Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. ROS Measurement
2.3. Western Blot Assays
2.4. Enzyme Activities
2.5. Determination of GSH and GSSG Levels
2.6. Metabolic Profiling
2.7. Cell Viability
2.8. Ethical Approval
2.9. Statistical Analysis
3. Results
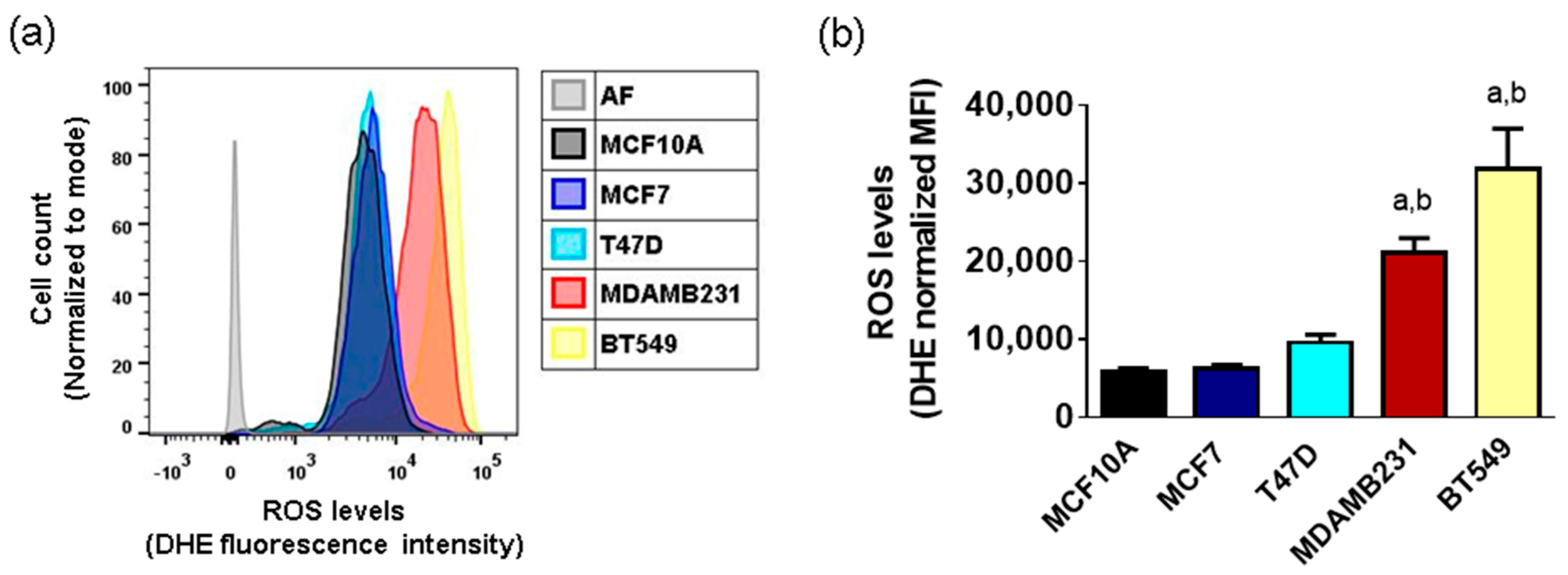
3.1. Basal ROS Production in Breast Cancer Cell Lines
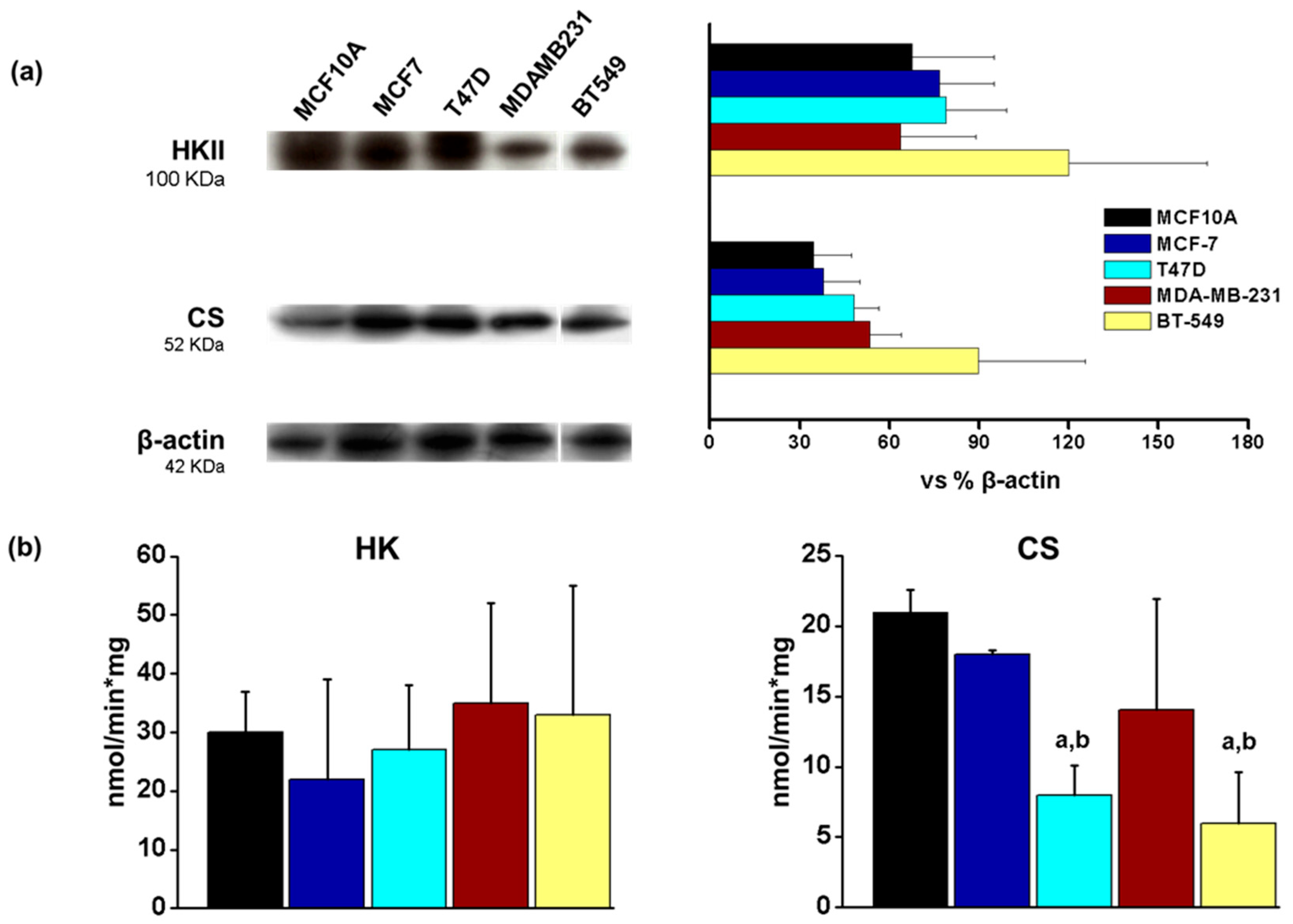
3.2. Antioxidant Enzyme Protein Levels in Breast Cancer Cell Lines
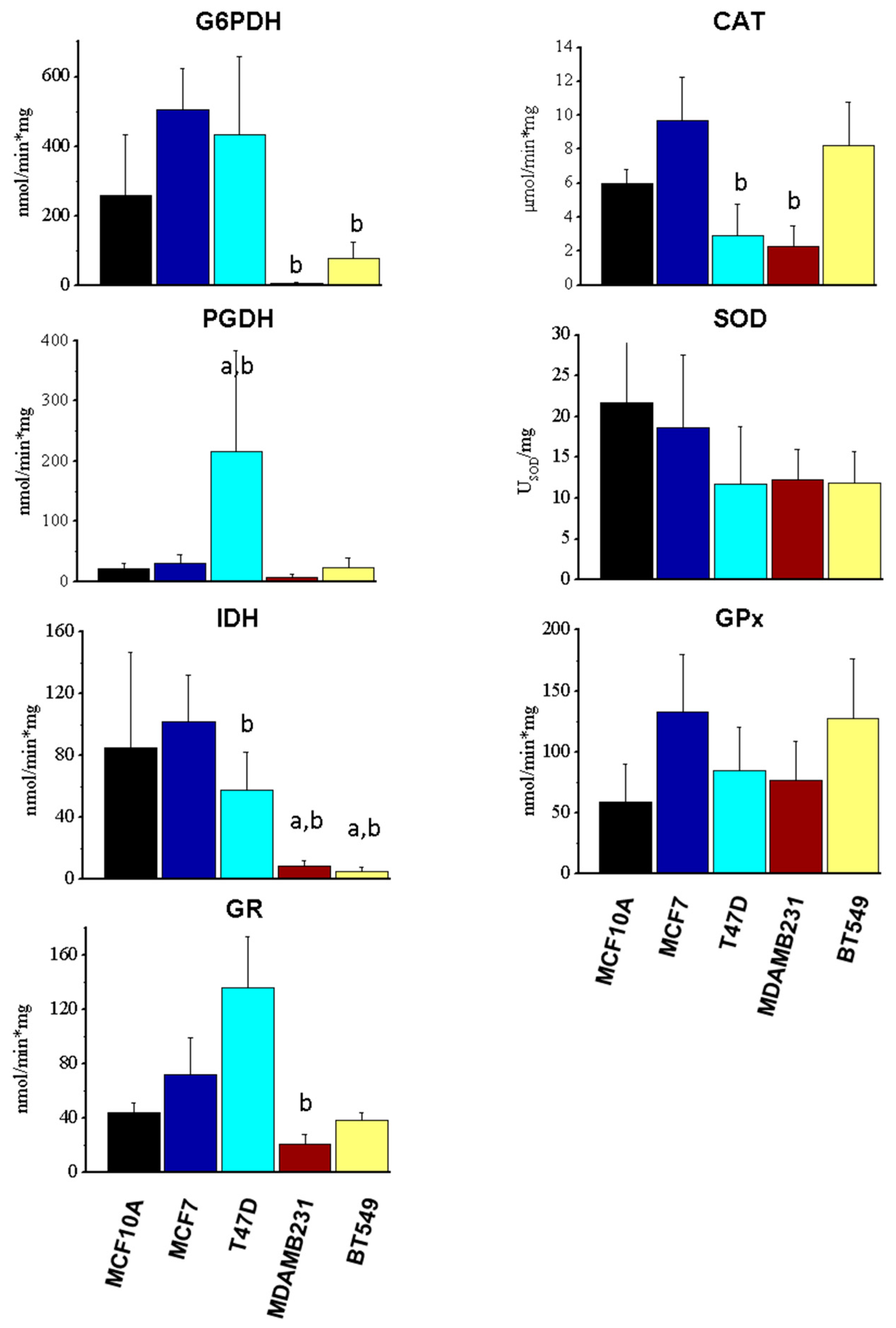
3.3. Enzyme Activity of Components of NADPH-Producing Enzymes and Antioxidant Systems
3.4. GSH and GSSG Levels in Breast Cells
3.5. Energetic Metabolism in Breast Cancer Cell Lines
3.6. Mitochondrial Parameters in Breast Cancer Cell Lines
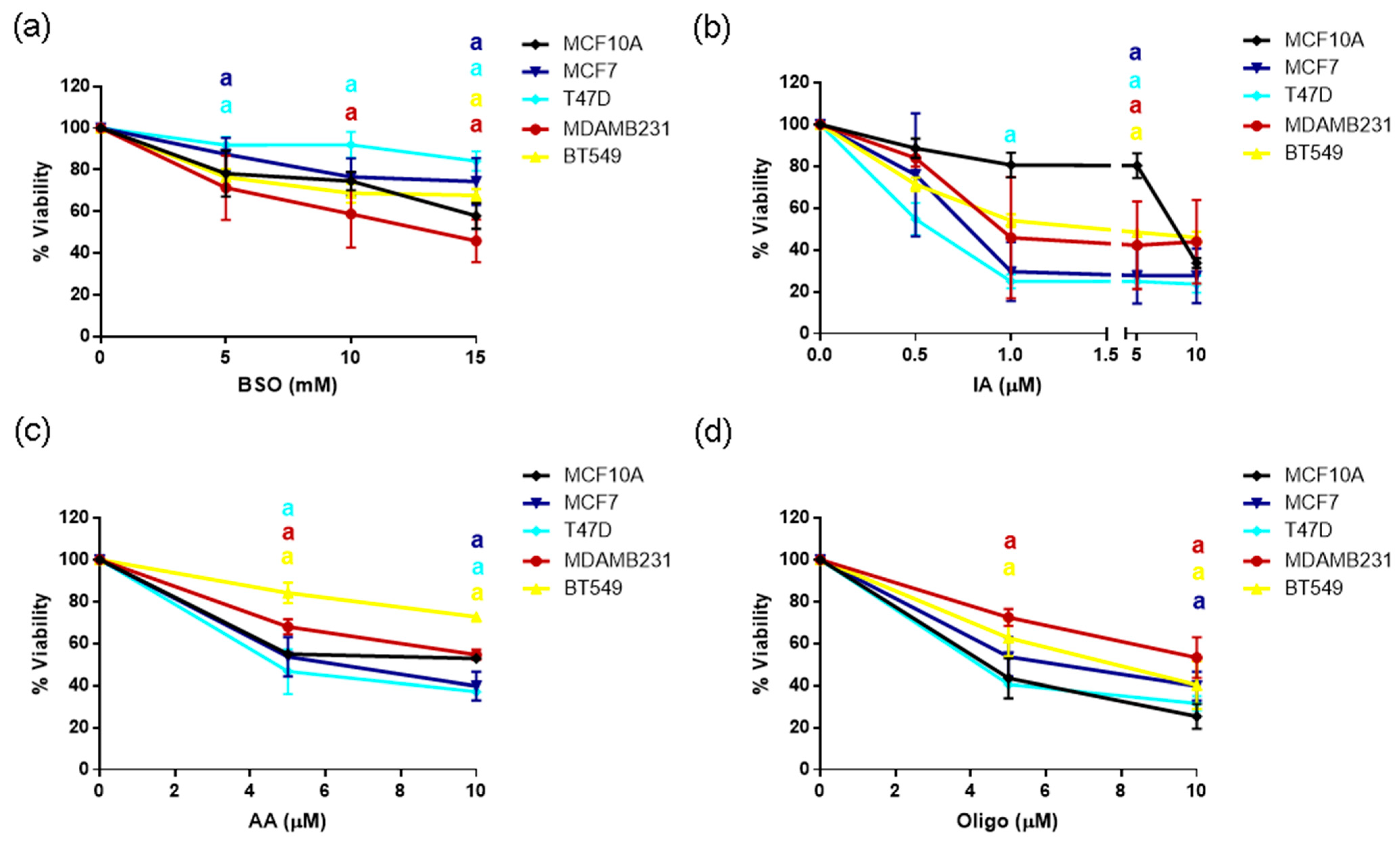
3.7. Sensitivity to GSH or Metabolic Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, R.; Marín-Hernández, A.; Saavedra, E.; Pardo, J.P.; Ralph, S.J.; Rodríguez-Enríquez, S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int. J. Biochem. Cell Biol. 2014, 50, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting mitochondrial metabolism for precision medicine in cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Trevino, S.; Rosas-Murrieta, N.H.; Millan-Perez-Pena, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1–NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Carter, W.O.; Naryanan, P.K.; Robinson, J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leukoc. Biol. 1994, 55, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Delgado-Magallon, A.; Montes-Alvarado, J.B.; Ramirez-Ramirez, D.; Flores-Alonso, J.C.; Cortes-Hernandez, P.; Reyes-Leyva, J.; Herrera-Camacho, I.; Anaya-Ruiz, M.; Pelayo, R.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front. Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Velazquez, S.C.; Robledo-Cadena, D.X.; Hernandez-Resendiz, I.; Gallardo-Perez, J.C.; Moreno-Sanchez, R.; Rodriguez-Enriquez, S. Energy Metabolism Drugs Block Triple Negative Breast Metastatic Cancer Cell Phenotype. Mol. Pharm. 2018, 15, 2151–2164. [Google Scholar] [CrossRef]
- Marin-Hernandez, A.; Gallardo-Perez, J.C.; Hernandez-Resendiz, I.; Del Mazo-Monsalvo, I.; Robledo-Cadena, D.X.; Moreno-Sanchez, R.; Rodriguez-Enriquez, S. Hypoglycemia Enhances Epithelial-Mesenchymal Transition and Invasiveness, and Restrains the Warburg Phenotype, in Hypoxic HeLa Cell Cultures and Microspheroids. J. Cell Physiol. 2017, 232, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanchez, R.; Gallardo-Perez, J.C.; Rodriguez-Enriquez, S.; Saavedra, E.; Marin-Hernandez, A. Control of the NADPH supply for oxidative stress handling in cancer cells. Free Radic. Biol. Med. 2017, 112, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Calleja, L.F.; Yoval-Sanchez, B.; Hernandez-Esquivel, L.; Gallardo-Perez, J.C.; Sosa-Garrocho, M.; Marin-Hernandez, A.; Jasso-Chavez, R.; Macias-Silva, M.; Salud Rodriguez-Zavala, J. Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. FEBS J. 2021, 288, 4064–4080. [Google Scholar] [CrossRef] [PubMed]
- Willmann, L.; Schlimpert, M.; Hirschfeld, M.; Erbes, T.; Neubauer, H.; Stickeler, E.; Kammerer, B. Alterations of the exo- and endometabolite profiles in breast cancer cell lines: A mass spectrometry-based metabolomics approach. Anal. Chim. Acta 2016, 925, 34–42. [Google Scholar] [CrossRef]
- Beatty, A.; Fink, L.S.; Singh, T.; Strigun, A.; Peter, E.; Ferrer, C.M.; Nicolas, E.; Cai, K.Q.; Moran, T.P.; Reginato, M.J.; et al. Metabolite Profiling Reveals the Glutathione Biosynthetic Pathway as a Therapeutic Target in Triple-Negative Breast Cancer. Mol. Cancer Ther. 2018, 17, 264–275. [Google Scholar] [CrossRef]
- Cao, L.Q.; Sun, H.; Xie, Y.; Patel, H.; Bo, L.; Lin, H.; Chen, Z.S. Therapeutic evolution in HR+/HER2- breast cancer: From targeted therapy to endocrine therapy. Front. Pharmacol. 2024, 15, 1340764. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, E.; Mesquita, A. Emerging Therapeutic Drugs in Metastatic Triple-Negative Breast Cancer. Breast Cancer Basic Clin. Res. 2021, 15, 11782234211002491. [Google Scholar] [CrossRef] [PubMed]
- Raninga, P.V.; Lee, A.C.; Sinha, D.; Shih, Y.Y.; Mittal, D.; Makhale, A.; Bain, A.L.; Nanayakarra, D.; Tonissen, K.F.; Kalimutho, M.; et al. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer. Int. J. Cancer 2020, 146, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Marin-Hernandez, A.; Rodriguez-Zavala, J.S.; Jasso-Chavez, R.; Saavedra, E.; Moreno-Sanchez, R. Protein acetylation effects on enzyme activity and metabolic pathway fluxes. J. Cell. Biochem. 2022, 123, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Kim, S.; Seo, Y.; Lee, J.; Jeon, S.; Vu, G.H.; Yeo, M.K.; Kim, C.S. The relative isoform expression levels of isocitrate dehydrogenase in breast cancer: IDH2 is a potential target in MDA-MB-231 cells. Korean J. Clin. Oncol. 2023, 19, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.J.; Yan, H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Cadoux-Hudson, T.; Schofield, C.J.; McCullagh, J.S.O. Isocitrate dehydrogenase gene variants in cancer and their clinical significance. Biochem. Soc. Trans. 2021, 49, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bai, R.K.; Trieu, R.; Wong, L.J. Mitochondrial dysfunction in human breast cancer cells and their transmitochondrial cybrids. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, G.K.; Wang, H.; Yang, X. Proton leak regulates mitochondrial reactive oxygen species generation in endothelial cell activation and inflammation—A novel concept. Arch. Biochem. Biophys. 2019, 662, 68–74. [Google Scholar] [CrossRef]
- Baffy, G. Mitochondrial uncoupling in cancer cells: Liabilities and opportunities. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 655–664. [Google Scholar] [CrossRef]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Valle, A.; Oliver, J.; Roca, P. UCP2 inhibition sensitizes breast cancer cells to therapeutic agents by increasing oxidative stress. Free. Radic. Biol. Med. 2015, 86, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Fahim, F.A.; Esmat, A.Y.; Mady, E.A.; Ibrahim, E.K. Antitumor activities of iodoacetate and dimethylsulphoxide against solid Ehrlich carcinoma growth in mice. Biol. Res. 2003, 36, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Akins, N.S.; Nielson, T.C.; Le, H.V. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr. Top. Med. Chem. 2018, 18, 494–504. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | GSH [nmol/mg prot] | GSSG [nmol/mg prot] | GSH/GSSG |
|---|---|---|---|
| MCF10A | 18.4 ± 7.8 | 0.19 ± 0.17 | 97 |
| MCF7 | 54 ± 14 a | 0.85 ± 0.47 | 63 |
| T47D | 4.9 ± 3.2 b | 0.15 ± 0.1 | 33 |
| MDAMB231 | 5.8 ± 2.3 b | 0.5 ± 0.46 | 12 |
| BT549 | 13.8 ± 4.3 b | 0.8 ± 0.54 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maycotte, P.; Sarmiento-Salinas, F.L.; García-Miranda, A.; Ovando-Ovando, C.I.; Robledo-Cadena, D.X.; Hernández-Esquivel, L.; Jasso-Chávez, R.; Marín-Hernández, A. Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines. Metabolites 2024, 14, 435. https://doi.org/10.3390/metabo14080435
Maycotte P, Sarmiento-Salinas FL, García-Miranda A, Ovando-Ovando CI, Robledo-Cadena DX, Hernández-Esquivel L, Jasso-Chávez R, Marín-Hernández A. Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines. Metabolites. 2024; 14(8):435. https://doi.org/10.3390/metabo14080435
Chicago/Turabian StyleMaycotte, Paola, Fabiola Lilí Sarmiento-Salinas, Alin García-Miranda, Cesar Ivan Ovando-Ovando, Diana Xochiquetzal Robledo-Cadena, Luz Hernández-Esquivel, Ricardo Jasso-Chávez, and Alvaro Marín-Hernández. 2024. "Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines" Metabolites 14, no. 8: 435. https://doi.org/10.3390/metabo14080435
APA StyleMaycotte, P., Sarmiento-Salinas, F. L., García-Miranda, A., Ovando-Ovando, C. I., Robledo-Cadena, D. X., Hernández-Esquivel, L., Jasso-Chávez, R., & Marín-Hernández, A. (2024). Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines. Metabolites, 14(8), 435. https://doi.org/10.3390/metabo14080435








