Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil
Abstract
:1. Introduction
2. Materials and Methods
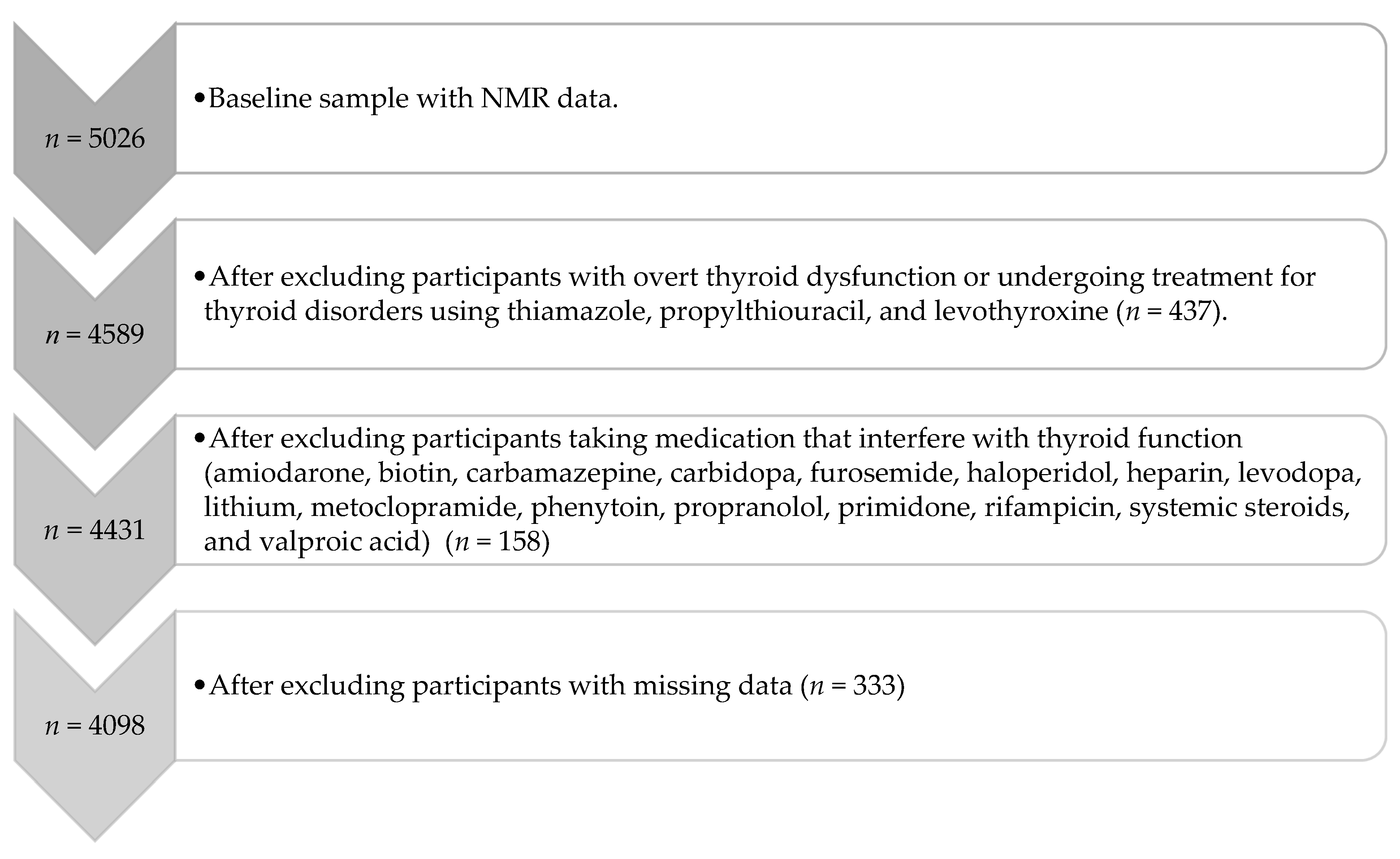
2.1. Participants
2.2. Thyroid-Related Parameters
2.3. Amino Acids Evaluation
2.4. Other Baseline Variables
2.5. Data Analyses
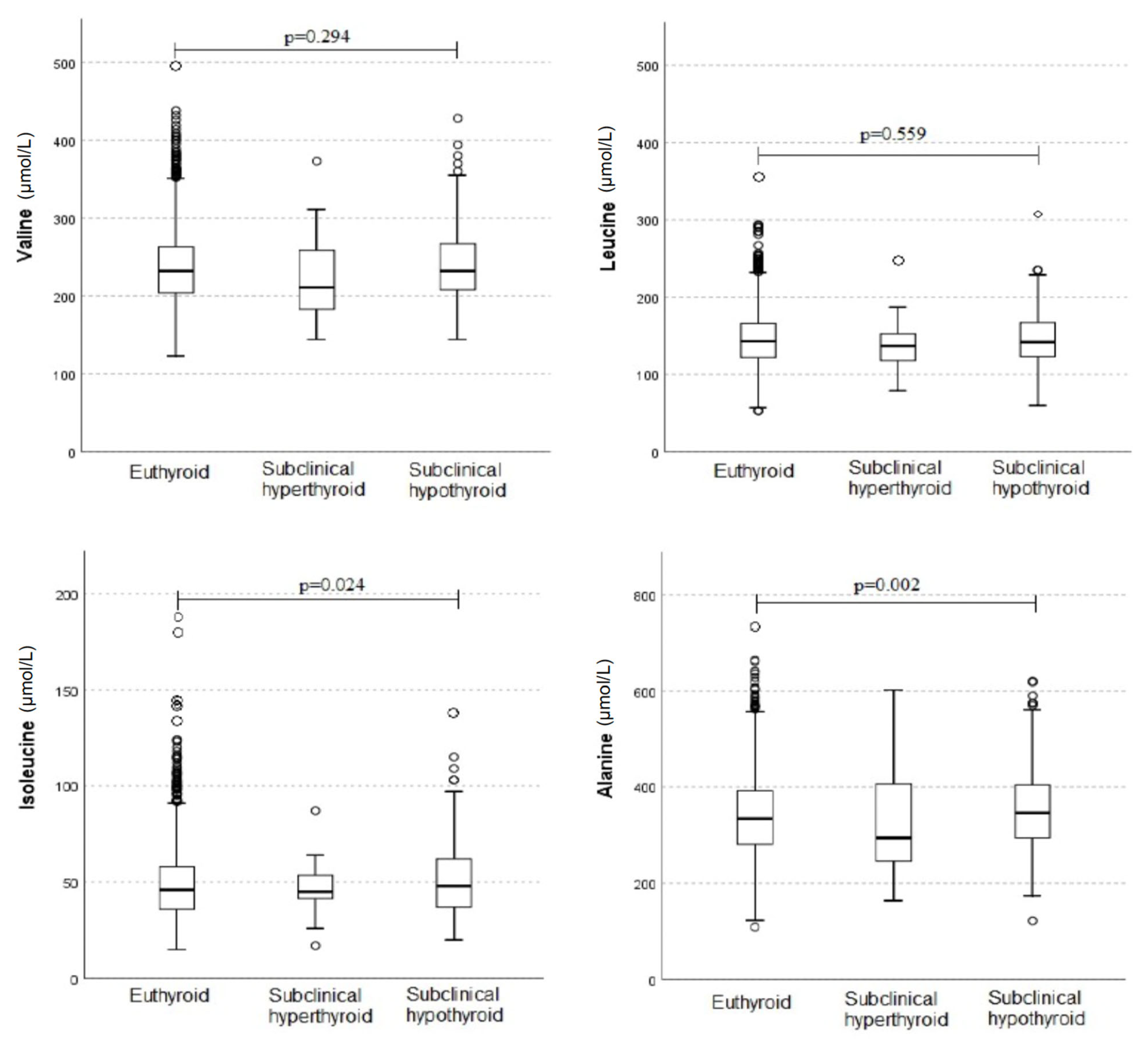
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamakawa, H.; Kato, T.S.; Noh, J.Y.; Yuasa, S.; Kawamura, A.; Fukuda, K.; Aizawa, Y. Thyroid hormone plays an important role in cardiac function: From bench to bedside. Front. Physiol. 2021, 12, 606931. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Fontes-Carvalho, R.; Borges-Canha, M.; Leite, A.R.; von Hafe, M.; Vale, C.; Martins, S.; Guimarães, J.T.; Carvalho, D.; Leite-Moreira, A.; et al. Association of thyroid function, within the euthyroid range, with cardiovascular risk: The epiporto study. Front. Endocrinol. 2022, 13, 1067801. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Salgado Nunez del Prado, S.; Celi, F.S. Thyroid hormone action and energy expenditure. J. Endocr. Soc. 2019, 3, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Wolak-Dinsmore, J.; Dullaart, R.P.F. Branched chain amino acids are associated with insulin resistance independent of leptin and adiponectin in subjects with varying degrees of glucose tolerance. Metab. Syndr. Relat. Disord. 2017, 15, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Bandt, J.-P.D.; Coumoul, X.; Barouki, R. Branched-chain amino acids and insulin resistance, from protein supply to diet-induced obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2018, 81, 139–164. [Google Scholar] [CrossRef]
- Batch, B.C.; Hyland, K.; Svetkey, L.P. Branch chain amino acids. Curr. Opin. Clin. Nutr. 2014, 17, 86–89. [Google Scholar] [CrossRef]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-chain amino acids and brain metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Santoro, N.; Tricò, D.; Giannini, C.; D’Adamo, E.; Zhao, H.; Peng, G.; Yu, X.; Lam, T.T.; Pierpont, B.; et al. A branched-chain amino acid-related metabolic signature characterizes obese adolescents with non-alcoholic fatty liver disease. Nutrients 2017, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- de Almeida-Pititto, B.; Dualib, P.M.; Jordão, M.C.; Izar Helfenstein Fonseca, M.; Jones, S.R.; Blaha, M.J.; Toth, P.P.; Santos, R.D.; Bensenor, I.M.; Ferreira, S.R.G.; et al. Branched-chain amino acids predict incident diabetes in the brazilian longitudinal study of adult health—Elsa-brasil. Diabetes Res. Clin. Pract. 2021, 174, 108747. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y. Protein turnover, ureagenesis and gluconeogenesis. Int. J. Vitam. Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Odessey, R.; Khairallah, E.A.; Goldberg, A.L. Origin and possible significance of alanine production by skeletal muscle. J. Biol. Chem. 1974, 249, 7623–7629. [Google Scholar] [CrossRef] [PubMed]
- de Souza Galia, W.B.; Biazi, G.R.; Frasson-Uemura, I.G.; Miksza, D.R.; Zaia, C.T.B.V.; Zaia, D.A.M.; de Souza, H.M.; Bertolini, G.L. Gluconeogenesis is reduced from alanine, lactate and pyruvate, but maintained from glycerol, in liver perfusion of rats with early and late sepsis. Cell Biochem. Funct. 2021, 39, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Pozefsk, T.; Marlis, E.; Cahill, G.F. Alanine: Key role in gluconeogenesis. Science 1970, 167, 1003–1004. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, E00416. [Google Scholar] [CrossRef]
- Haymond, M.W.; Miles, J.M. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 1982, 31, 86–89. [Google Scholar] [CrossRef]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolis 2013, 62, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Goh, H.J.; Verma, S.; Govindharajulu, P.; Sadananthan, S.A.; Michael, N.; Henry, C.J.; Goh, J.P.; Velan, S.S.; Leow, M.K. Brown adipose tissues mediate the metabolism of branched chain amino acids during the transitioning from hyperthyroidism to euthyroidism (tribute). Sci. Rep. 2022, 12, 3693. [Google Scholar] [CrossRef]
- Giacco, A.; Cioffi, F.; Cuomo, A.; Simiele, R.; Senese, R.; Silvestri, E.; Amoresano, A.; Fontanarosa, C.; Petito, G.; Moreno, M.; et al. Mild endurance exercise during fasting increases gastrocnemius muscle and prefrontal cortex thyroid hormone levels through differential bhb and bcaa-mediated bdnf-mtor signaling in rats. Nutrients 2022, 14, 1166. [Google Scholar] [CrossRef] [PubMed]
- van der Boom, T.; Gruppen, E.G.; Lefrandt, J.D.; Connelly, M.A.; Links, T.P.; Dullaart, R.P.F. Plasma branched chain amino acids are lower in short-term profound hypothyroidism and increase in response to thyroid hormone supplementation. Scand. J. Clin. Lab. Investig. 2020, 80, 562–566. [Google Scholar] [CrossRef]
- Rochon, C.; Tauveron, I.; Dejax, C.; Benoit, P.; Capitan, P.; Bayle, G.; Prugnaud, J.; Fabricio, A.; Berry, C.; Champredon, C.; et al. Response of leucine metabolism to hyperinsulinemia in hypothyroid patients before and after thyroxine replacement1. J. Clin. Endocrinol. Metab. 2000, 85, 697–706. [Google Scholar] [CrossRef]
- Adegoke, O.A.J.; Abdullahi, A.; Tavajohi-Fini, P. Mtorc1 and the regulation of skeletal muscle anabolism and mass. Appl. Physiol. Nutr. Metab. 2012, 37, 395–406. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-chain amino acids: Catabolism in skeletal muscle and implications for muscle and whole-body metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Martínez-Sánchez, N.; Gallego, R.; Vázquez, M.J.; Roa, J.; Gándara, M.; Schoenmakers, E.; Nogueiras, R.; Chatterjee, K.; Tena-Sempere, M.; et al. Hypothalamic mtor pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J. Pathol. 2012, 227, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (t3) stimulates brown adipose tissue activation via mitochondrial biogenesis and mtor-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef]
- Carew, L.B.; Evarts, K.G.; Alster, F.A. Growth and plasma thyroid hormone concentrations of chicks fed diets deficient in essential amino acids. Poult. Sci. 1997, 76, 1398–1404. [Google Scholar] [CrossRef]
- Carew, L.B.; Evarts, K.G.; Alster, F.A. Growth, feed intake, and plasma thyroid hormone levels in chicks fed dietary excesses of essential amino acids. Poult. Sci. 1998, 77, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, H.K.; Reddy, S.; Jayaraman, V.; Krishna, K.; Song, Q.; Rajasekaran, K.E.; Wang, T.; Bei, K.; Rajasekaran, J.J. Effect of micronutrients on thyroid parameters. J. Thyroid Res. 2021, 2021, 1865483. [Google Scholar] [CrossRef]
- Aquino, E.M.; Barreto, S.M.; Bensenor, I.M.; Carvalho, M.S.; Chor, D.; Duncan, B.B.; Lotufo, P.A.; Mill, J.G.; Molina Mdel, C.; Mota, E.L.; et al. Brazilian longitudinal study of adult health (elsa-brasil): Objectives and design. Am. J. Epidemiol. 2012, 175, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Duncan, B.B.; Mill, J.G.; Lotufo, P.A.; Chor, D.; Barreto, S.M.; Aquino, E.M.; Passos, V.M.; Matos, S.M.; Molina Mdel, C.; et al. Cohort profile: Longitudinal study of adult health (elsa-brasil). Int. J. Epidemiol. 2015, 44, 68–75. [Google Scholar] [CrossRef]
- Pereira, A.C.; Bensenor, I.M.; Fedeli, L.M.; Castilhos, C.; Vidigal, P.G.; Maniero, V.; Leite, C.M.; Pimentel, R.A.; Duncan, B.B.; Mill, J.G.; et al. Delineamento e implementação do biobanco do elsa-brasil: Estudo prospectivo na população brasileira. Rev. De Saúde Pública 2012, 47, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bensenor, I.M.; Griep, R.H.; Pinto, K.A.; de Faria, C.P.; Felisbino-Mendes, M.; Caetano, E.; Albuquerque, L.S.; Schmidt, M.I. Rotinas de organização de exames e entrevistas no centro de investigação elsa-brasil. Rev. De Saúde Pública 2012, 47, 37–47. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J. NMR measurement of LDL particle number using the vantera® clinical analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire; 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.; Stewart, S.M. Validity of the international physical activity questionnaire short form (ipaq-sf): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Klobučar, I.; Vidović, L.; Arih, I.; Lechleitner, M.; Pregartner, G.; Berghold, A.; Habisch, H.; Madl, T.; Frank, S.; Degoricija, V. Low valine serum levels predict increased 1-year mortality in acute heart failure patients. Biomolecules 2023, 13, 1323. [Google Scholar] [CrossRef]
- Otvos, J.D.; Shalaurova, I.; May, H.T.; Muhlestein, J.B.; Wilkins, J.T.; McGarrah, R.W., 3rd; Kraus, W.E. Multimarkers of metabolic malnutrition and inflammation and their association with mortality risk in cardiac catheterisation patients: A prospective, longitudinal, observational, cohort study. Lancet Healthy Longev. 2023, 4, E72–E82. [Google Scholar] [CrossRef]
- Wiklund, P.; Zhang, X.; Pekkala, S.; Autio, R.; Kong, L.; Yang, Y.; Keinänen-Kiukaanniemi, S.; Alen, M.; Cheng, S. Insulin resistance is associated with altered amino acid metabolism and adipose tissue dysfunction in normoglycemic women. Sci. Rep. 2016, 6, 24540. [Google Scholar] [CrossRef]
- Riis, A.L.; Jørgensen, J.O.; Ivarsen, P.; Frystyk, J.; Weeke, J.; Møller, N. Increased protein turnover and proteolysis is an early and primary feature of short-term experimental hyperthyroidism in healthy women. J. Clin. Endocrinol. Metab. 2008, 93, 3999–4005. [Google Scholar] [CrossRef] [PubMed]
- Riis, A.L.; Jørgensen, J.O.; Gjedde, S.; Nørrelund, H.; Jurik, A.G.; Nair, K.S.; Ivarsen, P.; Weeke, J.; Møller, N. Whole body and forearm substrate metabolism in hyperthyroidism: Evidence of increased basal muscle protein breakdown. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E1067–E1073. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Shimomura, Y.; Otsuka, M.; Popov, K.M.; Harris, R.A. Experimental hyperthyroidism causes inactivation of the branched-chain α-ketoacid dehydrogenase complex in rat liver. Arch. Biochem. Biophys. 2000, 375, 55–61. [Google Scholar] [CrossRef]
- Morrison, W.L.; Gibson, J.N.A.; Jung, R.T.; Rennie, M.J. Skeletal muscle and whole body protein turnover in thyroid disease. Eur. J. Clin. Investig. 1988, 18, 62–68. [Google Scholar] [CrossRef]
- Christoffolete, M.A.; Linardi, C.C.; de Jesus, L.; Ebina, K.N.; Carvalho, S.D.; Ribeiro, M.O.; Rabelo, R.; Curcio, C.; Martins, L.; Kimura, E.T.; et al. Mice with targeted disruption of the dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 2004, 53, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Cioffi, F.; De Matteis, R.; Petito, G.; de Lange, P.; Silvestri, E.; Lombardi, A.; Moreno, M.; Goglia, F.; Lanni, A. 3,5 Diiodo-l-thyronine (t2) promotes the browning of white adipose tissue in high-fat diet-induced overweight male rats housed at thermoneutrality. Cells 2019, 8, 256. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20160051. [Google Scholar] [CrossRef]
- Cioffi, F.; Gentile, A.; Silvestri, E.; Goglia, F.; Lombardi, A. Effect of iodothyronines on thermogenesis: Focus on brown adipose tissue. Front. Endocrinol. 2018, 9, 254. [Google Scholar] [CrossRef]
- Steinhoff, K.G.; Krause, K.; Linder, N.; Rullmann, M.; Volke, L.; Gebhardt, C.; Busse, H.; Stumvoll, M.; Blüher, M.; Sabri, O.; et al. Effects of hyperthyroidism on adipose tissue activity and distribution in adults. Thyroid 2021, 31, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lucia, F.; Bianco, A.C. T3 levels and thyroid hormone signaling. Front. Endocrinol. 2022, 13, 1044691. [Google Scholar] [CrossRef] [PubMed]
- Abbey, E.; Mcgready, J.; Simonsick, E.; Mammen, J. T3:T4 ratio can distinguish between adaptive changes and true subclinical hypothyroidism in older adults. Innov. Aging 2020, 4, 229. [Google Scholar] [CrossRef]
- Berghe, G. Disorders of gluconeogenesis. J. Inherit. Metab. Dis. 1996, 19, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Seitz, H.J. Thyroid hormone action on intermediary metabolism. Klin. Wochenschr. 1984, 62, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, F.Y.; Ahbab, S.; Ataoğlu, H.E.; Türker, B.Ç.; Çetin, F.; Türker, F.; Mamaç, R.Y.; Yenigün, M. Ft3/Ft4 ratio predicts non-alcoholic fatty liver disease independent of metabolic parameters in patients with euthyroidism and hypothyroidism. Clinics 2016, 71, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, R.; Midgley, J.E.M.; Larisch, R.; Dietrich, J.W. Homeostatic control of the thyroid–pituitary axis: Perspectives for diagnosis and treatment. Front. Endocrinol. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, S.E.; Jung, S.W.; Jin, H.S.; Park, I.B.; Ahn, S.V.; Lee, S. Free triiodothyronine/free thyroxine ratio rather than thyrotropin is more associated with metabolic parameters in healthy euthyroid adult subjects. Clin. Endocrinol. 2017, 87, 87–96. [Google Scholar] [CrossRef]
- Yu, T.; Tian, C.; Song, J.; He, D.; Wu, J.; Wen, Z.; Sun, Z.; Sun, Z. Value of the ft3/ft4 ratio and its combination with the grace risk score in predicting the prognosis in euthyroid patients with acute myocardial infarction undergoing percutaneous coronary intervention: A prospective cohort study. BMC Cardiovasc. Disord. 2018, 18, 181. [Google Scholar] [CrossRef]
- da Silva, L.E.S.; Gouvêa, E.C.D.P.; Stopa, S.R.; Tierling, V.L.; Sardinha, L.M.V.; Macario, E.M.; Claro, R.M. Data resource profile: Surveillance system of risk and protective factors for chronic diseases by telephone survey for adults in brazil (vigitel). Int. J. Epidemiol. 2021, 50, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
| All (n = 4098) | Thyroid Function | |||
|---|---|---|---|---|
| Euthyroid (n = 3693) | Subclinical Hyper (n = 23) | Subclinical Hypo (n = 382) | ||
| n (%) | n (%) | n (%) | n (%) | |
| Gender | ||||
| Men | 1988 (48.5%) | 1796 (48.6%) | 7 (30.4%) | 185 (48.4%) |
| Women | 2110 (51.5%) | 1897 (51.4%) | 16 (69.6%) | 197 (51.6%) |
| Education | ||||
| Up to some college | 2316 (56.5%) | 2091 (56.6%) | 12 (52.2%) | 213 (55.8%) |
| Completed college or more | 1782 (43.5%) | 1602 (43.4%) | 11 (47.8%) | 169 (44.2%) |
| Self-reported race a | ||||
| Non-white | 1725 (42.1%) | 1574 (42.6%) | 13 (56.5%) | 138 (36.1%) |
| White | 2373 (57.9%) | 2119 (57.4%) | 10(43.5%) | 244 (63.9%) |
| Physical activity | ||||
| Inactive | 2703 (66.0%) | 2430 (65.8%) | 13 (56.5%) | 260 (68.1%) |
| Insufficiently active | 459 (11.2%) | 412 (11.2%) | 2 (8.7%) | 45 (11.8%) |
| Active | 936 (22.8%) | 851 (23.0%) | 8 (34.8%) | 77 (20.2%) |
| Smoking | ||||
| Never smoked | 2170 (53.0%) | 1940 (52.5%) | 11 (47.8%) | 219 (57.3%) |
| Past or current smoker | 1928 (47.0%) | 1753 (47.5%) | 12 (52.2%) | 163 (42.7%) |
| Diabetes mellitus | ||||
| No | 3384 (82.6%) | 3041 (82.3%) | 19 (82.6%) | 324 (84.8%) |
| Yes | 714 (17.4%) | 652 (17.7%) | 4 (17.4%) | 58 (15.2%) |
| Valine | Leucine | Isoleucine | Alanine | |||||
|---|---|---|---|---|---|---|---|---|
| β (CI 95%) | p | β (CI 95%) | p | β (CI 95%) | p | β (CI 95%) | p | |
| Model 1—Crude | ||||||||
| TSH a | 4.31 (0.56; 8.07) | 0.024 | 2.96 (0.26; 5.67) | 0.032 | 1.87 (0.38; 3.36) | 0.014 | 19.46 (12.60; 26.32) | <0.001 |
| FT4 a | 13.04 (−5.08; 31.16) | 0.158 | 16.09 (3.04; 29.14) | 0.016 | 1.02 (−6.17; 8.21) | 0.781 | −16.31 (−49.53; 16.91) | 0.336 |
| FT3 a | 243.27 (201.49; 285.04) | <0.001 | 170.53 (140.40; 200.66) | <0.001 | 76.35 (59.68; 93.01) | <0.001 | 148.73 (71.03; 226.39) | <0.001 |
| T3:T4 ratio a | 158.34 (119.96; 196.72) | <0.001 | 98.31 (70.59; 126.02) | <0.001 | 55.61 (40.37; 70.86) | <0.001 | 148.60 (77.83; 219.37) | <0.001 |
| Model 2—Adjusted for sociodemographic variables (age, race, sex, educational level) | ||||||||
| TSH a | 2.89 (−0.47; 6.25) | 0.092 | 2.41 (−0.06; 4.87) | 0.055 | 1.53 (0.17; 2.89) | 0.028 | 15.89 (9.16; 22.62) | <0.001 |
| FT4 a | −30.81 (−47.11; −14.50) | <0.001 | −11.87 (−23.83; 0.10) | 0.052 | −14.45 (−21.05; −7.85) | <0.001 | −51.25 (−84.00; −18.50) | 0.002 |
| FT3 a | 79.07 (39.61; 118.54) | <0.001 | 52.88 (23.95; 81.81) | <0.001 | 11.84 (−4.17; 27.84) | 0.147 | 82.01 (3.69; 162.34) | 0.040 |
| T3:T4 ratio a | 111.54 (76.91; 146.18) | <0.001 | 59.04 (33.60; 84.48) | <0.001 | 35.14 (21.09; 49.19) | <0.001 | 157.26 (87.56; 226.95) | <0.001 |
| Model 3—Adjusted for model 2 + health conditions (BMI, physical activity, diabetes, and smoking) | ||||||||
| TSH a | 1.41 (−1.65; 4.47) | 0.367 | 2.00 (−0.34; 4.33) | 0.094 | 1.10 (−0.19; 2.40) | 0.095 | 14.44 (7.89; 20.99) | <0.001 |
| FT4 a | −11.30 (−26.23; 3.63) | 0.138 | −2.00 (−13.38; 9.37) | 0.730 | −8.75 (−15.05; −2.45) | 0.006 | −7.72 (−39.72; 28.29) | 0.637 |
| FT3 a | 61.75 (25.87; 97.64) | 0.001 | 43.39 (16.05; 70.73) | 0.002 | 6.58 (−8.59; 21.75) | 0.396 | 64.98 (−11.99; 141.95) | 0.098 |
| T3:T4 ratio a | 62.17 (30.20; 94.13) | <0.001 | 33.55 (9.18; 57.92) | 0.007 | 20.46 (6.95; 33.97) | 0.003 | 104.32 (35.78; 172.86) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janovsky, C.C.P.S.; Meneghini, V.; Tebar, W.; Martins, J.R.M.; Sgarbi, J.A.; Teixeira, P.d.F.d.S.; Jones, S.R.; Blaha, M.J.; Toth, P.P.; Lotufo, P.A.; et al. Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil. Metabolites 2024, 14, 437. https://doi.org/10.3390/metabo14080437
Janovsky CCPS, Meneghini V, Tebar W, Martins JRM, Sgarbi JA, Teixeira PdFdS, Jones SR, Blaha MJ, Toth PP, Lotufo PA, et al. Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil. Metabolites. 2024; 14(8):437. https://doi.org/10.3390/metabo14080437
Chicago/Turabian StyleJanovsky, Carolina Castro Porto Silva, Vandrize Meneghini, William Tebar, Joao Roberto Maciel Martins, José Augusto Sgarbi, Patrícia de Fatima dos Santos Teixeira, Steven R. Jones, Michael J. Blaha, Peter P. Toth, Paulo A. Lotufo, and et al. 2024. "Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil" Metabolites 14, no. 8: 437. https://doi.org/10.3390/metabo14080437
APA StyleJanovsky, C. C. P. S., Meneghini, V., Tebar, W., Martins, J. R. M., Sgarbi, J. A., Teixeira, P. d. F. d. S., Jones, S. R., Blaha, M. J., Toth, P. P., Lotufo, P. A., Bittencourt, M. S., Santos, R. D., Santos, I. S., Chaker, L., & Bensenor, I. M. (2024). Branched-Chain Amino Acids, Alanine, and Thyroid Function: A Cross-Sectional, Nuclear Magnetic Resonance (NMR)-Based Approach from ELSA-Brasil. Metabolites, 14(8), 437. https://doi.org/10.3390/metabo14080437







