Investigating the Link between Intermediate Metabolism, Sexual Dimorphism, and Cardiac Autonomic Dysfunction in Patients with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Anthropometric, Biochemical, and Clinical Variables
2.3. Assessment of Cardiovascular Autonomic Function: Ewing’s Score and Power Spectral Heart Rate Data
2.4. Proton Nuclear Magnetic Resonance Spectroscopy Metabolomics
2.5. Statistical Analysis
3. Results
3.1. Sex-Based Clinical and Biochemical Characteristics of Patients with Cardioautonomic Neuropathy
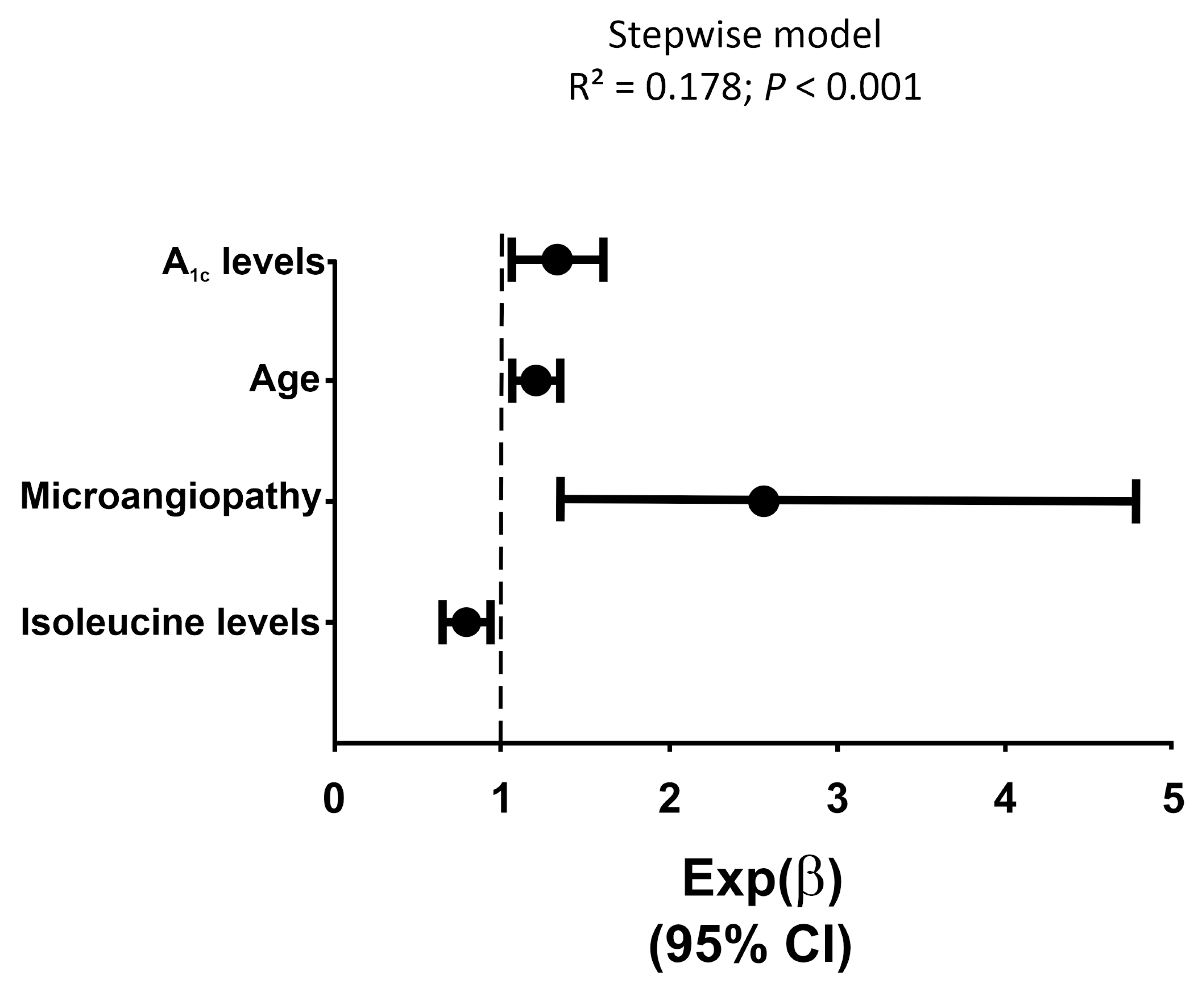
3.2. Association between Metabolomic Intermediate Metabolism with CAN
3.3. Correlation of LMWM Profile with Tests of Cardiovascular Autonomic Function
3.4. Interactions of Age, Sex, and CAN on LMWM Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regensteiner, J.G.; Golden, S.; Huebschmann, A.G.; Barrett-Connor, E.; Chang, A.Y.; Chyun, D.; Fox, C.S.; Kim, C.; Mehta, N.; Reckelhoff, J.F.; et al. Sex Differences in the Cardiovascular Consequences of Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 2424–2447. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Nevitt, S.; Eleftheriadou, A.; Kanagala, P.; Esa, H.; Cuthbertson, D.J.; Tahrani, A.; Alam, U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: A meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, e002480. [Google Scholar] [CrossRef] [PubMed]
- Gordan, R.; Gwathmey, J.K.; Xie, L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Nattero-Chávez, L.; Alonso Díaz, S.; Jiménez-Mendiguchia, L.; García-Cano, A.; Fernández-Durán, E.; Dorado Avendaño, B.; Escobar-Morreale, H.F.; Luque-Ramírez, M. Sexual Dimorphism and Sex Steroids Influence Cardiovascular Autonomic Neuropathy in Patients With Type 1 Diabetes. Diabetes Care 2019, 42, e175–e178. [Google Scholar] [CrossRef] [PubMed]
- Nattero-Chávez, L.; Insenser, M.; Quintero Tobar, A.; Fernández-Durán, E.; Dorado Avendaño, B.; Fiers, T.; Kaufman, J.M.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Sex differences and sex steroids influence on the presentation and severity of cardiovascular autonomic neuropathy of patients with type 1 diabetes. Cardiovasc. Diabetol. 2023, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.X.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta. 2010, 1802, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Strom, A.; Straßburger, K.; Knebel, B.; Bönhof, G.J.; Kotzka, J.; Szendroedi, J.; Roden, M. Association of cardiac autonomic dysfunction with higher levels of plasma lipid metabolites in recent-onset type 2 diabetes. Diabetologia 2021, 64, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Nattero-Chávez, L.; Insenser, M.; Amigó, N.; Samino, S.; Martínez-Micaelo, N.; Dorado Avendaño, B.; Quintero Tobar, A.; Escobar-Morreale, H.F.; Luque-Ramírez, M. Quantification of lipoproteins by proton nuclear magnetic resonance spectroscopy ((1)H-NMRS) improves the prediction of cardiac autonomic dysfunction in patients with type 1 diabetes. J. Endocrinol. Investig. 2024, 6, 023–02289. [Google Scholar] [CrossRef]
- Hansen, C.S.; Suvitaival, T.; Theilade, S.; Mattila, I.; Lajer, M.; Trošt, K.; Ahonen, L.; Hansen, T.W.; Legido-Quigley, C.; Rossing, P.; et al. Cardiovascular Autonomic Neuropathy in Type 1 Diabetes Is Associated With Disturbances in TCA, Lipid, and Glucose Metabolism. Front Endocrinol. 2022, 13, 831793. [Google Scholar] [CrossRef]
- Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.V.; Erbey, J.R.; Becker, D.; Arslanian, S.; Orchard, T.J. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000, 49, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Mazer, N.A. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: With illustrative examples from male and female populations. Steroids 2009, 74, 512–519. [Google Scholar] [CrossRef]
- Ewing, D.J.; Martyn, C.N.; Young, R.J.; Clarke, B.F. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985, 8, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Autonomic nervous system testing. Diabetes Care 1992, 15, 1095–1103. [Google Scholar] [PubMed]
- Gómez, J.; Brezmes, J.; Mallol, R.; Rodríguez, M.A.; Vinaixa, M.; Salek, R.M.; Correig, X.; Cañellas, N. Dolphin: A tool for automatic targeted metabolite profiling using 1D and 2D (1)H-NMR data. Anal. Bioanal. Chem. 2014, 406, 7967–7976. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, S.M. The normal menopause transition: An overview. Maturitas 1996, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Role of Impaired Glycolysis in Perturbations of Amino Acid Metabolism in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 1724. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zhang, S.; Ward, L.E.; Karakelides, H.; Raftery, D.; Nair, K.S. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS ONE 2010, 5, e10538. [Google Scholar] [CrossRef]
- Mathew, A.V.; Jaiswal, M.; Ang, L.; Michailidis, G.; Pennathur, S.; Pop-Busui, R. Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes. Diabetes 2019, 68, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Oh, S.F.; Wada, S.; Rowe, G.C.; Liu, L.; Chan, M.C.; Rhee, J.; Hoshino, A.; Kim, B.; Ibrahim, A.; et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016, 22, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef]
- Rojas, D.R.; Kuner, R.; Agarwal, N. Metabolomic signature of type 1 diabetes-induced sensory loss and nerve damage in diabetic neuropathy. J. Mol. Med. 2019, 97, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Cahill, G.F., Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ahola-Olli, A.V.; Mustelin, L.; Kalimeri, M.; Kettunen, J.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Havulinna, A.S.; Lehtimäki, T.; Kähönen, M.; et al. Circulating metabolites and the risk of type 2 diabetes: A prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 2019, 62, 2298–2309. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Tofte, N.; Suvitaival, T.; Trost, K.; Mattila, I.M.; Theilade, S.; Winther, S.A.; Ahluwalia, T.S.; Frimodt-Møller, M.; Legido-Quigley, C.; Rossing, P. Metabolomic Assessment Reveals Alteration in Polyols and Branched Chain Amino Acids Associated With Present and Future Renal Impairment in a Discovery Cohort of 637 Persons With Type 1 Diabetes. Front Endocrinol. 2019, 10, 818. [Google Scholar] [CrossRef]
- Shin, A.C.; Fasshauer, M.; Filatova, N.; Grundell, L.A.; Zielinski, E.; Zhou, J.Y.; Scherer, T.; Lindtner, C.; White, P.J.; Lapworth, A.L.; et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014, 20, 898–909. [Google Scholar] [CrossRef]
- Gannaban, R.B.; NamKoong, C.; Ruiz, H.H.; Choi, H.J.; Shin, A.C. Central Regulation of Branched-Chain Amino Acids Is Mediated by AgRP Neurons. Diabetes 2021, 70, 62–75. [Google Scholar] [CrossRef]
- Darst, B.F.; Koscik, R.L.; Hogan, K.J.; Johnson, S.C.; Engelman, C.D. Longitudinal plasma metabolomics of aging and sex. Aging 2019, 11, 1262–1282. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Martínez-García, M.; Insenser, M.; Cañellas, N.; Correig, X.; Luque-Ramírez, M. Serum metabolomics profiling by proton nuclear magnetic resonance spectrometry of the response to single oral macronutrient challenges in women with polycystic ovary syndrome (PCOS) compared with male and female controls. Biol. Sex Differ. 2023, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Martínez-García, M.; Insenser, M.; Cañellas, N.; Correig, X.; Luque-Ramírez, M. Serum metabolomics profiling by proton nuclear magnetic resonance spectroscopy reveals sexual dimorphism and masculinization of intermediate metabolism in women with polycystic ovary syndrome (PCOS). Biol. Sex Differ. 2023, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, R.L.; Peltonen, G.L.; Binns, S.E.; Shankaran, M.; Giordano, G.R.; Hartley, D.A.; Klochak, A.L.; Lonac, M.C.; Paris, H.L.; Szallar, S.E.; et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. Faseb. J. 2014, 28, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Women | Men | CAN | Sex | CAN*Sex | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | All | All women | no CAN | CAN | All men | no CAN | CAN | |||
| (n = 323) | (n = 147) | (n = 100) | (n = 47) | (n = 176) | (n = 133) | (n = 43) | ||||
| Clinical | ||||||||||
| Age, years | 42 (19) | 41 (21) | 38 (20) | 47 (26) | 43 (19) | 40 (21) | 46 (15) | <0.001 | 0.791 | 0.883 |
| Never smokers [N (%)] | 190 (59) | 86 (59) | 59 (59) | 27 (57) | 104 (59) | 82 (62) | 22 (51) | 0.225 | 0.55 | 0.469 |
| Antiaggregant therapy [N (%)] | 39 (12) | 15 (10) | 4 (4) | 11 (23) | 24 (14) | 13 (10) | 11 (26) | 0.011 | 0.855 | 0.259 |
| Statin therapy [N (%)] | 118 (37) | 46 (31) | 27 (27) | 19 (40) | 72 (41) | 48 (36) | 24 (56) | 0.024 | 0.146 | 0.701 |
| On antihypertensives [N (%)] | 50 (15) | 23 (16) | 10 (10) | 13 (28) | 27 (15) | 17 (13) | 10 (23) | 0.102 | 0.633 | 0.429 |
| Body mass index, kg/m2 | 24 (5) | 24 (5) | 24 (6) | 23 (4) | 25 (5) | 25 (5) | 26 (5) | 0.158 | 0.002 | 0.061 |
| Obesity [N (%)] | 30 (9) | 16 (11) | 11 (11) | 5 (11) | 14 (8) | 7 (5) | 7 (16) | 0.141 | 0.989 | 0.116 |
| Waist circumference, cm | 84 (17) | 77 (16) | 77 (16) | 78 (14) | 88 (16) | 87 (16) | 93 (15) | 0.041 | <0.001 | 0.134 |
| Hip, cm | 99 (12) | 98 (15) | 98 (17) | 101 (10) | 100 (11) | 100 (10) | 101 (14) | 0.482 | 0.504 | 0.834 |
| Waist to hip ratio | 0.85 (0.14) | 0.79 (0.10) | 0.78 (010) | 0.80 (0.13) | 0.90 (0.12) | 0.89 (0.11) | 0.94 (0.12) | 0.054 | <0.001 | 0.124 |
| Fat mass (%) | 24 ± 10 | 30 ± 8 | 30 ± 8 | 30 ± 7 | 18 ± 8 | 17 ± 7 | 21 ± 8 | 0.041 | <0.001 | 0.052 |
| Systolic blood pressure, mmHg | 120 (17) | 117 (19) | 115 (16) | 121 (24) | 122 (13) | 121 (13) | 128 (20) | 0.001 | <0.001 | 0.947 |
| Diastolic blood pressure, mmHg | 77 (15) | 73 (13) | 73 (13) | 74 (15) | 78 (15) | 78 (14) | 80 (18) | 0.101 | <0.001 | 0.443 |
| Diabetes | ||||||||||
| Age at diagnosis of T1D, years | 18 (16) | 19 (18) | 16 (18) | 22 (17) | 17 (13) | 17 (13) | 18 (17) | 0.009 | 0.531 | 0.514 |
| Duration of T1D, years | 18 (19) | 17 (18) | 15 (158) | 19 (20) | 20 (20) | 18 (20) | 23 (18) | 0.012 | 0.756 | 0.283 |
| DKA at diagnosis [N (%)] | 119 (37) | 52 (35) | 33 (33) | 19 (40) | 67 (38) | 54 (41) | 13 (30) | 0.226 | 0.314 | 0.139 |
| CSII [N (%)] | 79 (25) | 42 (29) | 29 (29) | 13 (28) | 37 (21) | 26 (20) | 11 (26) | 0.413 | 0.824 | 0.464 |
| Total insulin dose, units/day | 40 (24) | 34 (20) | 34 (20) | 32 (17) | 47 (23) | 45 (24) | 48 (17) | 0.785 | 0.001 | 0.614 |
| Daily insulin dose, units/kg/day | 0.56 (0.25) | 0.55 (0.23) | 0.55 (0.23) | 0.50 (0.24) | 0.57 (0.24) | 0.56 (0.26) | 0.62 (0.21) | 0.554 | 0.895 | 0.896 |
| eGDR, mg/kg/min | 9.6 (2.7) | 10.3 (2.2) | 10.5 (2.1) | 9.8 (3.4) | 9.1 (2.4) | 9.3 (2.1) | 8.2 (2.1) | 0.002 | 0.002 | 0.907 |
| Microangiopathy [N (%)] | 66 (20) | 31 (21) | 15 (15) | 16 (34) | 35 (20) | 19 (14) | 16 (37) | 0.002 | 0.754 | 0.735 |
| Macroangiopathy [N (%)] | 16 (5) | 7 (5) | 3 (3) | 4 (9) | 9 (5) | 4 (3) | 5 (12) | 0.038 | 0.624 | 0.743 |
| Metabolic parameters | ||||||||||
| A1c (%) | 7.2 (1.3) | 7.3 (1.7) | 7.2 (1.4) | 7.8 (1.2) | 7.1 (1.0) | 7.0 (1.0) | 7.4 (1.1) | 0.002 | 0.029 | 0.669 |
| A1c, mmol/mol | 55 (14) | 56 (19) | 55 (15) | 62 (13) | 54 (11) | 53 (11) | 57 (12) | 0.002 | 0.025 | 0.655 |
| Total cholesterol, mmol/L | 4.5 ± 0.82 | 4.7 ± 0.81 | 4.7 ± 0.75 | 4.6 ± 0.91 | 4.3 ± 0.80 | 4.2 ± 0.75 | 4.5 ± 0.91 | 0.678 | 0.005 | 0.092 |
| HDL cholesterol, mmol/L | 1.48 (0.47) | 1.68 (0.60) | 1.68 (0.52) | 1.65 (0.83) | 1.37 (0.39) | 1.40 (0.39) | 1.32 (0.46) | 0.208 | <0.001 | 0.356 |
| LDL cholesterol, mmol/L | 2.57 ± 0.65 | 2.60 ± 0.68 | 2.65 ± 0.68 | 2.50 ± 0.68 | 2.54 ± 0.63 | 2.48 ± 0.58 | 2.72 ± 0.75 | 0.593 | 0.733 | 0.02 |
| Triglycerides, mmol/L | 0.66 (0.31) | 0.63 (0.32) | 0.61 (0.31) | 0.69 (0.45) | 0.68 (0.32) | 0.65 (0.32) | 0.72 (0.24) | 0.011 | 0.053 | 0.748 |
| eGFR, mL/min/1.73 m2 | 89 (20) | 84 (15) | 86 (11) | 81 (20) | 94 (22) | 94 (22) | 88 (19) | 0.001 | <0.001 | 0.109 |
| Hormonal parameters | ||||||||||
| FSH, IU/L * | 4.6 (4.4) | 6.9 (45.0) | 5.8 (15.9) | 32.6 (57.2) | 3.5 (2.9) | 3.4 (2.9) | 4.4 (3.9) | 0.001 | <0.001 | 0.229 |
| LH, IU/L * | 3.8 (4.4) | 7.1 (17.8) | 5.5 (16.1) | 14.5 (18.3) | 3.2 (2.0) | 3.0 (2.1) | 3.6 (2.0) | <0.001 | <0.001 | 0.211 |
| Total T, nmol/L * | 15 (23) | 1.2 (0.7) | 1.0 (0.7) | 1.1 (0.8) | 23 (11) | 23 (11) | 22 (10) | 0.94 | <0.001 | 0.06 |
| Total E2, pmol/L * | 99 (94) | 218 (370) | 250 (358) | 89 (344) | 90 (115) | 87 (38) | 102 (45) | 0.026 | 0.046 | 0.001 |
| Total T/ E2 molar ratio * | 177 (254) | 7 (16) | 5 (11) | 12 (49) | 249 (123) | 258 (135) | 222 (100) | 0.019 | <0.001 | <0.001 |
| SHBG, nmol/L * | 62 (43) | 87 (54) | 83 (51) | 100 (61) | 50 (28) | 49 (28) | 51 (29) | 0.023 | <0.001 | 0.668 |
| Calculated free T, pmol/L * | 272 (395) | 10 (7) | 11 (8) | 8 (6) | 381 (173) | 392 (185) | 350 (117) | 0.114 | <0.001 | 0.126 |
| Calculated free E2, pmol/L * | 1.8 (1.6) | 3.0 (5.2) | 3.8 (4.6) | 1.3 (4.4) | 1.7 (0.8) | 1.6 (0.9) | 1.9 (0.6) | 0.026 | 0.046 | 0.001 |
| Calculated free T/E2, molar ratio * | 157 (239) | 4 (10) | 3 (7) | 8 (30) | 255 (109) | 244 (115) | 190 (90) | 0.014 | <0.001 | <0.001 |
| All Patients (n = 323) | No CAN (n = 233) | CAN (n = 90) | p | |
|---|---|---|---|---|
| Energy and Homeostasis Metabolites | ||||
| Acetate | 23 (15) | 23 (14) | 25 (13) | 0.086 |
| Acetone | 20 (21) | 22 (23) | 18 (16) | 0.339 |
| Creatine | 27 (20) | 27 (20) | 26 (18) | 0.381 |
| Creatinine | 62 (19) | 62 (18) | 64 (22) | 0.137 |
| Glucose | 7055 (4131) | 6952 (3745) | 7631 (5649) | 0.452 |
| Glycerol | 132 (72) | 133 (69) | 131 (77) | 0.809 |
| Lactate | 308(159) | 302(136) | 362 (233) | 0.006 |
| Hydroxybutyrate | 25 (66) | 27 (80) | 20 (48) | 0.530 |
| Amino Acids | ||||
| Alanine | 310 (82) | 307 (74) | 326 (91) | 0.063 |
| Glycine | 218 (69) | 217 (59) | 230 (97) | 0.178 |
| Glutamate | 66 (27) | 65 (26) | 69 (28) | 0.427 |
| Glutamine | 434 ± 58 | 432 ± 54 | 439 ± 66 | 0.289 |
| Histidine | 77 ± 12 | 76 ± 12 | 77 ± 13 | 0.569 |
| Isoleucine | 32 (17) | 33 (17) | 29 (17) | 0.002 |
| Leucine | 96 (27) | 99 (28) | 93 (29) | 0.178 |
| Threonine | 238 ± 51 | 242 ± 51 | 228 ± 50 | 0.033 |
| Tyrosine | 37 ± 9 | 36 ± 9 | 37 ± 10 | 0.285 |
| Valine | 210 ± 45 | 214 ± 48 | 200 ± 37 | 0.014 |
| ∆SBP | E/I Ratio | VAL Index | 30:15 Index | Normalized Low Frequency | Normalized High Frequency | Score Ewing Total | |
|---|---|---|---|---|---|---|---|
| Lactate | ρ = −0.057 p = 0.314 | ρ = −0.083 p = 0.150 | ρ = −0.136 p = 0.018 | ρ = −0.014 p = 0.801 | ρ = −0.173 p = 0.002 | ρ = −0.164 p = 0.004 | ρ = 0.163 p = 0.004 |
| Isoleucine | ρ = 0.085 p = 0.129 | ρ = 0.135 p = 0.017 | ρ = 0.156 p = 0.006 | ρ = 0.080 p = 0.156 | ρ = 0.067 p = 0.239 | ρ = 0.054 p = 0.343 | ρ = −0.158 p = 0.004 |
| Threonine | ρ = 0.127 p = 0.024 | ρ = 0.096 p = 0.093 | ρ = 0.146 p = 0.011 | ρ = 0.004 p = 0.943 | ρ = 0.069 p = 0.224 | ρ = 0.012 p = 0.829 | ρ = −0.109 p = 0.052 |
| Valine | ρ = 0.124 p = 0.026 | ρ = 0.123 p = 0.030 | ρ = 0.206 p < 0.001 | ρ = 0.035 p = 0.535 | ρ = 0.065 p = 0.248 | ρ = 0.024 p = 0.675 | ρ = −0.146 p = 0.009 |
| Variable | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 147) | ≤50 years (n = 103) | >50 years (n = 44) | All (n = 176) | ≤50 years (n = 135) | >50 years (n = 41) | Sex | Age | Sex*Age | |
| Energy and Homeostasis Metabolites | |||||||||
| Acetate | 22 (14) | 19 (13) | 28 (15) | 25 (17) | 24 (18) | 25 (13) | 0.397 | 0.131 | 0.018 |
| Acetone | 18 (23) | 18 (22) | 23 (31) | 26 (22) | 27 (22) | 22 (18) | 0.040 | 0.271 | 0.397 |
| Creatine | 30 (18) | 27 (19) | 31 (23) | 24 (19) | 25 (18) | 24 (22) | 0.002 | 0.806 | 0.196 |
| Creatinine | 54 (12) | 53 (12) | 58 (13) | 69 (16) | 68 (14) | 73 (20) | <0.001 | <0.001 | 0.806 |
| Glucose | 7597 (4606) | 7368 (4648) | 8159 (5141) | 7336 (3936) | 7729 (4573) | 7049 (3519) | 0.438 | 0.127 | 0.154 |
| Glycerol | 142 (64) | 138 (63) | 143 (96) | 136 (79) | 149 (80) | 117 (68) | 0.001 | 0.786 | 0.028 |
| Lactate | 313 (183) | 307 (187) | 351 (198) | 308 (138) | 301 (125) | 336 (155) | 0.451 | 0.034 | 0.307 |
| Hydroxybutyrate | 27 (95) | 27 (71) | 29 (139) | 31 (72) | 35 (80) | 13 (52) | 0.808 | 0.781 | 0.709 |
| Amino Acids | |||||||||
| Alanine | 314 (92) | 306 (91) | 319 (98) | 306 (68) | 297 (73) | 318 (67) | 0.339 | 0.023 | 0.706 |
| Glycine | 227 (81) | 217 (78) | 255 (52) | 209 (53) | 207 (48) | 220 (65) | 0.001 | <0.001 | 0.053 |
| Glutamate | 61 (27) | 62 (28) | 61 (25) | 70 (28) | 67 (29) | 78 (29) | <0.001 | 0.022 | 0.411 |
| Glutamine | 416 ± 58 | 408 ± 57 | 438 ± 57 | 445 ± 53 | 442 ± 53 | 455 ± 21 | 0.001 | 0.004 | 0.118 |
| Histidine | 76 ± 12 | 76 ± 13 | 75 ± 10 | 78 ± 13 | 77 ± 13 | 79 ± 11 | 0.153 | 0.858 | 0.200 |
| Isoleucine | 28 (15) | 28 (16) | 27 (10) | 35 (17) | 35 (17) | 36 (17) | <0.001 | 0.073 | 0.865 |
| Leucine | 92 (26) | 91 (27) | 93 (21) | 106 (30) | 108 (30) | 102 (29) | <0.001 | 0.767 | 0.204 |
| Threonine | 220 ± 46 | 218 ± 48 | 224 ± 41 | 257 ± 47 | 259 ± 50 | 250 ± 38 | <0.001 | 0.856 | 0.167 |
| Tyrosine | 36 ± 9 | 36 ± 9 | 34 ± 10 | 37 ± 9 | 36 ± 9 | 41 ± 9 | 0.228 | <0.001 | 0.462 |
| Valine | 198 ± 44 | 193 ± 43 | 196 ± 36 | 226 ± 44 | 228 ± 47 | 220 ± 36 | <0.001 | 0.750 | 0.228 |
| Metabolite | Sex (Men vs. Women) | Age (≤50 vs. >50 Years Old) | Interaction Sex*Age | CAN (No CAN vs. CAN) | Interaction CAN*Age |
|---|---|---|---|---|---|
| Energy and Homeostasis Metabolites | |||||
| Acetate | = | = | ↓ in men >50 years↑ in women >50 years | = | ↓ CAN only in >50 years |
| Acetone | ↑ in men | = | = | = | = |
| Creatine | ↑ in women | = | = | = | = |
| Creatinine | ↑ in men | ↑ in >50 years | = | = | = |
| D-glucose | = | = | = | = | = |
| Glycerol | ↑ in women | = | ↓ in men >50 years↑ in women >50 years | = | = |
| Hydroxybutyrate | = | = | = | = | = |
| Lactate | = | ↑ >50 years | = | ↑ in CAN | = |
| Amino Acids | |||||
| Alanine | = | ↑ >50 years | = | = | = |
| Glutamine | ↑ in women | ↑ >50 years | = | = | = |
| Glutamate | ↑ in men | ↑ >50 years | = | = | = |
| Glycine | ↑ in men | ↑ >50 years | = | = | = |
| Histidine | = | = | = | = | = |
| Isoleucine | ↑ in men | = | = | ↓ in CAN | = |
| Leucine | ↑ in men | = | = | = | = |
| Threonine | ↑ in men | = | = | ↓ in CAN | = |
| Tyrosine | = | ↑ >50 years | = | = | ↑ CAN only in >50 years |
| Valine | ↑ in men | = | = | ↓ in CAN | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insenser, M.R.; Nattero-Chávez, L.; Luque-Ramírez, M.; Quiñones, S.d.L.; Quintero-Tobar, A.; Samino, S.; Amigó, N.; Dorado Avendaño, B.; Fiers, T.; Escobar-Morreale, H.F. Investigating the Link between Intermediate Metabolism, Sexual Dimorphism, and Cardiac Autonomic Dysfunction in Patients with Type 1 Diabetes. Metabolites 2024, 14, 436. https://doi.org/10.3390/metabo14080436
Insenser MR, Nattero-Chávez L, Luque-Ramírez M, Quiñones SdL, Quintero-Tobar A, Samino S, Amigó N, Dorado Avendaño B, Fiers T, Escobar-Morreale HF. Investigating the Link between Intermediate Metabolism, Sexual Dimorphism, and Cardiac Autonomic Dysfunction in Patients with Type 1 Diabetes. Metabolites. 2024; 14(8):436. https://doi.org/10.3390/metabo14080436
Chicago/Turabian StyleInsenser, María Rosa, Lía Nattero-Chávez, Manuel Luque-Ramírez, Sara de Lope Quiñones, Alejandra Quintero-Tobar, Sara Samino, Núria Amigó, Beatriz Dorado Avendaño, Tom Fiers, and Héctor F. Escobar-Morreale. 2024. "Investigating the Link between Intermediate Metabolism, Sexual Dimorphism, and Cardiac Autonomic Dysfunction in Patients with Type 1 Diabetes" Metabolites 14, no. 8: 436. https://doi.org/10.3390/metabo14080436
APA StyleInsenser, M. R., Nattero-Chávez, L., Luque-Ramírez, M., Quiñones, S. d. L., Quintero-Tobar, A., Samino, S., Amigó, N., Dorado Avendaño, B., Fiers, T., & Escobar-Morreale, H. F. (2024). Investigating the Link between Intermediate Metabolism, Sexual Dimorphism, and Cardiac Autonomic Dysfunction in Patients with Type 1 Diabetes. Metabolites, 14(8), 436. https://doi.org/10.3390/metabo14080436







