Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Preoperative Education on Nutrition and Exercise Habits
2.3. Surgery and Postoperative Care
2.4. Measurements
2.5. Outcome Measures
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
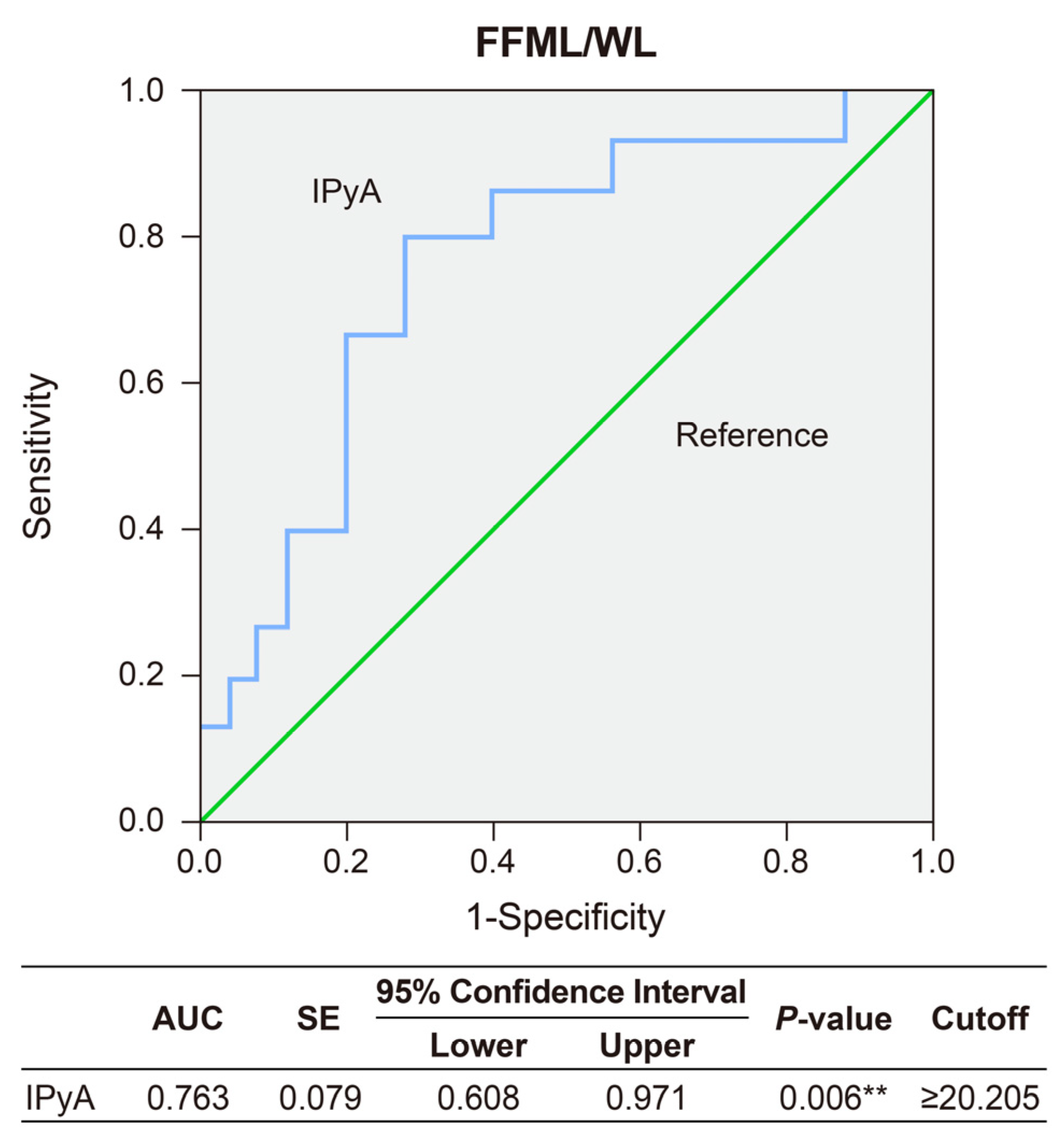
3.2. Predictors for FFM Gain Relative to Weight Loss between 3 and 6 Months after Sleeve Gastrectomy
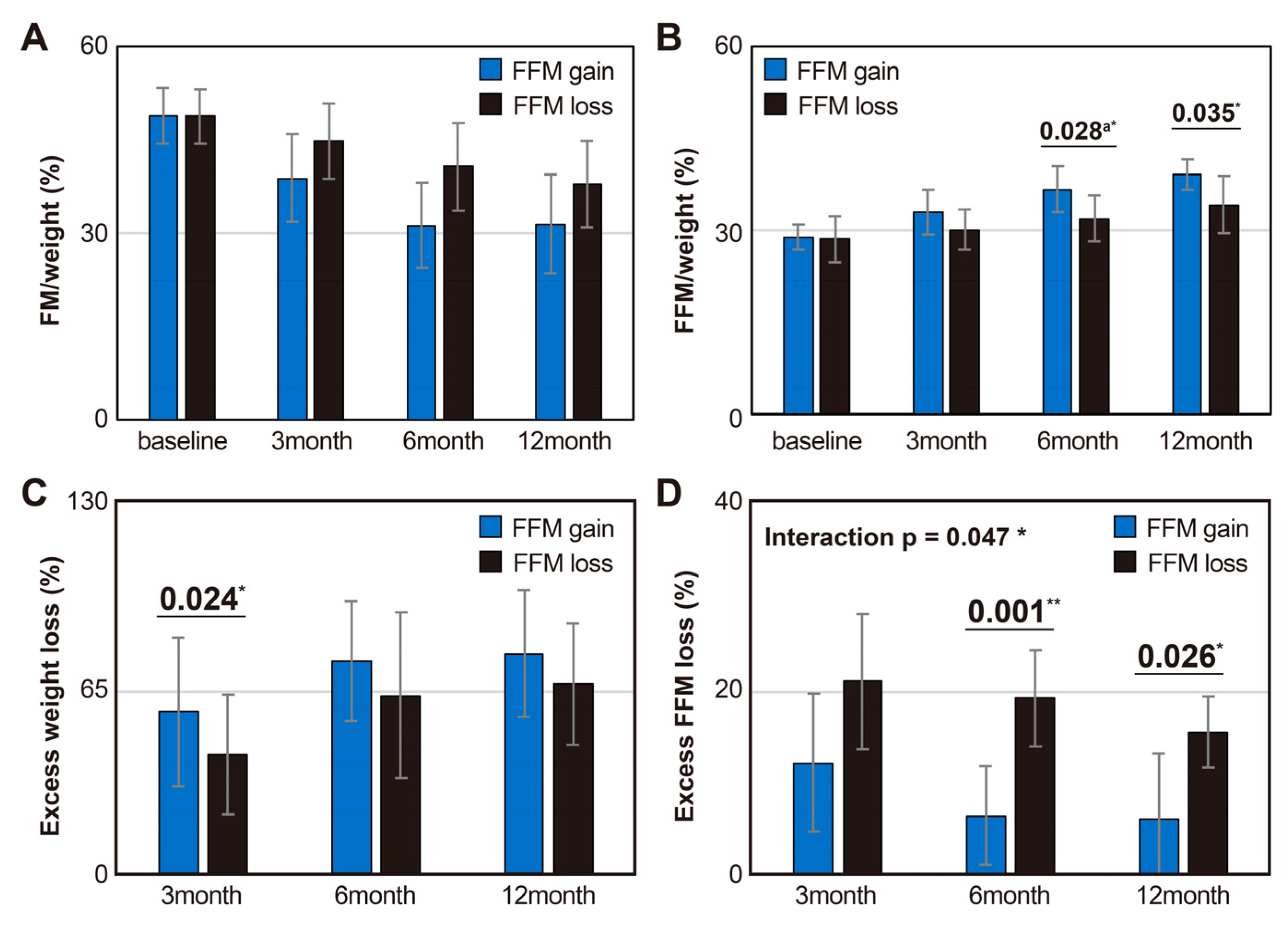
3.3. Body Composition and Weight Changes Up to 1 Year After Surgery between Groups Based on the Relative FFM Changes to Weight Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alalwan, A.A.; Friedman, J.; Park, H.; Segal, R.; Brumback, B.A.; Hartzema, A.G. US national trends in bariatric surgery: A decade of study. Surgery 2021, 170, 13–17. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014, 63 Pt B, 2985–3023. [Google Scholar] [CrossRef]
- Bhandari, M.; Fobi, M.A.L.; Buchwald, J.N. Standardization of Bariatric Metabolic Procedures: World Consensus Meeting Statement. Obes. Surg. 2019, 29 (Suppl. 4), 309–345. [Google Scholar] [CrossRef] [PubMed]
- Barzin, M.; Heidari Almasi, M.; Mahdavi, M.; Khalaj, A.; Valizadeh, M.; Hosseinpanah, F. Body Composition Changes Following Sleeve Gastrectomy Vs. One-Anastomosis Gastric Bypass: Tehran Obesity Treatment Study (TOTS). Obes. Surg. 2021, 31, 5286–5294. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shishikura, K.; Tanimoto, K.; Sakai, S.; Tanimoto, Y.; Terasaki, J.; Hanafusa, T. Association between skeletal muscle mass and insulin secretion in patients with type 2 diabetes mellitus. Endocr. J. 2014, 61, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Reinmann, A.; Gafner, S.C.; Hilfiker, R.; Bruyneel, A.V.; Pataky, Z.; Allet, L. Bariatric Surgery: Consequences on Functional Capacities in Patients With Obesity. Front. Endocrinol. 2021, 12, 646283. [Google Scholar] [CrossRef] [PubMed]
- Baad, V.M.A.; Bezerra, L.R.; de Holanda, N.C.P.; Dos Santos, A.C.O.; da Silva, A.A.M.; Bandeira, F.; Cavalcante, T.C.F. Body Composition, Sarcopenia and Physical Performance After Bariatric Surgery: Differences Between Sleeve Gastrectomy and Roux-En-Y Gastric Bypass. Obes. Surg. 2022, 32, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Bužga, M.; Pekar, M.; Uchytil, J.; Horká, V.; Malůš, J.; Vilímek, D.; Švagera, Z.; Kutáč, P.; Holéczy, P. Prevention of sarcopenia in patients with obesity after bariatric and metabolic surgery: The effect of programmed training on the muscle tissue and anthropometric functions—A randomized controlled trial (SarxOb study protocol). Bosn. J. Basic. Med. Sci. 2022, 23, 191–197. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Monpellier, V.M.; Eijsvogels, T.M.H.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. Rate and Determinants of Excessive Fat-Free Mass Loss After Bariatric Surgery. Obes. Surg. 2020, 30, 3119–3126. [Google Scholar] [CrossRef]
- Ha, J.; Kwon, Y.; Park, S. Metabolomics in Bariatric Surgery: Towards Identification of Mechanisms and Biomarkers of Metabolic Outcomes. Obes. Surg. 2021, 31, 4564–4574. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montoro, J.I.; Kuchay, M.S.; Balaguer-Román, A.; Martínez-Sánchez, M.A.; Frutos, M.D.; Fernández-García, J.C.; Ramos-Molina, B. Gut microbiota and related metabolites in the pathogenesis of nonalcoholic steatohepatitis and its resolution after bariatric surgery. Obes. Rev. 2022, 23, e13367. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Metabolomic Analysis of the Improvements in Insulin Secretion and Resistance After Sleeve Gastrectomy: Implications of the Novel Biomarkers. Obes. Surg. 2021, 31, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Amino Acid Metabolites and Slow Weight Loss in the Early Postoperative Period after Sleeve Gastrectomy. J. Clin. Med. 2020, 9, 2348. [Google Scholar] [CrossRef]
- Ha, J.; Jang, M.; Kwon, Y.; Park, Y.S.; Park, D.J.; Lee, J.-H.; Lee, H.-J.; Ha, T.K.; Kim, Y.-J.; Han, S.-M.; et al. Metabolomic Profiles Predict Diabetes Remission after Bariatric Surgery. J. Clin. Med. 2020, 9, 3897. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, D.L.; Knuth, N.D.; Huizenga, R.; Rood, J.C.; Ravussin, E.; Hall, K.D. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J. Clin. Endocrinol. Metab. 2012, 97, 2489–2496. [Google Scholar] [CrossRef]
- Guida, B.; Cataldi, M.; Busetto, L.; Aiello, M.L.; Musella, M.; Capone, D.; Parolisi, S.; Policastro, V.; Ragozini, G.; Belfiore, A. Predictors of fat-free mass loss 1 year after laparoscopic sleeve gastrectomy. J. Endocrinol. Investig. 2018, 41, 1307–1315. [Google Scholar] [CrossRef]
- Nuijten, M.A.H.; Eijsvogels, T.M.H.; Monpellier, V.M.; Janssen, I.M.C.; Hazebroek, E.J.; Hopman, M.T.E. The magnitude and progress of lean body mass, fat-free mass, and skeletal muscle mass loss following bariatric surgery: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13370. [Google Scholar] [CrossRef]
- Maïmoun, L.; Aouinti, S.; Puech, M.; Lefebvre, P.; Deloze, M.; de Santa Barbara, P.; Renard, E.; Christol, J.P.; Myzia, J.; Picot, M.C.; et al. Changes in Lean Tissue Mass, Fat Mass, Biological Parameters and Resting Energy Expenditure over 24 Months Following Sleeve Gastrectomy. Nutrients 2023, 15, 1201. [Google Scholar] [CrossRef]
- Shantavasinkul, P.C.; Omotosho, P.; Muehlbauer, M.J.; Natoli, M.; Corsino, L.; Tong, J.; Portenier, D.; Torquati, A. Metabolic profiles, energy expenditures, and body compositions of the weight regain versus sustained weight loss patients who underwent Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2021, 17, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Voorwinde, V.; Steenhuis, I.H.M.; Janssen, I.M.C.; Monpellier, V.M.; van Stralen, M.M. Definitions of Long-Term Weight Regain and Their Associations with Clinical Outcomes. Obes. Surg. 2020, 30, 527–536. [Google Scholar] [CrossRef]
- Sivakumar, J.; Chen, Q.; Sutherland, T.R.; Read, M.; Ward, S.; Chong, L.; Hii, M.W. Body Composition Differences Between Excess Weight Loss ≥ 50% and < 50% at 12 Months Following Bariatric Surgery. Obes. Surg. 2022, 32, 2556–2566. [Google Scholar] [CrossRef]
- Vrzalová, A.; Pečinková, P.; Illés, P.; Gurská, S.; Džubák, P.; Szotkowski, M.; Hajdúch, M.; Mani, S.; Dvořák, Z. Mixture Effects of Tryptophan Intestinal Microbial Metabolites on Aryl Hydrocarbon Receptor Activity. Int. J. Mol. Sci. 2022, 23, 10825. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y.; Agrawal, R.; Whiley, L.; Wood, T.K.; Hejndorf, S.; Ng, Y.Z.; Low, J.Z.Y.; Rossant, J.; et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef]
- Aoki, R.; Aoki-Yoshida, A.; Suzuki, C.; Takayama, Y. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J. Immunol. 2018, 201, 3683–3693. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cheng, L.; Jiang, Y.; Zhang, L.; Qian, L. Indole-3-pyruvic acid alleviates rheumatoid arthritis via the aryl hydrocarbon receptor pathway. Ann. Transl. Med. 2023, 11, 213. [Google Scholar] [CrossRef]
- Zuo, M.; Fang, J.; Huang, P.; Liu, S.; Hou, P.; Wang, S.; Liu, Z.; Feng, C.; Cao, L.; Li, P.; et al. IL4I1-catalyzed tryptophan metabolites mediate the anti-inflammatory function of cytokine-primed human muscle stem cells. Cell Death Discov. 2023, 9, 269. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Fukada, S.I.; Akimoto, T.; Sotiropoulos, A. Role of damage and management in muscle hypertrophy: Different behaviors of muscle stem cells in regeneration and hypertrophy. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118742. [Google Scholar] [CrossRef]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Scala, P.; Rehak, L.; Giudice, V.; Ciaglia, E.; Puca, A.A.; Selleri, C.; Della Porta, G.; Maffulli, N. Stem Cell and Macrophage Roles in Skeletal Muscle Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 10867. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Feng, C.; Chen, W.; Hou, P.; Liu, Z.; Zuo, M.; Han, Y.; Xu, C.; Melino, G.; Verkhratsky, A.; et al. Redressing the interactions between stem cells and immune system in tissue regeneration. Biol. Direct 2021, 16, 18. [Google Scholar] [CrossRef] [PubMed]
| Variables | Baseline (n = 42) |
|---|---|
| Age, years | 39.93 ± 10.91 |
| Female sex, no. (%) | 27 (64.2) |
| BMI, kg/m2 | 40.85 ± 6.53 |
| Weight, kg | 114.97 ± 23.40 |
| FM/weight, % | 48.80 ± 4.28 |
| FFM/weight, % | 32.03 ± 5.67 |
| Baseline | |||
|---|---|---|---|
| FFM Gain (n = 16) | FFM Loss (n = 26) | p-Value | |
| ΔFFM/ΔW | –15.59 ± 11.65 | 18.83 ± 17.76 | - |
| Age, years | 42.50 ± 9.85 | 38.21 ± 11.43 | 0.228 |
| Female sex, no. (%) | 9 (56.2) | 18 (69.2) | 0.406 |
| Anthropometric parameters | |||
| BMI, kg/m2 | 38.39 ± 5.16 | 42.27 ± 6.89 | 0.048 * |
| Weight, kg | 111.51 ± 21.39 | 116.97 ± 24.67 | 0.478 |
| FM/weight, % | 45.58 ± 7.16 | 48.80 ± 4.38 | 0.220 |
| FFM/weight, % | 30.44 ± 6.38 | 33.00 ± 5.19 | 0.992 |
| Serum analysis | |||
| AST, U/L | 39.40 ± 16.02 | 47.80 ± 23.24 | 0.117 |
| ALT, U/L | 59.80 ± 27.04 | 65.30 ± 30.88 | 0.635 |
| BUN, mg/dL | 14.52 ± 5.18 | 14.23 ± 5.59 | 0.893 |
| Creatinine, mg/dL | 0.90 ± 0.37 | 0.84 ± 0.24 | 0.707 |
| Bilirubin, mg/dL | 0.71 ± 0.44 | 0.75 ± 0.31 | 0.472 a |
| Uric acid, mg/dL | 5.99 ± 1.96 | 5.91 ± 1.68 | 0.922 |
| Fasting blood glucose, mg/dL | 144.10 ± 44.01 | 136.11 ± 42.68 | 0.644 |
| Cholesterol, mg/dL | 197.80 ± 35.37 | 177.62 ± 38.04 | 0.164 |
| HDL, mg/dL | 56.00 ± 21.61 | 41.57 ± 5.57 | 0.066 |
| LDL, mg/dL | 134.00 ± 42.72 | 111.57 ± 33.21 | 0.165 |
| TG, mg/dL | 178.78 ± 102.75 | 169.90 ± 86.52 | 0.824 |
| Aromatic amino acids, µmol/L | |||
| Tryptophan | 57.02 ± 11.69 | 54.05 ± 13.43 | 0.469 |
| IPyA | 27.62 ± 10.43 | 18.31 ± 8.52 | 0.007 ** |
| IAA | 1.68 ± 0.85 | 1.49 ± 1.03 | 0.133 a |
| ILA | 0.69 ± 0.14 | 0.62 ± 0.19 | 0.236 |
| IPA | 1.35 ± 2.92 | 0.46 ± 0.55 | 0.138 |
| 5-HTP | 0.21 ± 0.76 | 0.08 ± 0.30 | 0.428 |
| 5-HT | 0.24 ± 0.21 | 0.26 ± 0.24 | 0.834 |
| 5-HIAA | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.170 a |
| Phenylalanine | 67.12 ± 9.63 | 67.85 ± 12.40 | 0.841 |
| Tyrosine | 56.19 ± 16.61 | 58.94 ± 15.91 | 0.595 |
| L-DOPA | 0.58 ± 0.37 | 0.48 ± 0.21 | 0.247 |
| Dopamine | 0.11 ± 0.08 | 0.10 ± 0.07 | 0.506 |
| Model 1 | ||||
| Step 1 | B (s.e) | p-Value | Odds Ratio | 95% CI |
| BMI | −0.123 (0.070) | 0.080 | 0.080 | 0.770–1.015 |
| IPyA | 0.116 (0.048) | 0.015 * | 1.123 | 1.023–1.233 |
| R2 = 0.357, F = 8.00, p = 0.002 ** | ||||
| Model 2 | ||||
| Step 1 | B (s.e) | p-value | Odds Ratio | 95% CI |
| Age | −0.054 (0.090) | 0.550 | 0.948 | 0.794–1.130 |
| Sex | 1.216 (1.893) | 0.521 | 3.374 | 0.083–137.926 |
| BMI | 0.646 (0.435) | 0.138 | 1.908 | 0.813–4.476 |
| Weight | 0.470 (0.306) | 0.125 | 1.600 | 0.878–2.913 |
| FM | −0. 799 (0.377) | 0.034 * | 0.450 | 0.215–0.941 |
| FFM | −0. 892 (0.639) | 0.163 | 0.410 | 0.117–1.433 |
| IPyA | 0.217 (0.106) | 0.041 * | 1.242 | 1.009–1.529 |
| Step 4 | B (s.e) | p-value | Odds Ratio | 95% CI |
| BMI | 0.534 (0.354) | 0.131 | 1.706 | 0.853–3.412 |
| FM | −0.307 (0.178) | 0.084 | 0.736 | 0.519–1.042 |
| IPyA | 1.217 (0.091) | 0.030 * | 1.217 | 1.019–1.454 |
| R2 = 0.576, F = 8.00, p = 0.001 ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, E.; Kwon, Y.; Park, S. Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy. Metabolites 2024, 14, 444. https://doi.org/10.3390/metabo14080444
Seo E, Kwon Y, Park S. Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy. Metabolites. 2024; 14(8):444. https://doi.org/10.3390/metabo14080444
Chicago/Turabian StyleSeo, Eunhye, Yeongkeun Kwon, and Sungsoo Park. 2024. "Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy" Metabolites 14, no. 8: 444. https://doi.org/10.3390/metabo14080444
APA StyleSeo, E., Kwon, Y., & Park, S. (2024). Association Between Indole-3-Pyruvic Acid and Change in Fat-Free Mass Relative to Weight Loss in Patients Undergoing Sleeve Gastrectomy. Metabolites, 14(8), 444. https://doi.org/10.3390/metabo14080444








