Metabolomic and Physiological Analyses Reveal the Effects of Different Storage Conditions on Sinojackia xylocarpa Hu Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Seed Viability Assay
2.4. Physiological and Biochemical Assays
2.5. Transmission Electron Microscopy (TEM)
2.6. Metabolite Profiling
2.7. Data Processing and Statistical Analysis
3. Results
3.1. Impacts of Different Storage Conditions on the Seed Quality of S. xylocarpa Seed
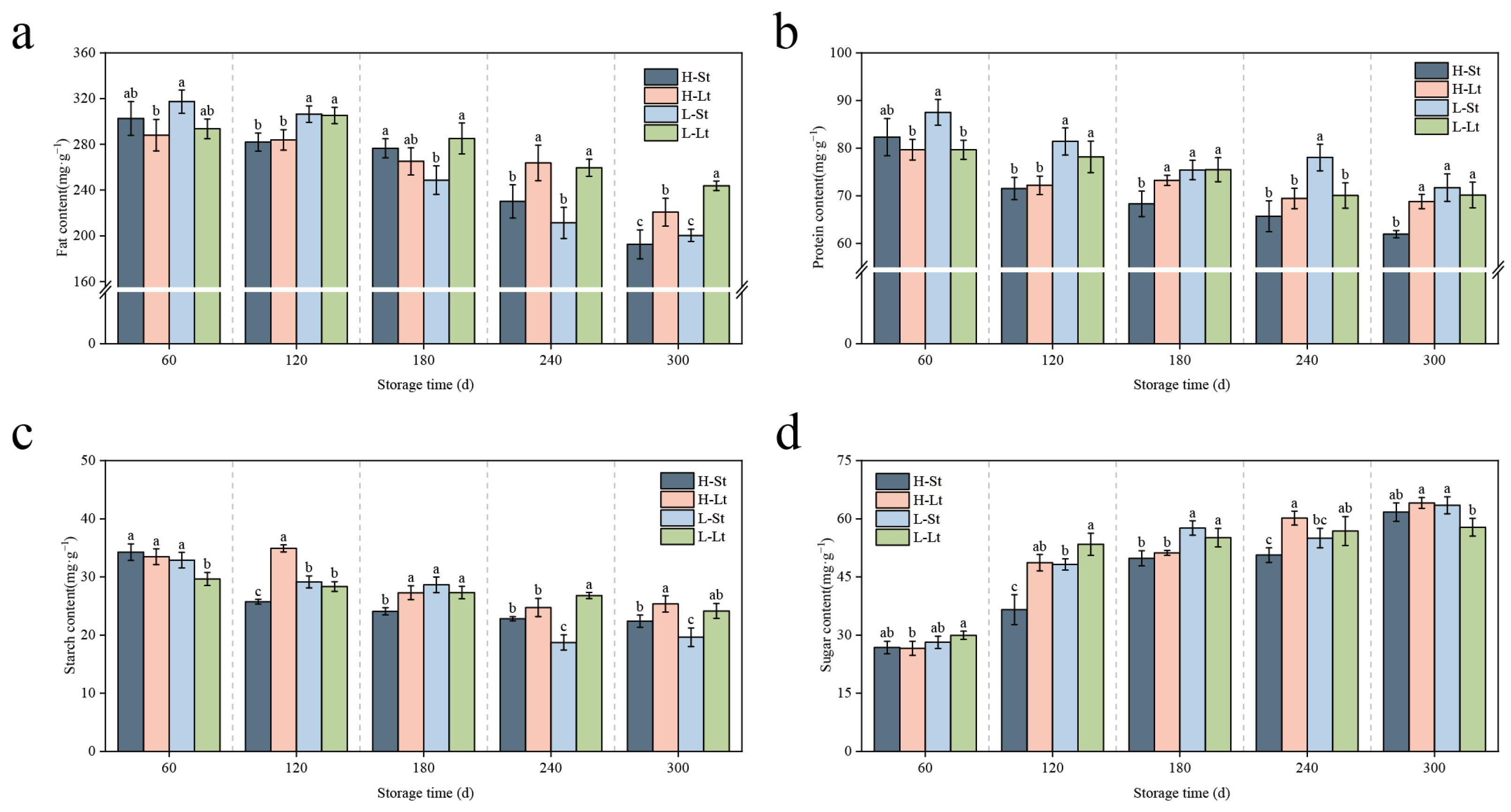
3.2. Impacts of Different Storage Conditions on Nutritional Components of S. xylocarpa Seeds
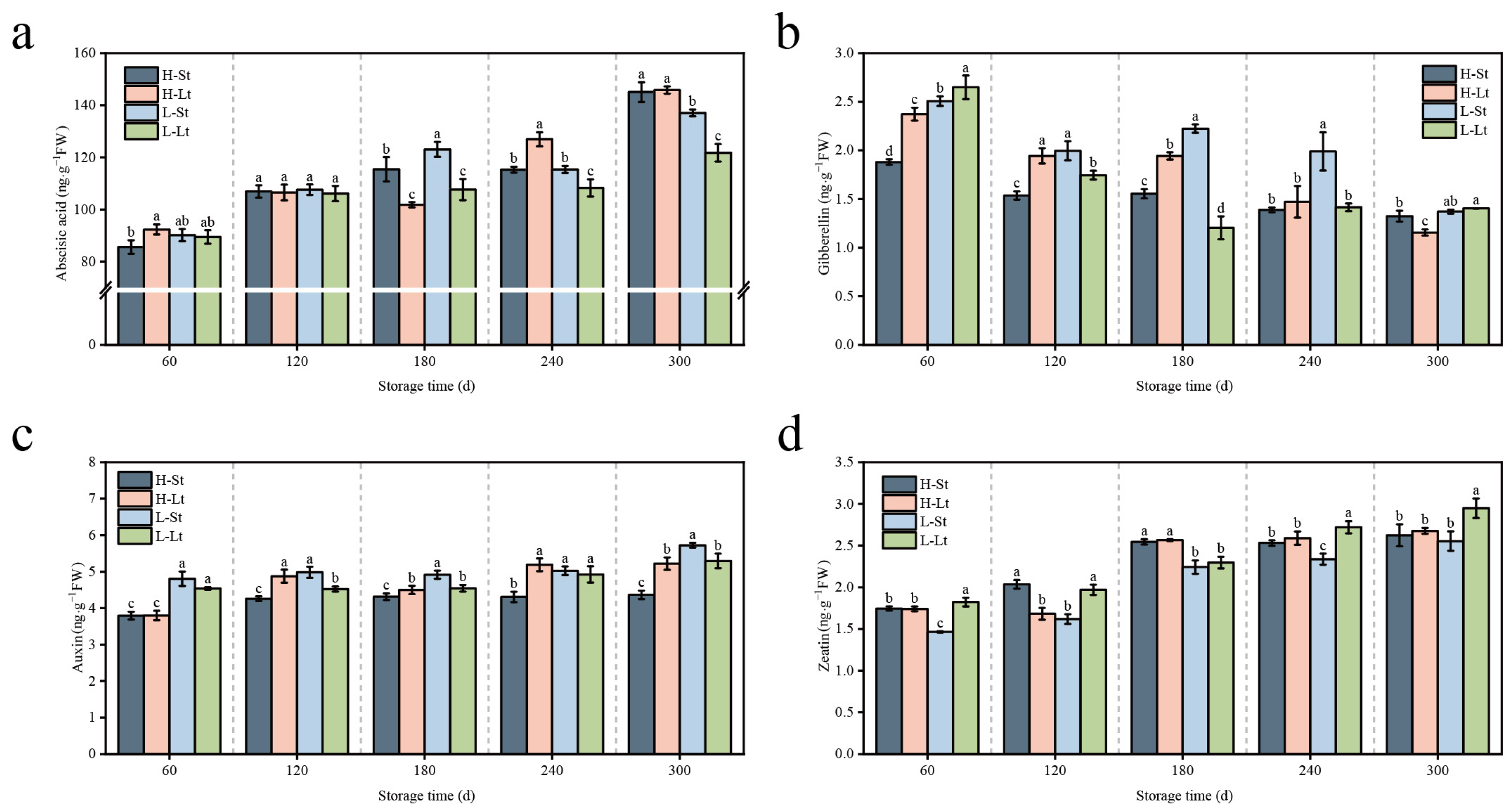
3.3. Effects of Different Storage Conditions on Endogenous Hormones in S. xylocarpa Seeds
3.4. Differences in Seed Metabolites and Metabolic Pathways under Different Storage Conditions
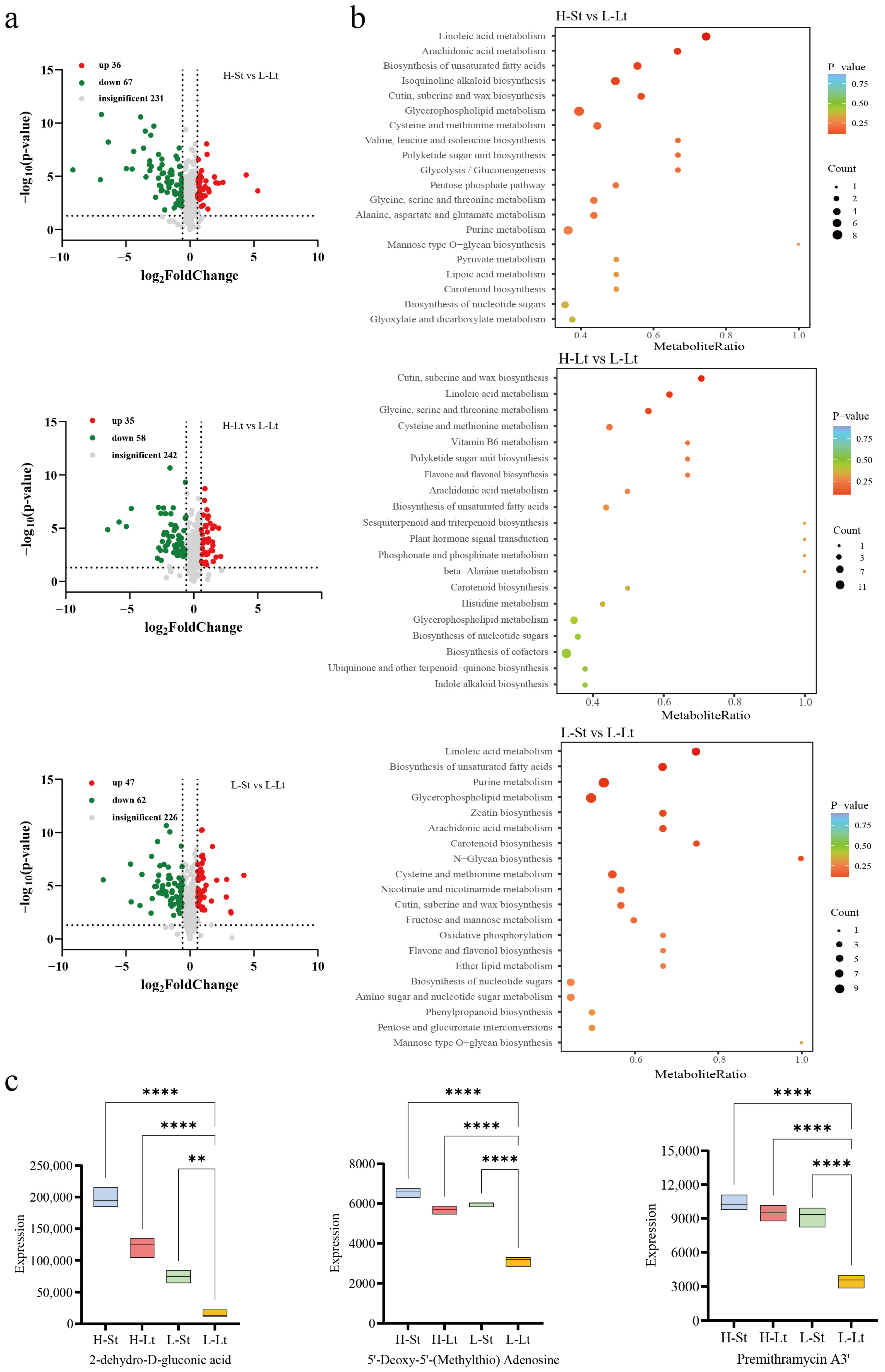
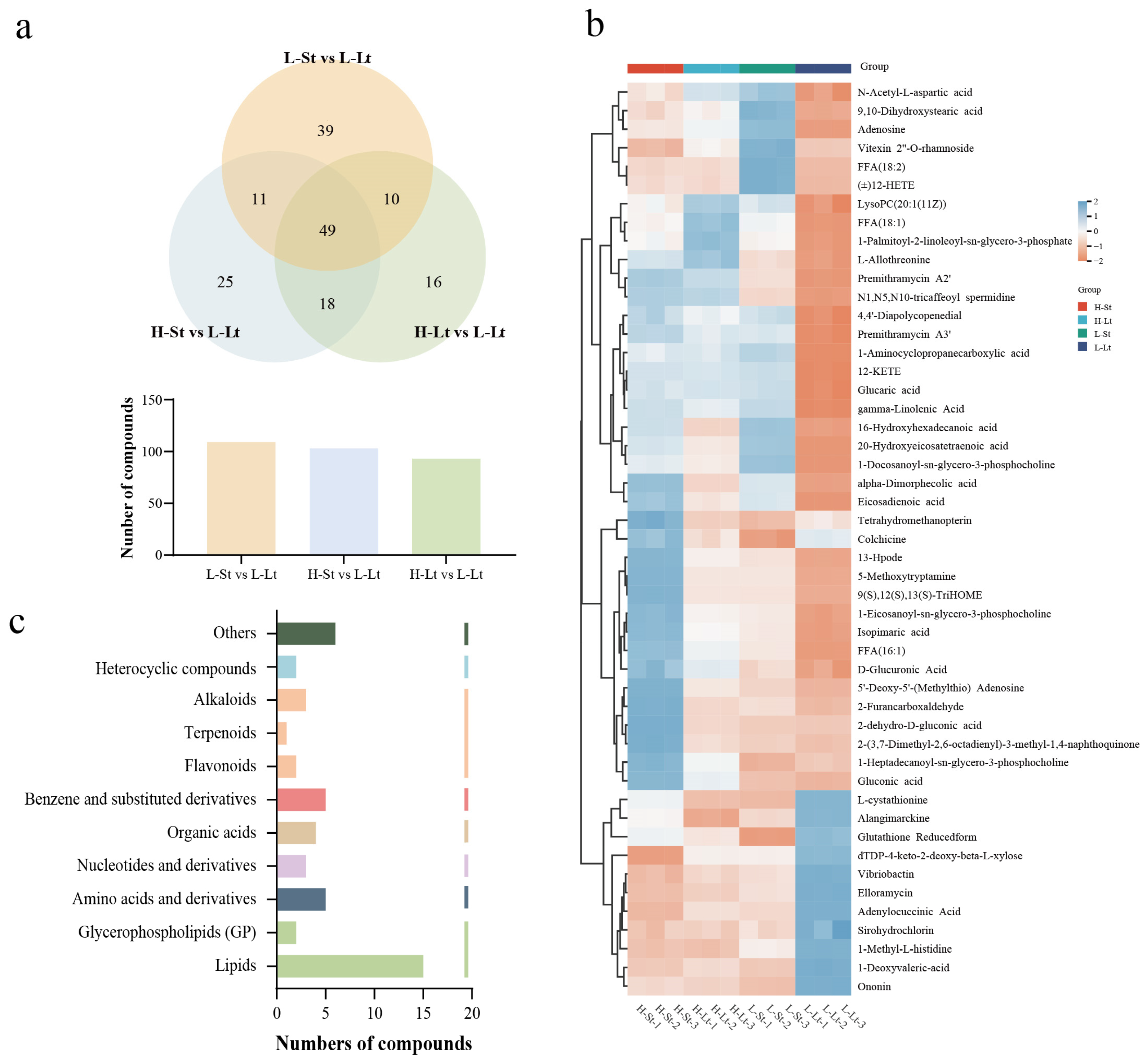
3.4.1. Differential Accumulation Analysis of Metabolites
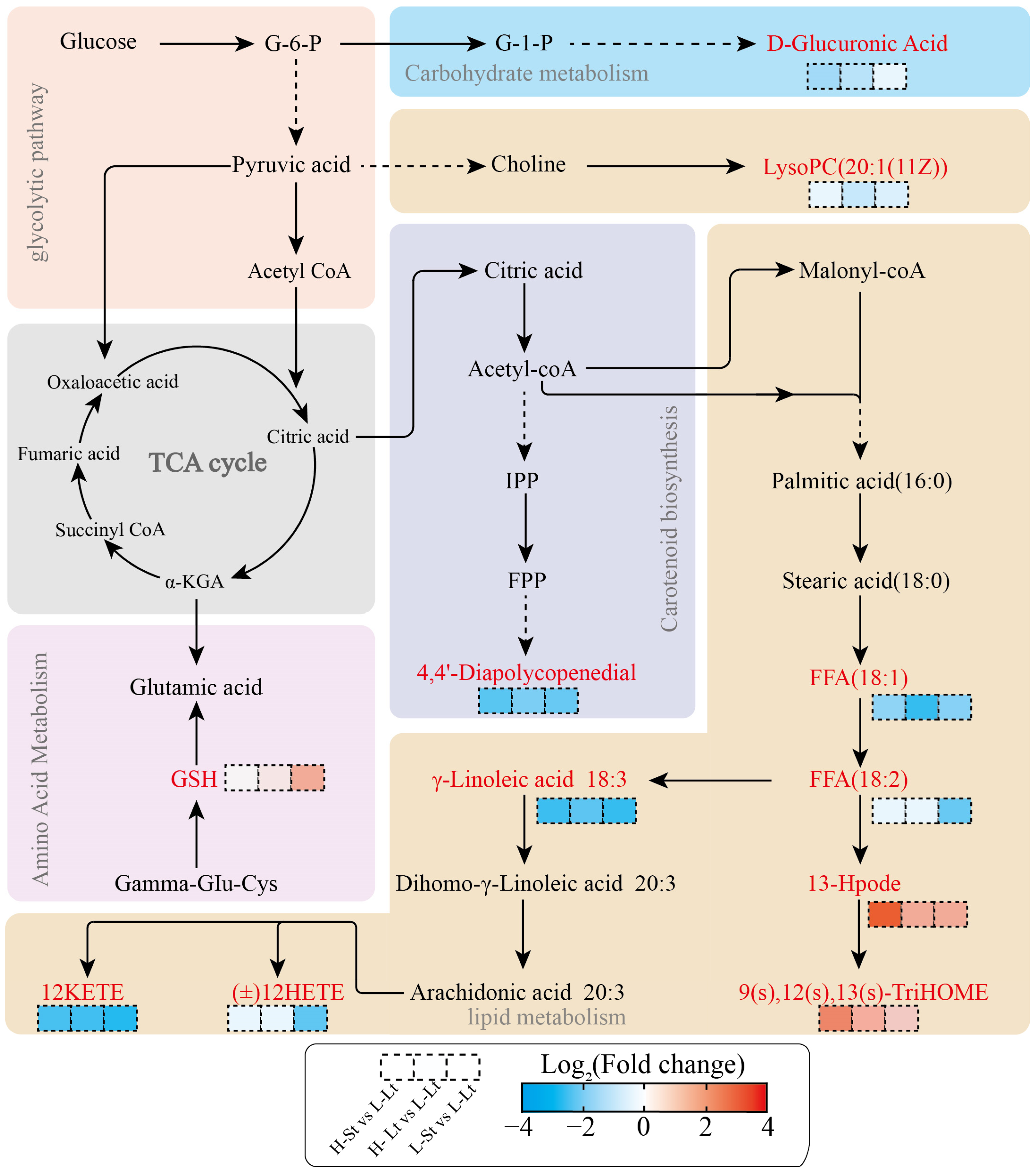
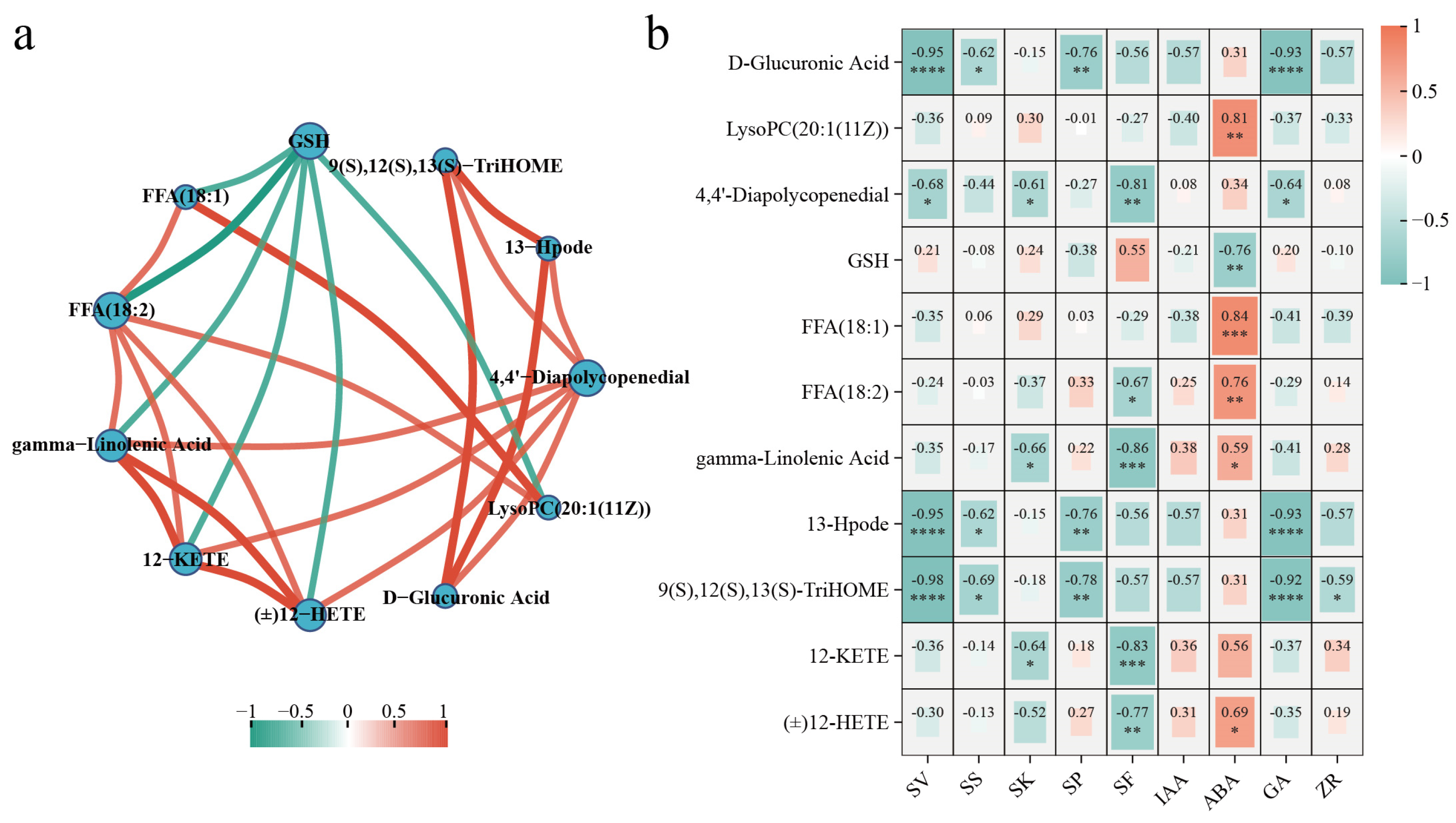
3.4.2. Analysis of the Important Metabolites and Metabolic Pathways
4. Discussion
4.1. Changes in Seed Quality During Storage
4.2. Changes in Nutritional Components during Seed Storage
4.3. Changes in Endogenous Hormones during Seed Storage
4.4. Prominent Metabolites and Metabolic Pathway Responses of S. xylocarpa Seeds under Different Storage Conditions
4.4.1. Lipid Metabolism
4.4.2. Amino Acid Metabolism
4.4.3. Carbohydrate Metabolism
4.4.4. Carotenoid Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, H.H. Sinojackia, a new genus of Styracaceae from southeastern China. J. Arnold Arbor. 1928, 9, 130–131. [Google Scholar] [CrossRef]
- Jian, X.; Wang, Y.; Li, Q.; Miao, Y. Plastid Phylogenetics, Biogeography, and Character Evolution of the Chinese Endemic Genus Sinojackia Hu. Diversity 2024, 16, 305. [Google Scholar] [CrossRef]
- Wu, Y.; Bao, W.Q.; Hu, H.; Shen, Y.B. Mechanical constraints in the endosperm and endocarp are major causes of dormancy in Sinojackia xylocarpa Hu (Styracaceae) seeds. J. Plant Growth Regul. 2023, 42, 644–657. [Google Scholar] [CrossRef]
- Zhu, S.; Wei, X.F.; Lu, Y.X.; Zhang, D.W.; Wang, Z.F.; Ge, J.; Li, S.L.; Song, Y.F.; Yang, Y.; Yi, X.G.; et al. The jacktree genome and population genomics provides insights for the mechanisms of the germination obstacle and the conservation of endangered ornamental plants. Hortic. Res. 2024, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Liao, S.; Cui, K. Appropriate ultra-low seed moisture content stabilizes the seed longevity of Calocedrus macrolepis, associated with changes in endogenous hormones, antioxidant enzymes, soluble sugars and unsaturated fatty acids. New For. 2019, 50, 455–468. [Google Scholar] [CrossRef]
- Zhang, H.S.; Hu, J. Seed Science, 2nd ed.; China Agriculture Press: Beijing, China, 2014; pp. 191–204. [Google Scholar]
- Hussain, S.; Zheng, M.; Khan, F.; Khaliq, A.; Fahad, S.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci. Rep. 2015, 5, 8101. [Google Scholar] [CrossRef]
- Thirusendura Selvi, D.; Saraswathy, S. Seed viability, seed deterioration and seed quality improvements in stored onion seeds: A review. J. Hortic. Sci. Biotechnol. 2018, 93, 1–7. [Google Scholar] [CrossRef]
- Afzal, I.; Jaffar, I.; Zahid, S.; Rehman, H.U.; Basra, S.M.A. Physiological and biochemical changes during hermetic storage of Moringa oleifera seeds. S. Afr. J. Bot. 2020, 129, 435–441. [Google Scholar] [CrossRef]
- Feng, J.; Shen, Y.B.; Shi, F.H.; Li, C. Changes in seed germination ability, lipid peroxidation and antioxidant enzyme activities of ginkgo biloba seed during desiccation. Forests 2017, 8, 286. [Google Scholar] [CrossRef]
- Chen, H.Y.; Shen, Y.B. Investigation of water distribution and mobility dynamics in recalcitrant Quercus acutissima Seeds during desiccation wsing magnetic resonance methods. Forests 2023, 14, 738. [Google Scholar] [CrossRef]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, V. Impact of seed color and storage time on the radish seed germination and sprout growth in plasma agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jia, B.; Zhang, W.; Li, A.; Li, T.; Jin, D.; Liu, Y.; Liu, H.; Wu, Y. Physiological characteristics and metabonomics analysis of blueberry leaves under salt stress. Plant Physiol. J. 2022, 58, 155–164. [Google Scholar] [CrossRef]
- Lv, H.X.; Xu, H.; Yang, K.; Yan, M. Comparative metabolomic analyses reveal metabolites associated with seed deterioration in Chinese cabbage. Sci. Hortic. 2024, 331, 113170. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). International Rules for Seed Testing. Chapter 6: Seed Health Testing; International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2017. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure. Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Du, M.; Eneji, A.E.; Wang, B.; Duan, L.; Li, Z.; Tian, X. Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. J. Exp. Bot. 2012, 63, 5887–5901. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Qu, M.; Cheng, F. Research on physicochemical properties, microscopic characterization and detection of different freezing-damaged corn seeds. Food Chem. X. 2022, 14, 100338. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, H.; Wang, Y.; Zhang, L.; Jia, J.; Ma, H. Storage time affects the viability, longevity, and germination of Eriochloa villosa (Thunb.) Kunth Seeds. Sustainability 2023, 15, 8576. [Google Scholar] [CrossRef]
- Mbofung, G.C.Y.; Goggi, A.S.; Leandro, L.F.S.; Mullen, R.E. Effects of storage temperature and relative humidity on viability and vigor of treated soybean seeds. Crop Sci. 2013, 53, 1086–1095. [Google Scholar] [CrossRef]
- Coradi, P.C.; Lima, R.E.; Padia, C.L.; Alves, C.Z.; Teodoro, P.E.; Carina da Silva Cândido, A. Soybean seed storage: Packaging technologies and conditions of storage environments. J. Stored Prod. Res. 2020, 89, 101709. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of orthodox seeds during ageing: Influencing factors, physiological alterations and the role of reactive oxygen species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Solberg, S.Ø.; Yndgaard, F.; Andreasen, C.; von Bothmer, R.; Loskutov, I.G.; Asdal, Å. Long-term storage and longevity of orthodox seeds: A systematic review. Front. Plant Sci. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Dobiesz, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J. Physiological and biochemical parameters of lupin seed subjected to 29 Years of storage. Crop Sci. 2017, 57, 2149–2159. [Google Scholar] [CrossRef]
- Narayana Murthy, U.M.; Sun, W.Q. Protein modification by amadori and maillard reactions during seed storage: Roles of sugar hydrolysis and lipid peroxidation. J. Exp. Bot. 2000, 51, 1221–1228. [Google Scholar] [CrossRef]
- Wang, W.; He, A.; Peng, S.; Huang, J.; Cui, K.; Nie, L. The effect of storage condition and duration on the deterioration of primed rice seeds. Front. Plant Sci. 2018, 9, 172. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Wahab, A.; Abdi, G.; Saleem, M.H.; Ali, B.; Ullah, S.; Shah, W.; Mumtaz, S.; Yasin, G.; Muresan, C.C.; Marc, R.A. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the Adverse effects of drought stress: A comprehensive review. Plants 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, F.; Luo, X.; Dai, Y.; Yang, Y.; Zheng, C.; Yang, W.; Shu, K. A matter of life and death: Molecular, physiological, and environmental regulation of seed longevity. Plant Cell Environ. 2020, 43, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [PubMed]
- Bueso, E.; Muñoz-Bertomeu, J.; Campos, F.; Brunaud, V.; Martínez, L.; Sayas, E.; Ballester, P.; Yenush, L.; Serrano, R. ARABIDOPSIS THALIANA HOMEOBOX25 uncovers a role for gibberellins in seed longevity. Plant Physiol. 2013, 164, 999–1010. [Google Scholar] [CrossRef]
- Lin, C.; Shen, H.; Guan, Y.; An, J.; Hu, W.; Hu, J. Changes of physiological, biochemistry and gene expression related to ABA and GA3 in hybrid rice seeds stored at different moisture contents and packing methods. Plant Physiol. J. 2017, 53, 1077–1086. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Neveu, M.; Lalanne, D.; Ly Vu, B.; Kanno, Y.; Seo, M.; Leprince, O.; Buitink, J. A role for auxin signaling in the acquisition of longevity during seed maturation. New Phytol. 2020, 225, 284–296. [Google Scholar] [CrossRef]
- Yan, S.; Huang, W.; Gao, J.; Fu, H.; Liu, J. Comparative metabolomic analysis of seed metabolites associated with seed storability in rice (Oryza sativa L.) during natural aging. Plant Physiol. Biochem. 2018, 127, 590–598. [Google Scholar] [CrossRef]
- Marangoni, A.G.; Palma, T.; Stanley, D.W. Membrane effects in postharvest physiology. Postharvest Biol. Technol. 1996, 7, 193–217. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiang, H.Z.; Miao, Y.; Zhang, L.; Guo, Z.F.; Zhao, X.H.; Lin, J.W.; Li, T.L. An overview of cold resistance in plants. J. Agron. Crop Sci. 2014, 200, 237–245. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Radio-frequency treatment for stabilization of wheat germ: Storage stability and physicochemical properties. Innov. Food Sci. Emerg. 2019, 52, 158–165. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant unsaturated fatty acids: Multiple roles in stress response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, G.; Li, Y.; Lv, H. Changes in quality characteristics and metabolites composition of wheat under different storage temperatures. J. Stored Prod. Res. 2024, 105, 102229. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Zhang, Y.H.; Xiao, A.F.; Zhang, A.H.; Fang, B.S. Key enzymes in fatty acid synthesis pathway for bioactive lipids biosynthesis. Front. Nutr. 2022, 9, 851402. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.; Ahmad, N.; Sun, Y.; Wang, Y.; Liu, X.; Yao, N.; Jing, Y.; Du, L.; Li, X.; et al. The Carthamus tinctorius L. genome sequence provides insights into synthesis of unsaturated fatty acids. BMC Genom. 2024, 25, 510. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [PubMed]
- Ricker, K.E.; Bostock, R.M. Eicosanoids in the Phytophthora infestans-potato interaction: Lipoxygenase metabolism of arachidonic acid and biological activities of selected lipoxygenase products. Physiol. Mol. Plant Pathol. 1994, 44, 65–80. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-sugar metabolism in plants: The legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, S. Nucleotide Sugars in Chemistry and Biology. Molecules 2020, 25, 5755. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Bhattacharjee, N.; Chakraborty, P.; Bhattacharjee, S. Carotenoids as Antioxidants. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 447–473. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Shen, Y. Metabolomic and Physiological Analyses Reveal the Effects of Different Storage Conditions on Sinojackia xylocarpa Hu Seeds. Metabolites 2024, 14, 503. https://doi.org/10.3390/metabo14090503
Cai H, Shen Y. Metabolomic and Physiological Analyses Reveal the Effects of Different Storage Conditions on Sinojackia xylocarpa Hu Seeds. Metabolites. 2024; 14(9):503. https://doi.org/10.3390/metabo14090503
Chicago/Turabian StyleCai, Hao, and Yongbao Shen. 2024. "Metabolomic and Physiological Analyses Reveal the Effects of Different Storage Conditions on Sinojackia xylocarpa Hu Seeds" Metabolites 14, no. 9: 503. https://doi.org/10.3390/metabo14090503
APA StyleCai, H., & Shen, Y. (2024). Metabolomic and Physiological Analyses Reveal the Effects of Different Storage Conditions on Sinojackia xylocarpa Hu Seeds. Metabolites, 14(9), 503. https://doi.org/10.3390/metabo14090503





