Alterations in Vagal Tone Are Associated with Changes in the Gut Microbiota of Adults with Anxiety and Depression Symptoms: Analysis of Fecal Metabolite Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Depression and Anxiety
2.3. Assessment of Heart Rate Variability
2.4. Fecal Sample Acquisition
2.5. Evaluation of Microbiota Composition through Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Metabolite Extraction
2.7. Metabolite Profiling Based on Nuclear Magnetic Resonance (NMR)
2.8. Data and Statistical Analysis
2.8.1. Depression and Anxiety
2.8.2. Heart Rate Variability (HRV)
2.8.3. Microbiota Composition
2.8.4. Metabolome Analysis
3. Results
3.1. Main Characteristics of Groups
3.2. Microbiota Analysis
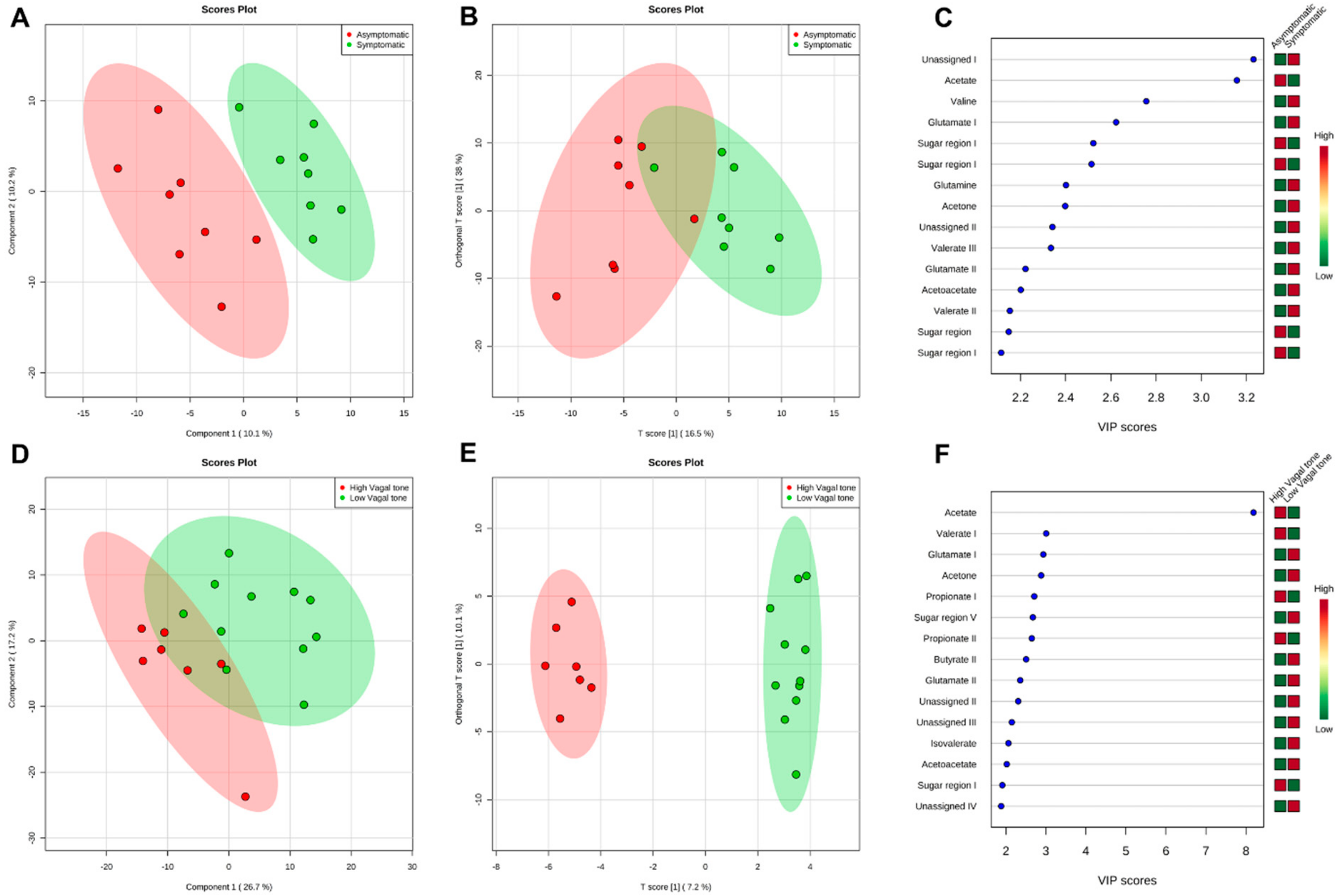
3.3. Metabolomics Analysis
4. Discussion
4.1. Short-Chain Fatty Acids (SCFAs)
4.2. Ketone Bodies
4.3. Amino Acids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Mental Health Report: Transforming Mental Health for All; WHO: Geneva, Switzerland, 2022; Available online: https://iris.who.int/bitstream/handle/10665/356119/9789240049338-eng.pdf?sequence=1 (accessed on 13 July 2024).
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/10665/254610/1/WHO-MSD-MER-2017.2-eng.pdf (accessed on 10 June 2020).
- Martins-de-Souza, D. Proteomics and Metabolomics in Psychiatry; Advances in Biological Psychiatry; S. Karger AG: Basel, Switzerland, 2014; Volume 29, ISBN 978-3-318-02599-6. [Google Scholar]
- American Psychiatric Society. Cautionary Statement for Forensic Use of DSM-5. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013; ISBN 978-0-89042-559-6. [Google Scholar]
- Wang, Y.-P.; Gorenstein, C. Psychometric Properties of the Beck Depression Inventory-II: A Comprehensive Review. Rev. Bras. Psiquiatr. 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, N.C.; Newman, M.G. Anxiety and Depression as Bidirectional Risk Factors for One Another: A Meta-Analysis of Longitudinal Studies. Psychol. Bull. 2017, 143, 1155–1200. [Google Scholar] [CrossRef]
- Volchan, E.; Rocha-Rego, V.; Bastos, A.F.; Oliveira, J.M.; Franklin, C.; Gleiser, S.; Berger, W.; Souza, G.G.L.; Oliveira, L.; David, I.A.; et al. Immobility Reactions under Threat: A Contribution to Human Defensive Cascade and PTSD. Neurosci. Biobehav. Rev. 2017, 76, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro Costa, L.N.; Carneiro, B.A.; Alves, G.S.; Lins Silva, D.H.; Faria Guimaraes, D.; Souza, L.S.; Bandeira, I.D.; Beanes, G.; Miranda Scippa, A.; Quarantini, L.C. Metabolomics of Major Depressive Disorder: A Systematic Review of Clinical Studies. Cureus 2022, 14, e23009. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yieh, L.; Yang, T.; Drinkenburg, W.; Peeters, P.; Steckler, T.; Narayan, V.A.; Wittenberg, G.; Ye, J. Metabolomic Biosignature Differentiates Melancholic Depressive Patients from Healthy Controls. BMC Genom. 2016, 17, 669. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zheng, P.; Zhao, L.; Jia, J.; Tang, S.; Xu, P.; Xie, P.; Gao, H. Predictive Diagnosis of Major Depression Using NMR-Based Metabolomics and Least-Squares Support Vector Machine. Clin. Chim. Acta 2017, 464, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.; Harwood, J.; Swade, C.; Coppen, A. Decreased Tryptophan Excretion by Depressive Patients. Clin. Chim. Acta 1978, 88, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Thipakorn, Y.; Vasupanrajit, A.; Abo Algon, A.A.; Tunvirachaisakul, C.; Hashim Aljanabi, A.A.; Oxenkrug, G.; Al-Hakeim, H.K.; Maes, M. The Tryptophan Catabolite or Kynurenine Pathway in Major Depressive and Bipolar Disorder: A Systematic Review and Meta-Analysis. Brain Behav. Immun. Health 2022, 26, 100537. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Ho, C.S.H.; Tay, G.W.N.; Wee, H.N.; Ching, J. The Utility of Amino Acid Metabolites in the Diagnosis of Major Depressive Disorder and Correlations with Depression Severity. Int. J. Mol. Sci. 2023, 24, 2231. [Google Scholar] [CrossRef]
- Bai, S.; Xie, J.; Bai, H.; Tian, T.; Zou, T.; Chen, J.-J. Gut Microbiota-Derived Inflammation-Related Serum Metabolites as Potential Biomarkers for Major Depressive Disorder. J. Inflamm. Res. 2021, 14, 3755–3766. [Google Scholar] [CrossRef]
- Wang, M.; Song, Z.; Lai, S.; Tang, F.; Dou, L.; Yang, F. Depression-Associated Gut Microbes, Metabolites and Clinical Trials. Front. Microbiol. 2024, 15, 1292004. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.-P.J.; Rezzi, S.; Peré-Trepat, E.; Kamlage, B.; Collino, S.; Leibold, E.; Kastler, J.; Rein, D.; Fay, L.B.; Kochhar, S. Metabolic Effects of Dark Chocolate Consumption on Energy, Gut Microbiota, and Stress-Related Metabolism in Free-Living Subjects. J. Proteome Res. 2009, 8, 5568–5579. [Google Scholar] [CrossRef] [PubMed]
- Phua, L.C.; Wilder-Smith, C.H.; Tan, Y.M.; Gopalakrishnan, T.; Wong, R.K.; Li, X.; Kan, M.E.; Lu, J.; Keshavarzian, A.; Chan, E.C.Y. Gastrointestinal Symptoms and Altered Intestinal Permeability Induced by Combat Training Are Associated with Distinct Metabotypic Changes. J. Proteome Res. 2015, 14, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Pieh, C.; Probst, T. Metabolomic Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 4784. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Early-Life Adversity and Brain Development: Is the Microbiome a Missing Piece of the Puzzle? Neuroscience 2017, 342, 37–54. [Google Scholar] [CrossRef]
- Tillisch, K.; Labus, J.; Kilpatrick, L.; Jiang, Z.; Stains, J.; Ebrat, B.; Guyonnet, D.; Legrain–Raspaud, S.; Trotin, B.; Naliboff, B.; et al. Consumption of Fermented Milk Product with Probiotic Modulates Brain Activity. Gastroenterology 2013, 144, 1394–1401.e4. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, Y.; Zhong, H.; Liu, Z.; Geng, J.; Wang, H.; Wang, W. Gut Microbes in Central Nervous System Development and Related Disorders. Front. Immunol. 2024, 14, 1288256. [Google Scholar] [CrossRef]
- Groen, R.N.; De Clercq, N.C.; Nieuwdorp, M.; Hoenders, H.J.R.; Groen, A.K. Gut Microbiota, Metabolism and Psychopathology: A Critical Review and Novel Perspectives. Crit. Rev. Clin. Lab. Sci. 2018, 55, 283–293. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.-D.I.; Soković Bajić, S.; Vojnović Milutinović, D.; Tomić, M.; Golić, N.; Tolinački, M. Gut Microbiota Dysbiosis Associated with Altered Production of Short Chain Fatty Acids in Children with Neurodevelopmental Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.; Kubo, C.; Koga, Y. Postnatal Microbial Colonization Programs the Hypothalamic–Pituitary–Adrenal System for Stress Response in Mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Crumeyrolle-Arias, M.; Jaglin, M.; Bruneau, A.; Vancassel, S.; Cardona, A.; Daugé, V.; Naudon, L.; Rabot, S. Absence of the Gut Microbiota Enhances Anxiety-like Behavior and Neuroendocrine Response to Acute Stress in Rats. Psychoneuroendocrinology 2014, 42, 207–217. [Google Scholar] [CrossRef]
- Ohira, H.; Matsunaga, M.; Osumi, T.; Fukuyama, S.; Shinoda, J.; Yamada, J.; Gidron, Y. Vagal Nerve Activity as a Moderator of Brain–Immune Relationships. J. Neuroimmunol. 2013, 260, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Tracey, K.J. The Vagus Nerve and the Inflammatory Reflex—Linking Immunity and Metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Butler, M.I.; Wagner-Skacel, J. Gut-Brain-Crosstalk- the Vagus Nerve and the Microbiota-Gut-Brain Axis in Depression. A Narrative Review. J. Affect. Disord. Rep. 2023, 13, 100607. [Google Scholar] [CrossRef]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence Diagram of Physiological and Environmental Factors Affecting Heart Rate Variability: An Extended Literature Overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.; Lee, S.-H.; Kim, D.-H.; Hwang, B. Comparative Study of Heart Rate Variability in Patients with Schizophrenia, Bipolar Disorder, Post-Traumatic Stress Disorder, or Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2013, 11, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jangpangi, D. Alteration of Heart Rate Variability in Patients of Depression. J. Clin. Diagn. Res. 2016, 10, CM04–CM06. [Google Scholar] [CrossRef]
- Scott, B.G.; Weems, C.F. Resting Vagal Tone and Vagal Response to Stress: Associations with Anxiety, Aggression, and Perceived Anxiety Control among Youths. Psychophysiology 2014, 51, 718–727. [Google Scholar] [CrossRef]
- Friedman, B.H. An Autonomic Flexibility–Neurovisceral Integration Model of Anxiety and Cardiac Vagal Tone. Biol. Psychol. 2007, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Smarr, K.L.; Keefer, A.L. Measures of Depression and Depressive Symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res. 2011, 63, S454–S466. [Google Scholar] [CrossRef]
- Julian, L.J. Measures of Anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63, S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, S.; Dantzer, C.; Mondillon, L.; Trocme, C.; Gauchez, A.-S.; Ducros, V.; Mathieu, N.; Toussaint, B.; Fournier, A.; Canini, F.; et al. Relationship between Vagal Tone, Cortisol, TNF-Alpha, Epinephrine and Negative Affects in Crohn’s Disease and Irritable Bowel Syndrome. PLoS ONE 2014, 9, e105328. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II 2011; APA: Washington, DC, USA, 1996. [Google Scholar] [CrossRef]
- Skapinakis, P. Spielberger State-Trait Anxiety Inventory. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 6261–6264. ISBN 978-94-007-0752-8. [Google Scholar]
- Kogan, A.; Gruber, J.; Shallcross, A.J.; Ford, B.Q.; Mauss, I.B. Too Much of a Good Thing? Cardiac Vagal Tone’s Nonlinear Relationship with Well-Being. Emotion 2013, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.L.A.; Da Rocha, A.F.; De Oliveira Nascimento, F.A.; Neto, J.S.; Junqueira, L.F. Development of a Matlab Software for Analysis of Heart Rate Variability. In Proceedings of the 6th International Conference on Signal Processing, Beijing, China, 26–30 August 2002; IEEE: Beijing, China, 2002; Volume 2, pp. 1488–1491. [Google Scholar]
- Perakakis, P.; Joffily, M.; Taylor, M.; Guerra, P.; Vila, J. KARDIA: A Matlab Software for the Analysis of Cardiac Interbeat Intervals. Comput. Methods Programs Biomed. 2010, 98, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.; Camm, A.; Kleiger, R. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Nunes, C.F.; Nogueira, J.S.; Vianna, P.H.O.; Ciambarella, B.T.; Rodrigues, P.M.; Miranda, K.R.; Lobo, L.A.; Domingues, R.M.C.P.; Busch, M.; Atella, G.C.; et al. Probiotic Treatment during Neonatal Age Provides Optimal Protection against Experimental Asthma through the Modulation of Microbiota and T Cells. Int. Immunol. 2018, 30, 155–169. [Google Scholar] [CrossRef]
- Lamichhane, S.; Yde, C.C.; Schmedes, M.S.; Jensen, H.M.; Meier, S.; Bertram, H.C. Strategy for Nuclear-Magnetic-Resonance-Based Metabolomics of Human Feces. Anal. Chem. 2015, 87, 5930–5937. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2012, 41, D801–D807. [Google Scholar] [CrossRef]
- Ruscio, A.M.; Ruscio, J. The Latent Structure of Analogue Depression: Should the Beck Depression Inventory Be Used to Classify Groups? Psychol. Assess. 2002, 14, 135–145. [Google Scholar] [CrossRef]
- Fioravanti, A.C.M.; Santos, L.F.; Maissonette, S.; Cruz, A.P.M.; Landeira-Fernandez, J. Avaliação da estrutura fatorial da Escala de Ansiedade-Traço do IDATE. Avaliação Psicol. 2006, 5, 217–224. [Google Scholar]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-Regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.R.P.; Martins, C.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; De Oliveira Torres, R.; Soares, A.L.; Almeida, F.C.L.; Valente, A.P.; De Souza, I.P.R. Salivary Metabolite Fingerprint of Type 1 Diabetes in Young Children. J. Proteome Res. 2016, 15, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in Gut Microbiota and Fecal Metabolic Phenotype Associated with Depression by 16S rRNA Gene Sequencing and LC/MS-Based Metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef]
- Paige, L.A.; Mitchell, M.W.; Krishnan, K.R.R.; Kaddurah-Daouk, R.; Steffens, D.C. A Preliminary Metabolomic Analysis of Older Adults with and without Depression. Int. J. Geriat. Psychiatry 2007, 22, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Van De Wiele, T.; De Henauw, S. Chronic Psychosocial Stress and Gut Health in Children: Associations with Calprotectin and Fecal Short-Chain Fatty Acids. Psychosom. Med. 2017, 79, 927–935. [Google Scholar] [CrossRef]
- Ortega, M.A.; Alvarez-Mon, M.A.; García-Montero, C.; Fraile-Martinez, O.; Guijarro, L.G.; Lahera, G.; Monserrat, J.; Valls, P.; Mora, F.; Rodríguez-Jiménez, R.; et al. Gut Microbiota Metabolites in Major Depressive Disorder—Deep Insights into Their Pathophysiological Role and Potential Translational Applications. Metabolites 2022, 12, 50. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Van De Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Furet, J.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut Microbiota Richness Promotes Its Stability upon Increased Dietary Fibre Intake in Healthy Adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean Diet Is Associated with the Gut Microbiota Pattern and Gastrointestinal Characteristics in an Adult Population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, O.; Hestad, K.A.; Hanssen, J.F.; Rudi, K. Isovaleric Acid in Stool Correlates with Human Depression. Nutr. Neurosci. 2016, 19, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The Neuropharmacology of Butyrate: The Bread and Butter of the Microbiota-Gut-Brain Axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Valvassori, S.; Varela, R.; Arent, C.; Dal-Pont, G.; Bobsin, T.; Budni, J.; Reus, G.; Quevedo, J. Sodium Butyrate Functions as an Antidepressant and Improves Cognition with Enhanced Neurotrophic Expression in Models of Maternal Deprivation and Chronic Mild Stress. Curr. Neurovascular Res. 2014, 11, 359–366. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; López-Maside, L.; Donapetry-García, C.; Fernández-Fernández, C.; Sixto-Leal, C. Enzymes Involved in Branched-Chain Amino Acid Metabolism in Humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, A.; Sada, N.; Juge, N.; Wakasa, A.; Moriyama, Y.; Inoue, T. Neuronal Inhibition and Seizure Suppression by Acetoacetate and Its Analog, 2-phenylbutyrate. Epilepsia 2017, 58, 845–857. [Google Scholar] [CrossRef]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals with Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet. Res. 2017, 19, e36. [Google Scholar] [CrossRef]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.R.; Koutnik, A.P.; Poff, A.M.; Kesl, S.L.; D’Agostino, D.P. Corrigendum: Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front. Mol. Neurosci. 2017, 10, 137. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-Endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [PubMed]
- Janik, R.; Thomason, L.A.M.; Stanisz, A.M.; Forsythe, P.; Bienenstock, J.; Stanisz, G.J. Magnetic Resonance Spectroscopy Reveals Oral Lactobacillus Promotion of Increases in Brain GABA, N-Acetyl Aspartate and Glutamate. NeuroImage 2016, 125, 988–995. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel Metabolic and Physiological Functions of Branched Chain Amino Acids: A Review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef]
- Baranyi, A.; Amouzadeh-Ghadikolai, O.; Von Lewinski, D.; Rothenhäusler, H.-B.; Theokas, S.; Robier, C.; Mangge, H.; Reicht, G.; Hlade, P.; Meinitzer, A. Branched-Chain Amino Acids as New Biomarkers of Major Depression—A Novel Neurobiology of Mood Disorder. PLoS ONE 2016, 11, e0160542. [Google Scholar] [CrossRef] [PubMed]
- Windmueller, H.G.; Spaeth, A.E. Metabolism of Absorbed Aspartate, Asparagine, and Arginine by Rat Small Intestine in Vivo. Arch. Biochem. Biophys. 1976, 175, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Linking the Biological Underpinnings of Depression: Role of Mitochondria Interactions with Melatonin, Inflammation, Sirtuins, Tryptophan Catabolites, DNA Repair and Oxidative and Nitrosative Stress, with Consequences for Classification and Cognition. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 255–266. [Google Scholar] [CrossRef]
- Mudd, S.H.; Ebert, M.H.; Scriver, C.R. Labile Methyl Group Balances in the Human: The Role of Sarcosine. Metabolism 1980, 29, 707–720. [Google Scholar] [CrossRef]
- Chen, K.-T.; Wu, C.-H.; Tsai, M.-H.; Wu, Y.-C.; Jou, M.-J.; Huang, C.-C.; Wei, I.-H. Antidepressant-like Effects of Long-Term Sarcosine Treatment in Rats with or without Chronic Unpredictable Stress. Behav. Brain Res. 2017, 316, 1–10. [Google Scholar] [CrossRef]
- Krystal, J.H.; Tolin, D.F.; Sanacora, G.; Castner, S.A.; Williams, G.V.; Aikins, D.E.; Hoffman, R.E.; D’Souza, D.C. Neuroplasticity as a Target for the Pharmacotherapy of Anxiety Disorders, Mood Disorders, and Schizophrenia. Drug Discov. Today 2009, 14, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Methionine Metabolism in Mammals. J. Nutr. Biochem. 1990, 1, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-Adenosyl Methionine (SAMe) for Depression in Adults. Cochrane Database Syst. Rev. 2016, 2016, CD011286. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Klarer, M.; Arnold, M.; Günther, L.; Winter, C.; Langhans, W.; Meyer, U. Gut Vagal Afferents Differentially Modulate Innate Anxiety and Learned Fear. J. Neurosci. 2014, 34, 7067–7076. [Google Scholar] [CrossRef] [PubMed]

| Asymptomatic (n = 8) | Symptomatic (n = 8) | High Vagal Tone (n = 11) | Low Vagal Tone (n = 7) | |
|---|---|---|---|---|
| Gender (M:F) | 5:3 | 2:6 | 3:4 | 5:6 |
| Age ± SD | 23 ± 1.8 | 22.3 ± 1.8 | 21.7 ± 1.8 | 23.1 ± 1.8 |
| BMI ± SD | 24.3 ± 5.0 | 23.0 ± 1.9 | 22.4 ± 2.2 | 24.5 ± 4.3 |
| Exercise (Y:N) | 7:1 ** | 1:7 ** | 5:2 | 4:7 |
| Eating habits (O:V) | 8:0 | 6:2 | 5:2 | 10:1 |
| HF ± SD | 1008.1 ± 875.8 | 1023.6 ± 915.5 | 2036.1 ± 910.9 *** | 490.7 ± 405.4 *** |
| RMSSD ± SD | 43.6 ± 13.3 | 38.2 ± 13.6 | 60.6 ± 13.8 *** | 31.9 ± 8.2 *** |
| BDI-II Scores ± SD | 5.8 ± 3.8 *** | 22.3 ± 7.0 *** | 10.8 ± 11.1 | 15.4 ± 8.6 |
| STAI-T Scores ± SD | 33.8 ± 7.1 *** | 53.2 ± 8.2 *** | 40.1 ± 14.8 | 46.4 ± 9.2 |
| RMSSD | BDI-II | STAI-T | HF | |
|---|---|---|---|---|
| RMSSD | −0.24 | −0.09 | 0.86 | |
| BDI-II | −0.24 | 0.82 | 0.004 | |
| STAI-T | −0.09 | 0.82 | 0.19 | |
| HF | 0.86 | 0.004 | 0.19 | |
| Butyrate (HMDB0000883) | −0.05 | 0.29 | 0.32 | −0.04 |
| Acetate (HMDB00042) | 0.14 | −0.05 | −0.27 | 0.04 |
| Propionate (HMDB00237) | 0.26 | 0.10 | −0.17 | 0.10 |
| Glutamate (HMDB00148) | −0.45 | 0.14 | 0.25 | −0.26 |
| Glutamine (HMDB0003423) | −0.09 | 0.01 | 0.22 | −0.02 |
| Acetoacetate (HMDB0304256) | −0.53 | 0.44 | 0.58 | −0.45 |
| Valerate (HMDB00892) | 0.30 | 0.25 | −0.03 | 0.20 |
| Aspartate (HMDB00191) | −0.37 | 0.43 | 0.39 | −0.33 |
| Valine (HMDB0000883) | −0.21 | 0.53 | 0.41 | −0.01 |
| Isovalerate (HMDB0000718) | −0.39 | 0.00 | 0.21 | −0.42 |
| Methionine (HMDB00696) | −0.58 | 0.24 | 0.19 | −0.46 |
| Malate (HMDB0031518) | −0.47 | 0.46 | 0.32 | −0.38 |
| Sarcosine (HMDB00271) | −0.12 | 0.32 | 0.41 | −0.13 |
| Acetone (HMDB01659) | −0.58 | 0.43 | 0.37 | −0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasqualette, L.; Fidalgo, T.K.d.S.; Freitas-Fernandes, L.B.; Souza, G.G.L.; Imbiriba, L.A.; Lobo, L.A.; Volchan, E.; Domingues, R.M.C.P.; Valente, A.P.; Miranda, K.R. Alterations in Vagal Tone Are Associated with Changes in the Gut Microbiota of Adults with Anxiety and Depression Symptoms: Analysis of Fecal Metabolite Profiles. Metabolites 2024, 14, 450. https://doi.org/10.3390/metabo14080450
Pasqualette L, Fidalgo TKdS, Freitas-Fernandes LB, Souza GGL, Imbiriba LA, Lobo LA, Volchan E, Domingues RMCP, Valente AP, Miranda KR. Alterations in Vagal Tone Are Associated with Changes in the Gut Microbiota of Adults with Anxiety and Depression Symptoms: Analysis of Fecal Metabolite Profiles. Metabolites. 2024; 14(8):450. https://doi.org/10.3390/metabo14080450
Chicago/Turabian StylePasqualette, Laura, Tatiana Kelly da Silva Fidalgo, Liana Bastos Freitas-Fernandes, Gabriela Guerra Leal Souza, Luís Aureliano Imbiriba, Leandro Araujo Lobo, Eliane Volchan, Regina Maria Cavalcanti Pilotto Domingues, Ana Paula Valente, and Karla Rodrigues Miranda. 2024. "Alterations in Vagal Tone Are Associated with Changes in the Gut Microbiota of Adults with Anxiety and Depression Symptoms: Analysis of Fecal Metabolite Profiles" Metabolites 14, no. 8: 450. https://doi.org/10.3390/metabo14080450
APA StylePasqualette, L., Fidalgo, T. K. d. S., Freitas-Fernandes, L. B., Souza, G. G. L., Imbiriba, L. A., Lobo, L. A., Volchan, E., Domingues, R. M. C. P., Valente, A. P., & Miranda, K. R. (2024). Alterations in Vagal Tone Are Associated with Changes in the Gut Microbiota of Adults with Anxiety and Depression Symptoms: Analysis of Fecal Metabolite Profiles. Metabolites, 14(8), 450. https://doi.org/10.3390/metabo14080450








