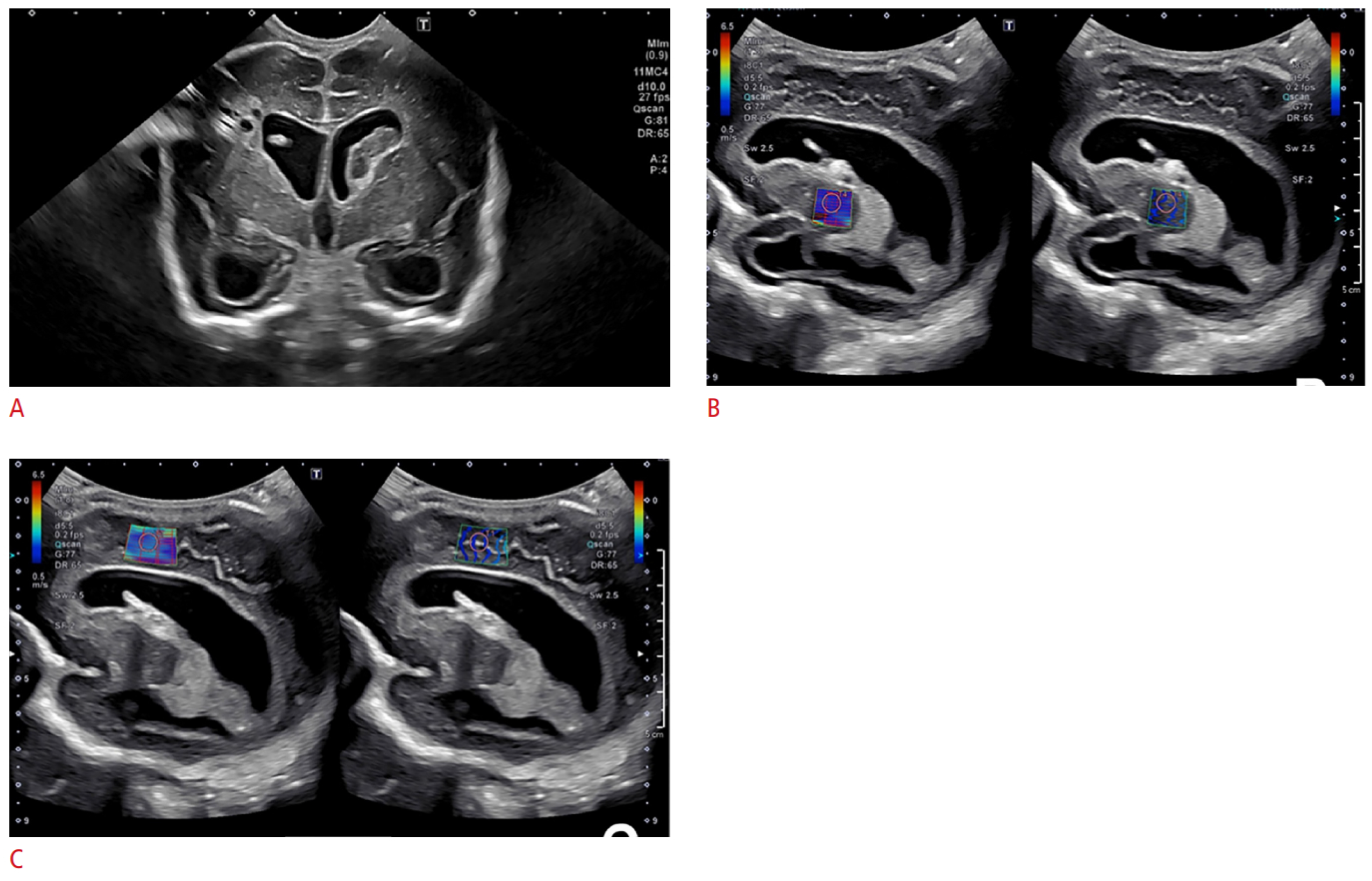
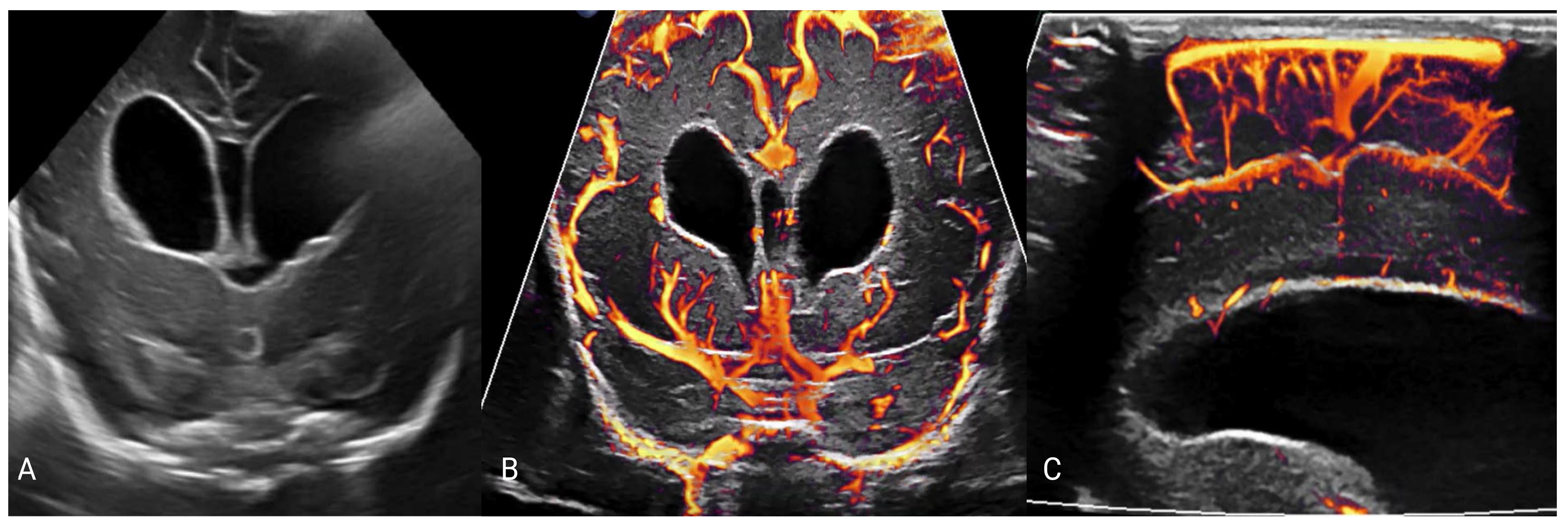
5. Imaging of Neurometabolic Derangement and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Mekary, R.; Glancz, L.J.; Yunusa, I.; Baticulon, R.E.; Fieggen, G.; Wellons, J.C.; Park, K.B.; Warf, B.C. Global hydrocephalus epidemiology and incidence: Systematic review and meta-analysis. J. Neurosurg. 2018, 130, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Flanders, T.M.; Billinghurst, L.; Flibotte, J.; Heuer, G.G. Neonatal Hydrocephalus. NeoReviews 2018, 19, e467–e477. [Google Scholar] [CrossRef]
- Rostgaard, N.; Olsen, M.H.; Lolansen, S.D.; Norager, N.H.; Plomgaard, P.; MacAulay, N.; Juhler, M. Ventricular CSF proteomic profiles and predictors of surgical treatment outcome in chronic hydrocephalus. Acta Neurochir. 2023, 165, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Dandy, W.E. Experimental hydrocephalus. Ann. Surg. 1919, 70, 129–142. [Google Scholar] [CrossRef]
- Greitz, D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: Rationale for third ventriculostomy in communicating hydrocephalus. Child’s Nerv. Syst. 2007, 23, 487–489. [Google Scholar] [CrossRef]
- Bering, E.A. Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J. Neurosurg. 1962, 19, 405–413. [Google Scholar] [CrossRef]
- Karimy, J.K.; Duran, D.; Hu, J.K.; Gavankar, C.; Gaillard, J.R.; Bayri, Y.; Rice, H.; DiLuna, M.L.; Gerzanich, V.; Marc Simard, J.; et al. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg. Focus 2016, 41, E10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, D.; Liu, P.; Liu, Q.; Li, M.; Han, W.; Wang, X.; Zhang, W.; Song, H.; Li, Z.; et al. Quantitative assessment of renal perfusion in children with UPJO by contrast enhanced ultrasound: A pilot study. J. Pediatr. Urol. 2022, 18, 75.e1–75.e7. [Google Scholar] [CrossRef]
- Yuan, L.; Zou, D.; Yang, X.; Chen, X.; Lu, Y.; Zhang, A.; Zhang, P.; Wei, F. Proteomics and functional study reveal kallikrein-6 enhances communicating hydrocephalus. Clin. Proteom. 2021, 18, 30. [Google Scholar] [CrossRef]
- Waybright, T.; Avellino, A.M.; Ellenbogen, R.G.; Hollinger, B.J.; Veenstra, T.D.; Morrison, R.S. Characterization of the human ventricular cerebrospinal fluid proteome obtained from hydrocephalic patients. J. Proteom. 2010, 73, 1156–1162. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Ning, B. Reactive astrocytes in central nervous system injury: Subgroup and potential therapy. Front. Cell. Neurosci. 2021, 15, 792764. [Google Scholar] [CrossRef] [PubMed]
- Karimy, J.K.; Reeves, B.C.; Damisah, E.; Duy, P.Q.; Antwi, P.; David, W.; Wang, K.; Schiff, S.J.; Limbrick, D.D.; Alper, S.L.; et al. Inflammation in acquired hydrocephalus: Pathogenic mechanisms and therapeutic targets. Nat. Rev. Neurol. 2020, 16, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cherian, S.; Whitelaw, A.; Thoresen, M.; Love, S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004, 14, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A. Histopathological study of congenital and acquired experimental hydrocephalus. Brain Dev. 1980, 2, 171–189. [Google Scholar] [CrossRef]
- Holste, K.G.; Xia, F.; Ye, F.; Keep, R.F.; Xi, G. Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: A review. Fluids Barriers CNS 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Xu, H.; Hu, F.; Braun, A.; Smith, K.; Rivera, A.; Lou, N.; Ungvari, Z.; Goldman, S.A.; Csiszar, A.; et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat. Med. 2007, 13, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Howard, S. Glial differentiation in the germinal layer of fetal and preterm infant brain: An immunocytochemical study. Pediatr. Pathol./Affil. Int. Paediatr. Pathol. Assoc. 1988, 8, 25–36. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Linder, N.; Haskin, O.; Levit, O.; Klinger, G.; Prince, T.; Naor, N.; Turner, P.; Karmazyn, B.; Sirota, L. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: A retrospective case-control study. Pediatrics 2003, 111, e590–e595. [Google Scholar] [CrossRef]
- Miyake, K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007, 19, 3–10. [Google Scholar] [CrossRef]
- Kwon, M.S.; Woo, S.K.; Kurland, D.B.; Yoon, S.H.; Palmer, A.F.; Banerjee, U.; Iqbal, S.; Ivanova, S.; Gerzanich, V.; Simard, J.M. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int. J. Mol. Sci. 2015, 16, 5028–5046. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tan, X.; Xia, F.; Hua, Y.; Keep, R.F.; Xi, G. Hydrocephalus Induced by Intraventricular Peroxiredoxin-2: The Role of Macrophages in the Choroid Plexus. Biomolecules 2021, 11, 654. [Google Scholar] [CrossRef]
- Huang, S.; Hu, W.; Rao, D.; Wu, X.; Bai, Q.; Wang, J.; Chu, Z.; Xu, Y. RIPK3-Dependent Necroptosis Activates MCP-1-Mediated Inflammation in Mice after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2022, 31, 106213. [Google Scholar] [CrossRef]
- Guo, F.; Hua, Y.; Wang, J.; Keep, R.F.; Xi, G. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl. Stroke Res. 2012, 3, 130–137. [Google Scholar] [CrossRef]
- Ascenzi, P.; Bocedi, A.; Visca, P.; Altruda, F.; Tolosano, E.; Beringhelli, T.; Fasano, M. Hemoglobin and heme scavenging. IUBMB Life 2005, 57, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Wang, C.; Lu, Q.; Cao, Y.; Yu, W.; Li, W.; Liu, B.; Gao, X.; Lü, J.; Pan, X. Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic. Biol. Med. 2022, 180, 108–120. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Kim, J.B.; Sig Choi, J.; Yu, Y.M.; Nam, K.; Piao, C.S.; Kim, S.W.; Lee, M.H.; Han, P.L.; Park, J.S.; Lee, J.K. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J. Neurosci. 2006, 26, 6413–6421. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. HMGB1-Mediated Neuroinflammatory Responses in Brain Injuries: Potential Mechanisms and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 4609. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Cui, W.; Cao, Y.; Zhao, L.; Wang, H.; Liu, X.; Fan, S.; Huang, K.; Tong, A.; et al. Inhibition of neuronal necroptosis mediated by RIP1/RIP3/MLKL provides neuroprotective effects on kaolin-induced hydrocephalus in mice. Cell Prolif. 2021, 54, e13108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, Q.; Guo, P.; Huang, S.; Jia, Z.; Liu, X.; Feng, H.; Chen, Y. NLRP3 inflammasome-mediated choroid plexus hypersecretion contributes to hydrocephalus after intraventricular hemorrhage via phosphorylated NKCC1 channels. J. Neuroinflamm. 2022, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018, 11, 359–374. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Cheng, S.; Gao, W.; Xu, X.; Fan, H.; Wu, Y.; Li, F.; Zhang, J.; Zhu, X.; Zhang, Y. Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res. Bull. 2016, 127, 226–233. [Google Scholar] [CrossRef]
- Robert, S.M.; Reeves, B.C.; Kiziltug, E.; Duy, P.Q.; Karimy, J.K.; Mansuri, M.S.; Marlier, A.; Allington, G.; Greenberg, A.B.W.; DeSpenza, T.; et al. The choroid plexus links innate immunity to CSF dysregulation in hydrocephalus. Cell 2023, 186, 764–785.e21. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, X.; Lu, J.; Shi, H.; Huang, L.; Shao, A.; Zhang, A.; Liu, Y.; Ren, R.; Lenahan, C.; et al. Inhibition of caspase-1-mediated inflammasome activation reduced blood coagulation in cerebrospinal fluid after subarachnoid haemorrhage. EBioMedicine 2022, 76, 103843. [Google Scholar] [CrossRef]
- Simard, P.F.; Tosun, C.; Melnichenko, L.; Ivanova, S.; Gerzanich, V.; Simard, J.M. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl. Stroke Res. 2011, 2, 227–231. [Google Scholar] [CrossRef]
- Purins, K.; Enblad, P.; Wiklund, L.; Lewén, A. Brain tissue oxygenation and cerebral perfusion pressure thresholds of ischemia in a standardized pig brain death model. Neurocritical Care 2012, 16, 462–469. [Google Scholar] [CrossRef]
- Zoremba, N.; Schnoor, J.; Berens, M.; Kuhlen, R.; Rossaint, R. Brain metabolism during a decrease in cerebral perfusion pressure caused by an elevated intracranial pressure in the porcine neocortex. Anesth. Analg. 2007, 105, 744–750. [Google Scholar] [CrossRef]
- Magnoni, S.; Ghisoni, L.; Locatelli, M.; Caimi, M.; Colombo, A.; Valeriani, V.; Stocchetti, N. Lack of improvement in cerebral metabolism after hyperoxia in severe head injury: A microdialysis study. J. Neurosurg. 2003, 98, 952–958. [Google Scholar] [CrossRef]
- Ljunggren, B.; Schutz, H.; Siesjö, B.K. Changes in energy state and acid-base parameters of the rat brain during complete compression ischemia. Brain Res. 1974, 73, 277–289. [Google Scholar] [CrossRef]
- Marklund, N.; Salci, K.; Lewén, A.; Hillered, L. Glycerol as a marker for post-traumatic membrane phospholipid degradation in rat brain. NeuroReport 1997, 8, 1457–1461. [Google Scholar] [CrossRef]
- Choi, D.W.; Hartley, D.M. Calcium and glutamate-induced cortical neuronal death. Res. Publ.-Assoc. Res. Nerv. Ment. Dis. 1993, 71, 23–34. [Google Scholar]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- du Plessis, A.J. Cerebral blood flow and metabolism in the developing fetus. Clin. Perinatol. 2009, 36, 531–548. [Google Scholar] [CrossRef]
- Foss-Skiftesvik, J.; Andresen, M.; Juhler, M. Childhood hydrocephalus - is radiological morphology associated with etiology. SpringerPlus 2013, 2, 11. [Google Scholar] [CrossRef]
- Horst, K.K.; Leschied, J.R.; Janitz, E.M.; Kim, J.S.; Narayanan, S.; Setty, B.N.; Birkemeier, K.; Sintim-Damoa, A.; Lampl, B.S.; Pomeranz, C.B.; et al. Neonatal neurosonography practices: A survey of active Society for Pediatric Radiology members. Pediatr. Radiol. 2023, 53, 112–120. [Google Scholar] [CrossRef]
- Behrendt, N.; Zaretsky, M.V.; West, N.A.; Galan, H.L.; Crombleholme, T.M.; Meyers, M.L. Ultrasound versus MRI: Is there a difference in measurements of the fetal lateral ventricles? J. Matern.-Fetal Neonatal Med. 2017, 30, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hwang, M.; Kilbaugh, T.J.; Sridharan, A.; Katz, J. Cerebral microcirculation mapped by echo particle tracking velocimetry quantifies the intracranial pressure and detects ischemia. Nat. Commun. 2022, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Medow, J.E.; Iskandar, B.J.; Wang, F.; Shokoueinejad, M.; Koueik, J.; Webster, J.G. Invasive and noninvasive means of measuring intracranial pressure: A review. Physiol. Meas. 2017, 38, R143–R182. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Cho, J.; Chiang, G.C.Y.; Kovanlikaya, I.; Heier, L.A.; Dyke, J.P.; Wang, Y. Cerebral oxygen extraction fraction declines with ventricular enlargement in patients with normal pressure hydrocephalus. Clin. Imaging 2023, 97, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chalak, L.F.; Lu, H. Non-invasive assessment of neonatal brain oxygen metabolism: A review of newly available techniques. Early Hum. Dev. 2014, 90, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Alperin, N.J.; Lee, S.H.; Loth, F.; Raksin, P.B.; Lichtor, T. MR-Intracranial pressure (ICP): A method to measure intracranial elastance and pressure noninvasively by means of MR imaging: Baboon and human study. Radiology 2000, 217, 877–885. [Google Scholar] [CrossRef]
- Glick, R.P.; Niebruegge, J.; Lee, S.H.; Egibor, O.; Lichtor, T.; Alperin, N. Early experience from the application of a noninvasive magnetic resonance imaging-based measurement of intracranial pressure in hydrocephalus. Neurosurgery 2006, 59, 1052–1060, discussion 1060. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Bailey, C.; Huisman, T.A.G.M.; de Jong, R.M.; Hwang, M. Contrast-Enhanced Ultrasound and Elastography Imaging of the Neonatal Brain: A Review. J. Neuroimaging 2017, 27, 437–441. [Google Scholar] [CrossRef]
- Xu, Z.S.; Yao, A.; Chu, S.S.; Paun, M.K.; McClintic, A.M.; Murphy, S.P.; Mourad, P.D. Detection of mild traumatic brain injury in rodent models using shear wave elastography: Preliminary studies. J. Ultrasound Med. 2014, 33, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Schneiderman, J.; Helenowski, I.B.; Morgan, E.; Dilley, K.; Danner-Koptik, K.; Hatahet, M.; Shimada, H.; Cohn, S.L.; Kletzel, M.; et al. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma. Pediatr. Blood Cancer 2014, 61, 1350–1356. [Google Scholar] [CrossRef]
- Su, Y.; Ma, J.; Du, L.; Xia, J.; Wu, Y.; Jia, X.; Cai, Y.; Li, Y.; Zhao, J.; Liu, Q. Application of acoustic radiation force impulse imaging (ARFI) in quantitative evaluation of neonatal brain development. Clin. Exp. Obstet. Gynecol. 2015, 42, 797–800. [Google Scholar] [CrossRef] [PubMed]
- El-Ali, A.M.; Subramanian, S.; Krofchik, L.M.; Kephart, M.C.; Squires, J.H. Feasibility and reproducibility of shear wave elastography in pediatric cranial ultrasound. Pediatr. Radiol. 2020, 50, 990–996. [Google Scholar] [CrossRef]
- deCampo, D.; Hwang, M. Characterizing the neonatal brain with ultrasound elastography. Pediatr. Neurol. 2018, 86, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pavan, L.; Gasser, B.; Maronezi, M.C.; Silva, P.; Uscategui, R.A.R.; Padilha-Nakaghi, L.C.; Lima, B.B.; Miranda, B.S.P.d.; Feliciano, M.A.R. Ultrasonography and elastography of the brain and cerebellum of English Bulldog fetuses. Theriogenology 2023, 198, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Quarello, E.; Lacoste, R.; Mancini, J.; Melot-Dusseau, S.; Gorincour, G. Feasibility and reproducibility of ShearWave(TM) elastography of fetal baboon organs. Prenat. Diagn. 2015, 35, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Zhang, Z.; Katz, J.; Freeman, C.; Kilbaugh, T. Brain contrast-enhanced ultrasonography and elastography in infants. Ultrasonography 2022, 41, 633–649. [Google Scholar] [CrossRef]
- Kolarovszki, B.; Zubor, P.; Kolarovszka, H.; Benco, M.; Richterova, R.; Matasova, K. The assessment of intracranial dynamics by transcranial Doppler sonography in perioperative period in paediatric hydrocephalus. Arch. Gynecol. Obstet. 2013, 287, 229–238. [Google Scholar] [CrossRef]
- Hanlo, P.W.; Gooskens, R.H.J.M.; Nijhuis, I.J.M.; Faber, J.A.J.; Peters, R.J.A.; van Huffelen, A.C.; Tulleken, C.A.F.; Willemse, J. Value of transcranial Doppler indices in predicting raised ICP in infantile hydrocephalus. Child’s Nerv. Syst. 1995, 11, 595–603. [Google Scholar] [CrossRef]
- Barletta, A.; Balbi, M.; Surace, A.; Caroli, A.; Radaelli, S.; Musto, F.; Saruggia, M.; Mangili, G.; Gerevini, S.; Sironi, S. Cerebral superb microvascular imaging in preterm neonates: In vivo evaluation of thalamic, striatal, and extrastriatal angioarchitecture. Neuroradiology 2021, 63, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.W.; Hwang, M. Advanced ultrasound techniques for neuroimaging in pediatric critical care: A review. Children 2022, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Hingot, V.; Heiles, B.; Muleki-Seya, P.; Tanter, M. Ultrasound Localization Microscopy and Super-Resolution: A State of the Art. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Viessmann, O.M.; Eckersley, R.J.; Christensen-Jeffries, K.; Tang, M.X.; Dunsby, C. Acoustic super-resolution with ultrasound and microbubbles. Phys. Med. Biol. 2013, 58, 6447–6458. [Google Scholar] [CrossRef]
- Ackermann, D.; Schmitz, G. Detection and Tracking of Multiple Microbubbles in Ultrasound B-Mode Images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016, 63, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Poelma, C. Ultrasound Imaging Velocimetry: A review. Exp. Fluids 2017, 58, 3. [Google Scholar] [CrossRef]
- Kim, H.B.; Hertzberg, J.R.; Shandas, R. Development and validation of echo PIV. Exp. Fluids 2004, 36, 455–462. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, H.; Cai, B.; He, Y.; Huang, S.; Zhang, Y. Feasibility of contrast-enhanced ultrasonography (CEUS) in evaluating renal microvascular perfusion in pediatric patients. BMC Med. Imaging 2022, 22, 194. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.S.; Kim, B.S.; Kim, D.R.; Kang, K.S. Quantitative Analysis of Pancreatic Fat in Children with Obesity Using Magnetic Resonance Imaging and Ultrasonography. Pediatr. Gastroenterol. Hepatol. Nutr. 2021, 24, 555–563. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, L.M.; Hwang, M. Metabolic Pathways in Hydrocephalus: Profiling with Proteomics and Advanced Imaging. Metabolites 2024, 14, 412. https://doi.org/10.3390/metabo14080412
Davis LM, Hwang M. Metabolic Pathways in Hydrocephalus: Profiling with Proteomics and Advanced Imaging. Metabolites. 2024; 14(8):412. https://doi.org/10.3390/metabo14080412
Chicago/Turabian StyleDavis, Laura May, and Misun Hwang. 2024. "Metabolic Pathways in Hydrocephalus: Profiling with Proteomics and Advanced Imaging" Metabolites 14, no. 8: 412. https://doi.org/10.3390/metabo14080412
APA StyleDavis, L. M., & Hwang, M. (2024). Metabolic Pathways in Hydrocephalus: Profiling with Proteomics and Advanced Imaging. Metabolites, 14(8), 412. https://doi.org/10.3390/metabo14080412





