Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
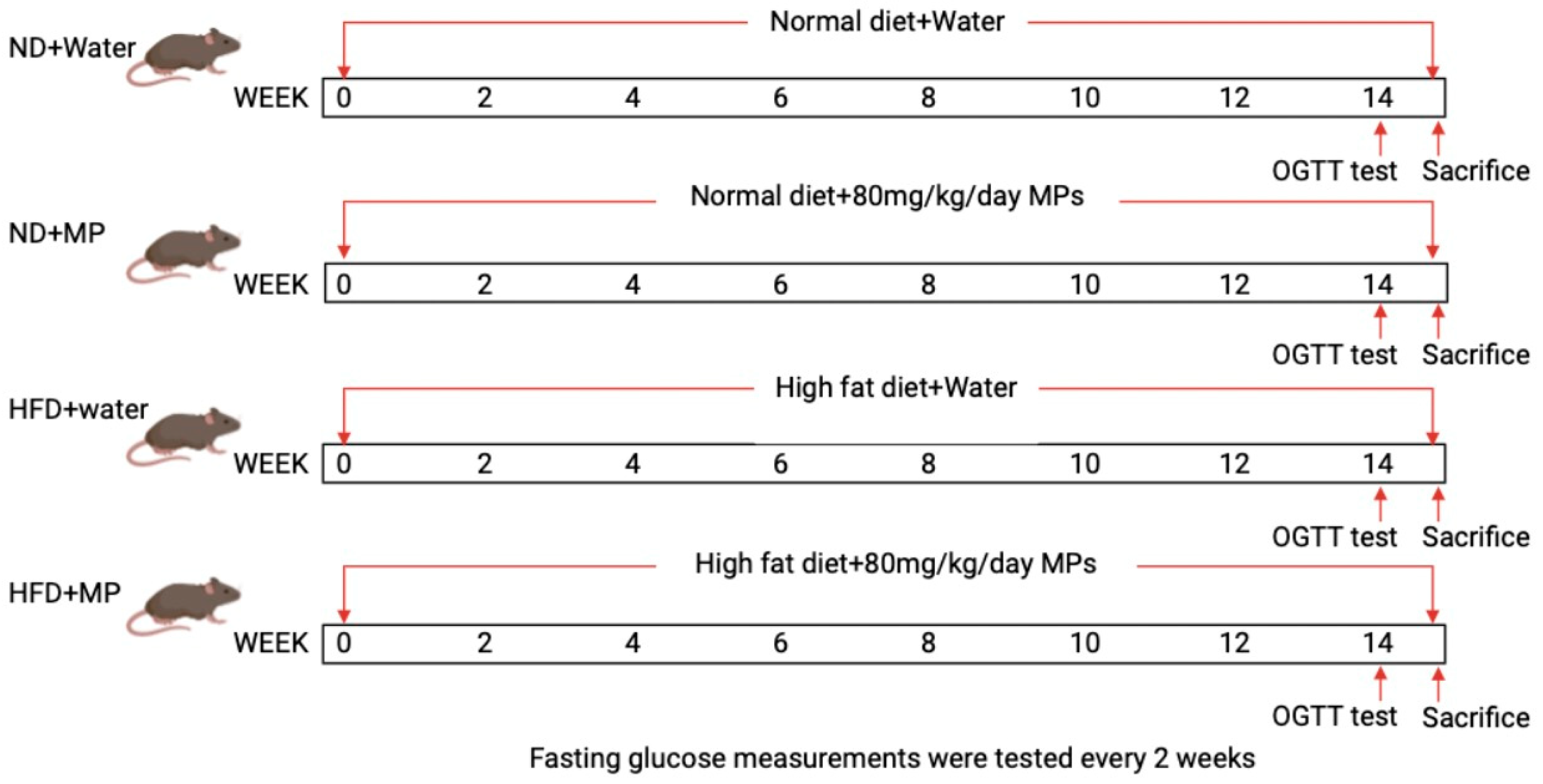
2.2. Experimental Animals and Exposure Protocol
2.3. Oral Glucose Tolerance Test
2.4. 16S rRNA High-Throughput Sequencing
2.5. Gut Microbiota Analysis
2.6. Statistical Analysis
3. Results
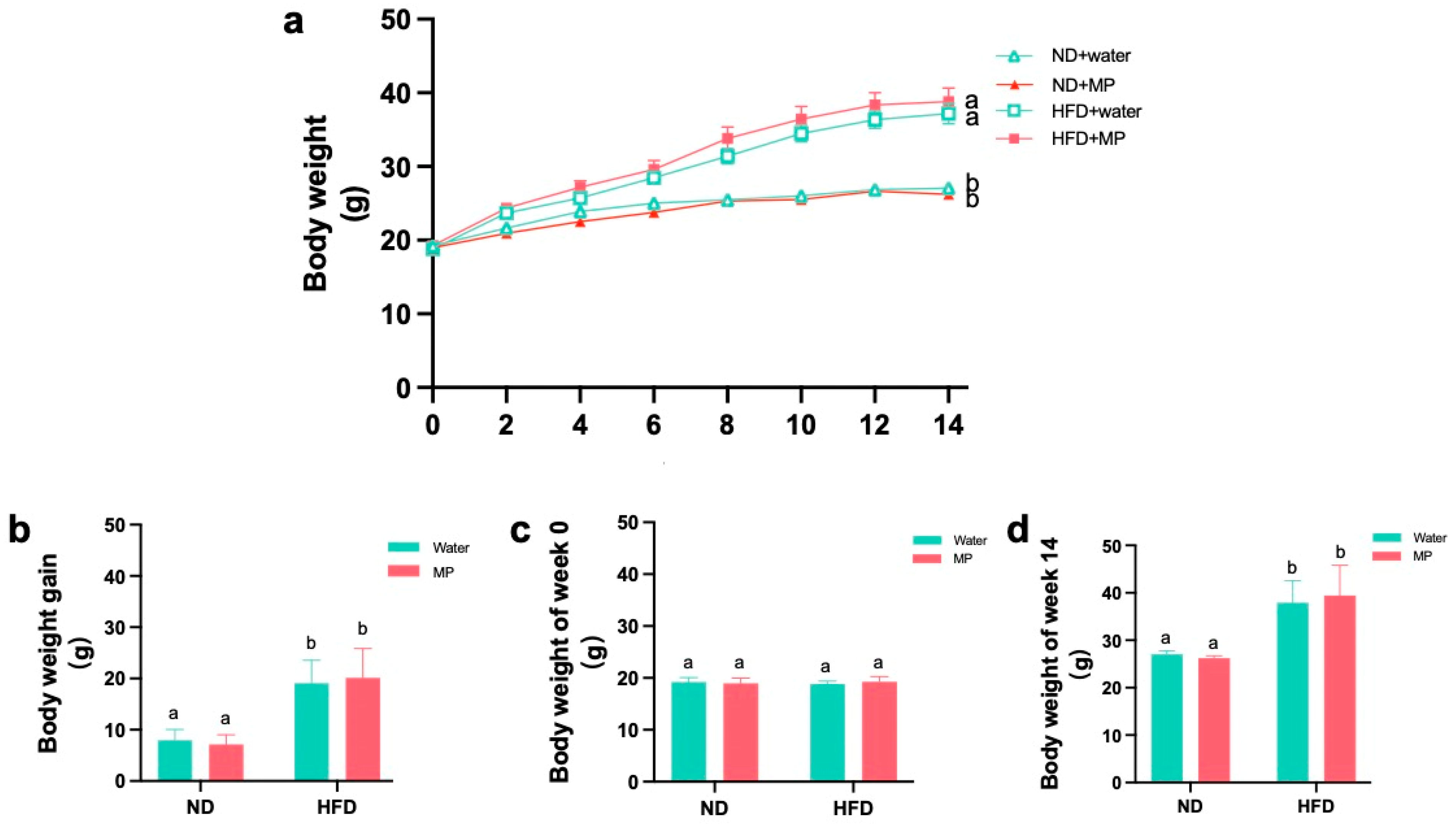
3.1. Effects of Polystyrene Microplastic Exposure on Mouse Body Weight Gain, Energy Intake, and Organ Weights
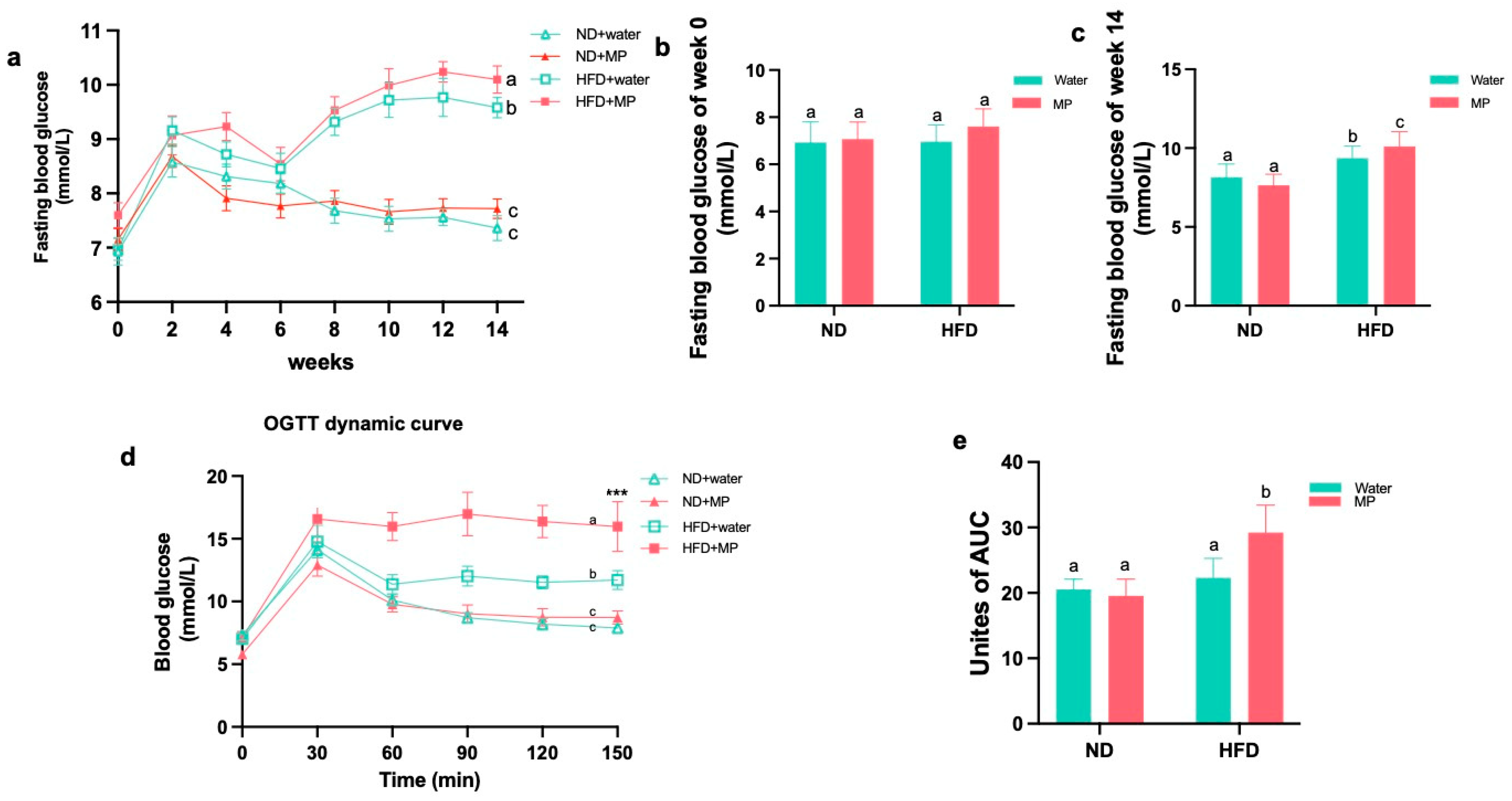
3.2. Polystyrene Microplastic Induces Abnormal Fasting Blood Glucose in Mice
3.3. Polystyrene Microplastic Induces Abnormal Glucose Tolerance in Mice
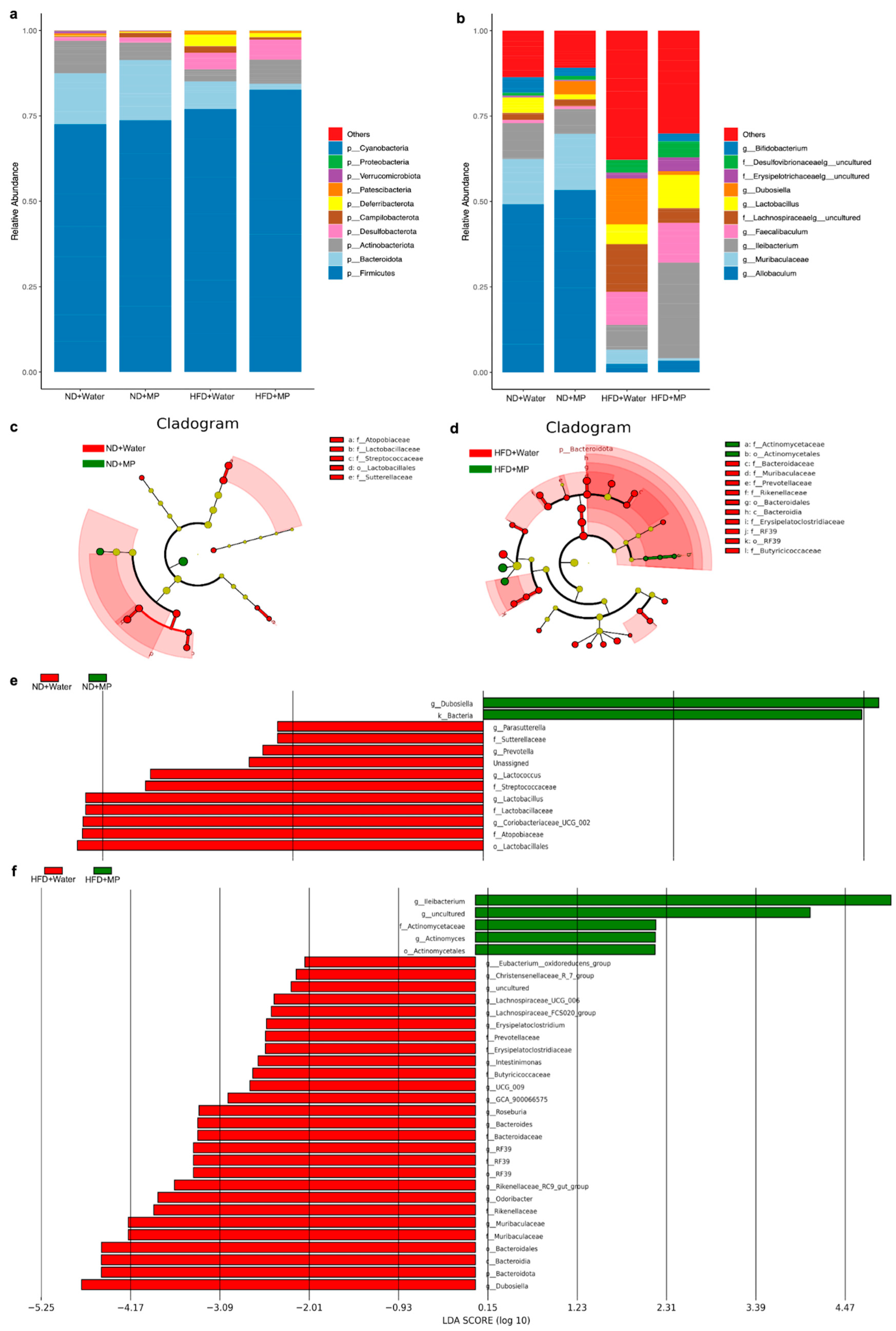
3.4. Differences in Gut Microbiota Diversity
3.5. Identification of Characteristic Gut Microbiota Differences
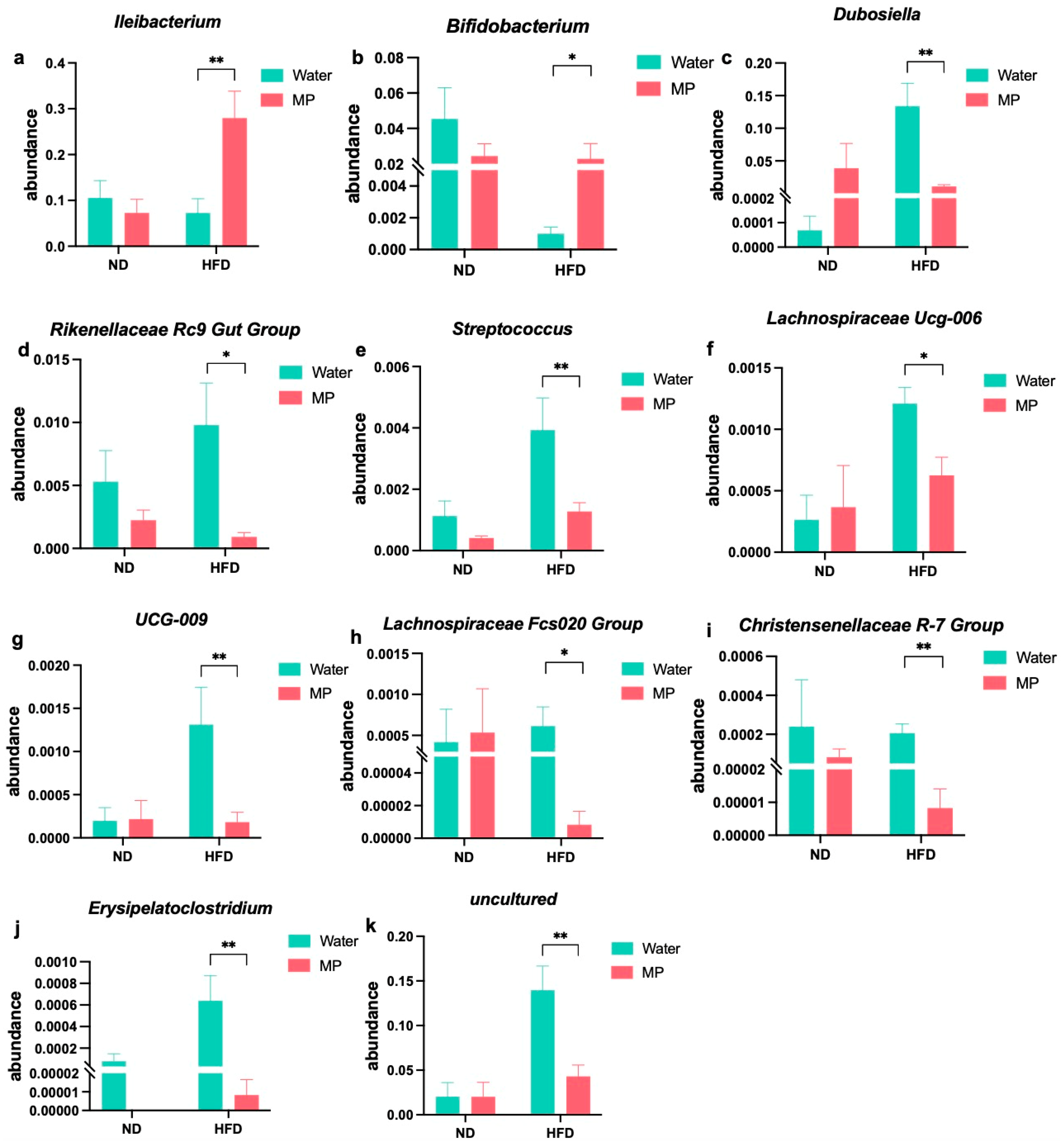
3.6. Changes in Gut Microbiota Genera upon Microplastic Exposure under Different Dietary Models
3.7. Gut Microbiota-Mediated Indirect Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.; Olsen, Y.; Mitchell, R.; Davis, A.; Rowland, S.; John, A.; McGonigle, D.; Russell, A. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Kelly, F. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Won, E.; Kang, H.; Lee, M.; Hwang, D.; Hwang, U.; Zhou, B.; Souissi, S.; Lee, S.; Lee, J. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus Koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Rong, J.; Guan, X.; Zha, S.; Shi, W.; Han, Y.; Du, X.; Wu, F.; Huang, W.; Liu, G. Immunotoxicity of Microplastics and Two Persistent Organic Pollutants Alone or in Combination to a Bivalve Species. Environ. Pollut. 2020, 258, 113845. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene Microplastics Affect the Distribution of Gut Microbiota and Inflammation Development in Mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene Microplastics Induce Mitochondrial Damage in Mouse GC-2 Cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut−Liver Axis Indicating Oral Exposure to Polystyrene Microplastic Potentially Increases the Risk of Insulin Resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Li, M.; Cai, Z.; Gong, H.; Yan, M. Microplastics as an Aquatic Pollutant Affect Gut Microbiota within Aquatic Animals. J. Hazard. Mater. 2022, 423, 127094. [Google Scholar] [CrossRef]
- Liebmann, B.; Köppel, S.; Königshofer, P.; Bucsics, T.; Reiberger, T.; Schwabl, P. Assessment of Microplastic Concentrations in Human Stool Final Results of a Prospective Study. United Eur. Gastroenterol. 2018, 2018 (Suppl. S1), 2884. [Google Scholar]
- Wang, Y.; Wei, Z.; Xu, K.; Wang, X.; Gao, X.; Han, Q.; Wang, S.; Chen, M. The Effect and a Mechanistic Evaluation of Polystyrene Nanoplastics on a Mouse Model of Type 2 Diabetes. Food Chem. Toxicol. 2023, 173, 113642. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.; Samuel, V.; Petersen, K.; Shulman, G. The Role of Hepatic Lipids in Hepatic Insulin Resistance and Type 2 Diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, Y.; Zhang, W.; Shen, T.; Li, H.; Wu, J.; Zhang, L.; Qin, L.; Chen, R.; Gu, W.; et al. Lipidomics and Transcriptomics Insight into Impacts of Microplastics Exposure on Hepatic Lipid Metabolism in Mice. Chemosphere 2022, 308, 136591. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, Y.; Long, J.; Yang, X.; Bao, L.; Yang, Z.; Wu, B.; Si, R.; Zhao, W.; Peng, C.; et al. Polystyrene Microplastic Exposure Induces Insulin Resistance in Mice via Dysbacteriosis and Pro-Inflammation. Sci. Total Environ. 2022, 838, 155937. [Google Scholar] [CrossRef] [PubMed]
- Assmann, G.; Sacks, F.; Awad, A.; Ascherio, A.; Bonanome, A.; Berra, B.; Booyse, F.; Carmena, R.; Dahlan, W.; DeLorgeril, M.; et al. Dietary Fat Consensus Statements. Am. J. Med. 2002, 113, 5–8. [Google Scholar]
- Lee, A.G.; Kang, S.; Yoon, H.J.; Im, S.; Oh, S.J.; Pak, Y.K. Polystyrene Microplastics Exacerbate Systemic Inflammation in High-Fat Diet−Induced Obesity. Int. J. Mol. Sci. 2023, 24, 12421. [Google Scholar] [CrossRef]
- Okamura, T.; Hamaguchi, M.; Hasegawa, Y.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Asano, M.; Yamazaki, M.; et al. Oral Exposure to Polystyrene Microplastics of Mice on a Normal or High-Fat Diet and Intestinal and Metabolic Outcomes. Environ. Health Perspect. 2023, 131, 27006. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- de Vos, W.; Tilg, H.; Van Hul, M.; Cani, P. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Ley, R.; Mahowald, M.; Magrini, V.; Mardis, E.; Gordon, J. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Xiang, S.; Chen, Z.; Song, L.; Li, Y.; Liao, Z.; Ge, B.; Zhou, B. The Probiotic Effects of AB23A on High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice May Be Associated with Suppressing the Serum Levels of Lipopolysaccharides and Branched-Chain Amino Acids. Arch. Biochem. Biophys. 2021, 714, 109080. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fei, J.; Xu, Q.; Tao, Y.; Zhou, Z.; Wang, Y.; Wu, J.; Gu, H.F. Interaction between Plasma Metabolomics and Intestinal Microbiome in Db/Db Mouse, an Animal Model for Study of Type 2 Diabetes and Diabetic Kidney Disease. Metabolites 2022, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Ruan, S.; Mo, Q.; Zhao, M.; Feng, F. The Effect of Sodium Benzoate on Host Health: Insight into Physiological Indexes and Gut Microbiota. Foods 2023, 12, 4081. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic Function of Lactobacillus Fermentum MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front. Microbiol. 2021, 12, 682290. [Google Scholar] [CrossRef]
- Liu, T.; Chen, M.; Tu, W.; Liang, Q.; Tao, W.; Jin, Z.; Xiao, Y.; Chen, L. Network and 16S rRNA Sequencing-Combined Approach Provides Insightal Evidence of Vitamin K2 for Salt-Sensitive Hypertension. Front. Nutr. 2021, 8, 639467. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented Carrot Juice Attenuates Type 2 Diabetes by Mediating Gut Microbiota in Rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Du, Y.; Guo, L.; Chen, M.; Huang, X.; Yang, F.; Hong, J.; Kong, X. Konjaku Flour Reduces Obesity in Mice by Modulating the Composition of the Gut Microbiota. Int. J. Obes. 2019, 43, 1631–1643. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoii, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Ma, Q.; Zhai, R.; Xie, X.; Chen, T.; Zhang, Z.; Liu, H.; Nie, C.; Yuan, X.; Tu, A.; Tian, B.; et al. Hypoglycemic Effects of Lycium Barbarum Polysaccharide in Type 2 Diabetes Mellitus Mice via Modulating Gut Microbiota. Front. Nutr. 2022, 9, 916271. [Google Scholar] [CrossRef]

| Effect Size | Bootstrap 95% CI | p | Proportion Mediated | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Direct effect | 0.5361 | 0.0353 | 1.2 | 0.026 | 0.3692 |
| Indirect effect | 0.3139 | −0.465 | 1.08 | 0.444 | 0.6308 |
| Total effect | 0.85 | 0.0632 | 1.68 | 0.024 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Niu, H.; Wu, L.; Xing, M.; Mo, Z.; Chen, Z.; Li, X.; Lou, X. Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions. Metabolites 2024, 14, 504. https://doi.org/10.3390/metabo14090504
Xu M, Niu H, Wu L, Xing M, Mo Z, Chen Z, Li X, Lou X. Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions. Metabolites. 2024; 14(9):504. https://doi.org/10.3390/metabo14090504
Chicago/Turabian StyleXu, Manjin, Huixia Niu, Lizhi Wu, Mingluan Xing, Zhe Mo, Zhijian Chen, Xueqing Li, and Xiaoming Lou. 2024. "Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions" Metabolites 14, no. 9: 504. https://doi.org/10.3390/metabo14090504
APA StyleXu, M., Niu, H., Wu, L., Xing, M., Mo, Z., Chen, Z., Li, X., & Lou, X. (2024). Impact of Microplastic Exposure on Blood Glucose Levels and Gut Microbiota: Differential Effects under Normal or High-Fat Diet Conditions. Metabolites, 14(9), 504. https://doi.org/10.3390/metabo14090504







