Investigating the Mechanisms of 15-PGDH Inhibitor SW033291 in Improving Type 2 Diabetes Mellitus: Insights from Metabolomics and Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Experimental Protocol for Animals
2.3. Sample Collection
2.4. Metabolomics Investigation Utilizing Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.4.1. Sample Preparation and Extraction
2.4.2. Instrumentation and Data Analysis
2.5. Transcriptomic Analysis
2.5.1. Total RNA Extraction
2.5.2. mRNA Library Construction, Sequencing, and Data Analysis
2.6. Integrated Analyses of Transcriptomics and Metabolomics
2.7. Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
3.1. Analysis of Serum Metabolomics
3.2. Analysis of Liver Metabolomics
3.3. Analysis of Liver Transcriptomics
3.4. Integrated Analyses of Metabolomics and Transcriptomics Unveiled the Mechanisms Underlying SW033291’s Effects in the Treatment of T2DM
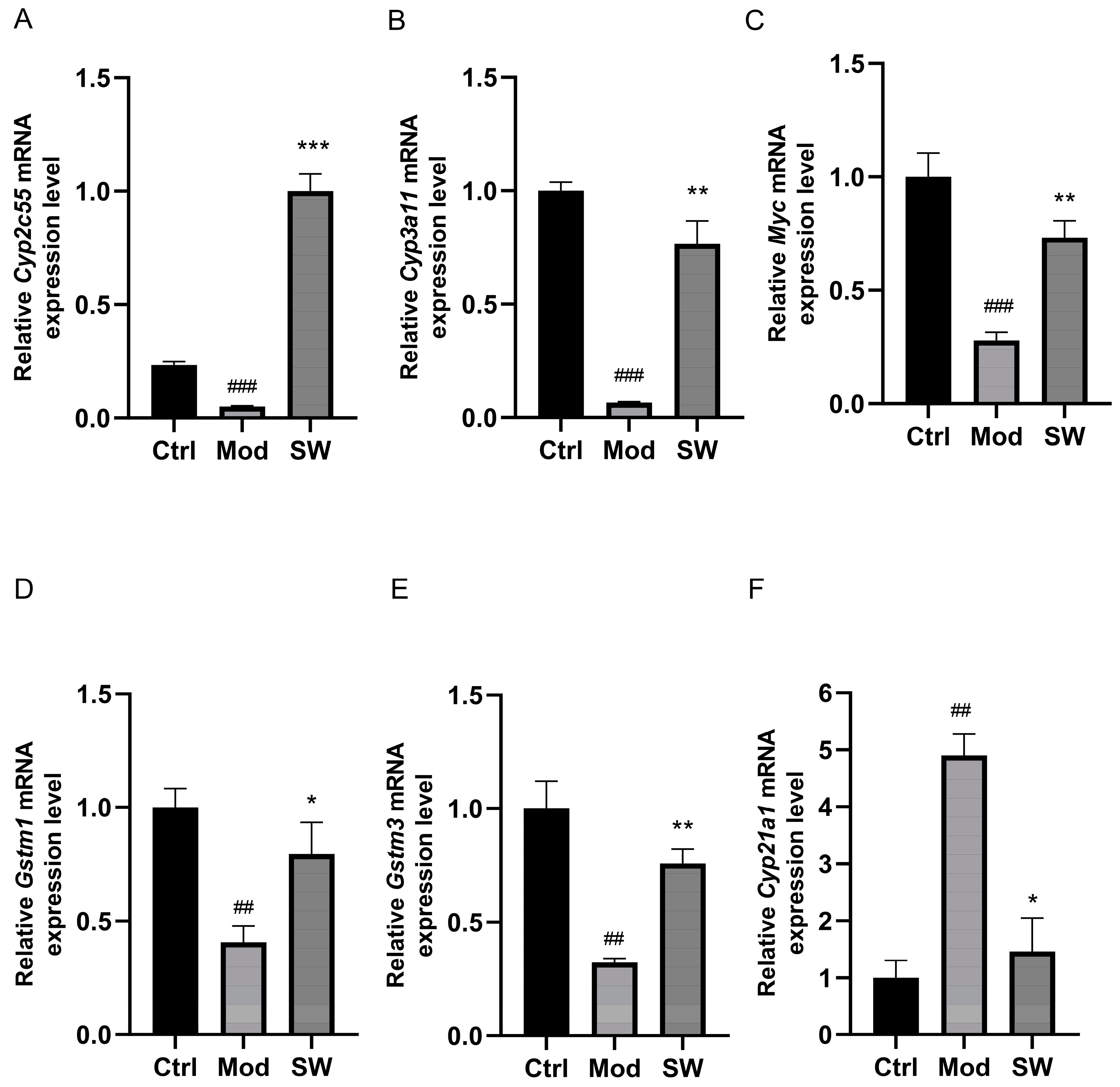
3.5. Verification of Significantly Altered Genes by qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-Diabetic Agent and Its Underlying Mechanism Analysis. Cells 2019, 8, 204. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 18, 8836–8854. [Google Scholar] [CrossRef]
- Mallipeddi, P.L.; Zhang, Y.; Li, H.; Markowitz, S.D.; Posner, B. Structural Insights into Novel 15-Prostaglandin Dehydrogenase Inhibitors. Molecules 2021, 26, 5903. [Google Scholar] [CrossRef] [PubMed]
- Coll, T.; Palomer, X.; Blanco-Vaca, F.; Escolà-Gil, J.C.; Sánchez, R.M.; Laguna, J.C.; Vázquez-Carrera, M. Cyclooxygenase 2 inhibition exacerbates palmitate-induced inflammation and insulin resistance in skeletal muscle cells. Endocrinology 2010, 2, 537–548. [Google Scholar] [CrossRef]
- Leighton, B.; Budohoski, L.; Lozeman, F.J.; Challiss, R.A.; Newsholme, E.A. The effect of prostaglandins E1, E2 and F2 alpha and indomethacin on the sensitivity of glycolysis and glycogen synthesis to insulin in stripped soleus muscles of the rat. Biochem. J. 1985, 1, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Frede, K.; Schanze, N.; Vogel, H.; Schürmann, A.; Spruss, A.; Bergheim, I.; Püschel, G.P. Stimulation of fat accumulation in hepatocytes by PGE2-dependent repression of hepatic lipolysis, β-oxidation and VLDL-synthesis. Lab. Invest. 2012, 11, 1597–1606. [Google Scholar] [CrossRef]
- Henkel, J.; Coleman, C.D.; Schraplau, A.; Jöhrens, K.; Weiss, T.S.; Jonas, W.; Schürmann, A.; Püschel, G.P. Augmented liver inflammation in a microsomal prostaglandin E synthase 1 (mPGES-1)-deficient diet-induced mouse NASH model. Sci. Rep. 2018, 1, 16127. [Google Scholar] [CrossRef]
- Xu, D.; Cai, J.; Wan, Z.K.; Gao, H.; Sun, Y. Pathophysiological role of prostaglandin E synthases in liver diseases. Prostaglandins Other Lipid Mediat. 2021, 154, 106552. [Google Scholar] [CrossRef] [PubMed]
- Francés, D.E.; Ingaramo, P.I.; Mayoral, R.; Través, P.; Casado, M.; Valverde, Á.M.; Martín-Sanz, P.; Carnovale, C.E. Cyclooxygenase-2 over-expression inhibits liver apoptosis induced by hyperglycemia. J. Cell Biochem. 2013, 3, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; de Boode, W.P.; Weisz, D.E.; Shah, P.S. Interventions for patent ductus arteriosus (PDA) in preterm infants: An overview of Cochrane Systematic Reviews. Cochrane Database Syst. Rev. 2023, 14, Cd013588. [Google Scholar]
- Miao, S.; Lv, C.; Liu, Y.; Zhao, J.; Li, T.; Wang, C.; Xu, Y.; Wang, X.; Xiao, X.; Zhang, H. Pharmacologic Blockade of 15-PGDH Protects Against Acute Renal Injury Induced by LPS in Mice. Front. Physiol. 2020, 11, 138. [Google Scholar] [CrossRef]
- Xie, M.; He, R.; Wang, H.; Zhao, S.; Zheng, J.; Wu, J.; Wen, H. Effects of small molecule inhibitor SW033291 on hepatic ischemia-reperfusion injury in mice. Biochem. Biophys. Res. Commun. 2022, 615, 70–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Desai, A.; Yang, S.Y.; Bae, K.B.; Antczak, M.I.; Fink, S.P.; Tiwari, S.; Willis, J.E.; Williams, N.S.; Dawson, D.M.; et al. TISSUE REGENERATION. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 2015, 348, aaa2340. [Google Scholar] [CrossRef]
- Palla, A.R.; Ravichandran, M.; Wang, Y.X.; Alexandrova, L.; Yang, A.V.; Kraft, P.; Holbrook, C.A.; Schürch, C.M.; Ho, A.T.V.; Blau, H.M. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science 2021, 371, eabc8059. [Google Scholar] [CrossRef]
- Liang, M.; Wang, L.; Wang, W. The 15-hydroxyprostaglandin dehydrogenase inhibitor SW033291 ameliorates abnormal hepatic glucose metabolism through PGE(2)-EP4 receptor-AKT signaling in a type 2 diabetes mellitus mouse model. Cell Signal 2023, 108, 110707. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhou, J.; Zheng, Y.; Xu, Z.; Li, Y.; Zhou, S.; Zhang, C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020, 127, 110182. [Google Scholar] [CrossRef]
- Leclercq, I.A.; Da Silva Morais, A.; Schroyen, B.; Van Hul, N.; Geerts, A. Insulin resistance in hepatocytes and sinusoidal liver cells: Mechanisms and consequences. J. Hepatol. 2007, 1, 142–156. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 1, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Karslioglu, E.; Kleinberger, J.W.; Salim, F.G.; Cox, A.E.; Takane, K.K.; Scott, D.K.; Stewart, A.F. cMyc is a principal upstream driver of beta-cell proliferation in rat insulinoma cell lines and is an effective mediator of human beta-cell replication. Mol. Endocrinol. 2011, 10, 1760–1772. [Google Scholar] [CrossRef]
- Riu, E.; Ferre, T.; Hidalgo, A.; Mas, A.; Franckhauser, S.; Otaegui, P.; Bosch, F. Overexpression of c-myc in the liver prevents obesity and insulin resistance. Faseb J 2003, 12, 1715–1717. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 8, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Yan, Y.J.; Li, Y.H.; Lv, L. Reversing effects of ginsenosides on LPS-induced hepatic CYP3A11/3A4 dysfunction through the pregnane X receptor. J. Ethnopharmacol. 2019, 229, 246–255. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yoshida, S.; Sun, Y.J.; Shirasawa, N.; Naito, A. Gastric estradiol-17β (E2) and liver ERα correlate with serum E2 in the cholestatic male rat. J. Endocrinol. 2013, 1, 39–49. [Google Scholar] [CrossRef]
- Albarakati, N.; Khayyat, D.; Dallol, A.; Al-Maghrabi, J.; Nedjadi, T. The prognostic impact of GSTM1/GSTP1 genetic variants in bladder Cancer. BMC Cancer 2019, 1, 991. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Das, S.; Bhowmik, A.; Ghosh, S.K.; Choudhury, Y. The GSTM1 and GSTT1 Null Genotypes Increase the Risk for Type 2 Diabetes Mellitus and the Subsequent Development of Diabetic Complications: A Meta-analysis. Curr. Diabetes Rev. 2019, 1, 31–43. [Google Scholar] [CrossRef]
- Tin, A.; Scharpf, R.; Estrella, M.M.; Yu, B.; Grove, M.L.; Chang, P.P.; Matsushita, K.; Köttgen, A.; Arking, D.E.; Boerwinkle, E.; et al. The Loss of GSTM1 Associates with Kidney Failure and Heart Failure. J. Am. Soc. Nephrol. 2017, 11, 3345–3352. [Google Scholar] [CrossRef]
- Chen, T.; Jinlin, D.; Wang, F.; Yuan, Z.; Xue, J.; Lu, T.; Huang, W.; Liu, Y.; Zhang, Y. GSTM3 deficiency impedes DNA mismatch repair to promote gastric tumorigenesis via CAND1/NRF2-KEAP1 signaling. Cancer Lett. 2022, 538, 215692. [Google Scholar] [CrossRef]
- Pass, G.J.; Becker, W.; Kluge, R.; Linnartz, K.; Plum, L.; Giesen, K.; Joost, H.G. Effect of hyperinsulinemia and type 2 diabetes-like hyperglycemia on expression of hepatic cytochrome p450 and glutathione s-transferase isoforms in a New Zealand obese-derived mouse backcross population. J. Pharmacol. Exp. Ther. 2002, 2, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Kaji, K.; Sato, S.; Ogawa, H.; Takagi, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J. Nutr. Biochem. 2021, 89, 108573. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Yin, Y.; Chen, X.; Sun, Z. GSTM3 reverses the resistance of hepatoma cells to radiation by regulating the expression of cell cycle/apoptosis-related molecules. Oncol. Lett. 2014, 4, 1435–1440. [Google Scholar] [CrossRef]
- Naiki, Y.; Miyado, M.; Shindo, M.; Horikawa, R.; Hasegawa, Y.; Katsumata, N.; Takada, S.; Akutsu, H.; Onodera, M.; Fukami, M. Adeno-Associated Virus-Mediated Gene Therapy for Patients’ Fibroblasts, Induced Pluripotent Stem Cells, and a Mouse Model of Congenital Adrenal Hyperplasia. Hum. Gene Ther. 2022, 15–16, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.P.; Soldin, O.P.; Guo, T.; Soldin, S.J. Steroid hormones: Relevance and measurement in the clinical laboratory. Clin. Lab. Med. 2004, 1, 105–118. [Google Scholar] [CrossRef]
- Pott, J.; Bae, Y.J.; Horn, K.; Teren, A.; Kühnapfel, A.; Kirsten, H.; Ceglarek, U.; Loeffler, M.; Thiery, J.; Kratzsch, J.; et al. Genetic Association Study of Eight Steroid Hormones and Implications for Sexual Dimorphism of Coronary Artery Disease. J. Clin. Endocrinol. Metab. 2019, 11, 5008–5023. [Google Scholar] [CrossRef]
- Naiki, Y.; Miyado, M.; Horikawa, R.; Katsumata, N.; Onodera, M.; Pang, S.; Ogata, T.; Fukami, M. Extra-adrenal induction of Cyp21a1 ameliorates systemic steroid metabolism in a mouse model of congenital adrenal hyperplasia. Endocr. J. 2016, 10, 897–904. [Google Scholar] [CrossRef]
- Hofmann, A.; Peitzsch, M.; Brunssen, C.; Mittag, J.; Jannasch, A.; Frenzel, A.; Brown, N.; Weldon, S.M.; Eisenhofer, G.; Bornstein, S.R.; et al. Elevated Steroid Hormone Production in the db/db Mouse Model of Obesity and Type 2 Diabetes. Horm. Metab. Res. 2017, 1, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Merk, V.M.; Renzulli, P.; Vrugt, B.; Fleischmann, A.; Brunner, T. Glucocorticoids are differentially synthesized along the mu-rine and human respiratory tree. Allergy. 2023, 78, 2428–2440. [Google Scholar] [CrossRef]
- Li, J.X.; Cummins, C.L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 2022, 9, 540–557. [Google Scholar] [CrossRef]
- Dammann, C.; Stapelfeld, C.; Maser, E. Expression and activity of the cortisol-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 is tissue and species-specific. Chem. Biol. Interact. 2019, 303, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.S.; Klett, E.L. Linoleic acid and the regulation of glucose homeostasis: A review of the evidence. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.M.; Djordjilović, V.; Pan, H.; Bussberg, V.; MacDonald, A.M.; Narain, N.R.; Kiebish, M.A.; Blüher, M.; Tseng, Y.H.; Lynes, M.D. ScreenDMT reveals DiHOMEs are replicably inversely associated with BMI and stimulate adipocyte calcium influx. Commun. Biol. 2024, 1, 996. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, A.P.A.; Muñoz, V.R.; Cintra, D.E.; Pauli, J.R. 12,13-diHOME as a new therapeutic target for metabolic diseases. Life Sci. 2022, 290, 120229. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 5, 631–637. [Google Scholar] [CrossRef]
- Pinckard, K.M.; Shettigar, V.K.; Wright, K.R.; Abay, E.; Baer, L.A.; Vidal, P.; Dewal, R.S.; Das, D.; Duarte-Sanmiguel, S.; Hernández-Saavedra, D.; et al. A Novel Endocrine Role for the BAT-Released Lipokine 12,13-diHOME to Mediate Cardiac Function. Circulation 2021, 2, 145–159. [Google Scholar] [CrossRef]
- Smith, J.N.P.; Otegbeye, F.; Jogasuria, A.P.; Christo, K.F.; Antczak, M.I.; Ready, J.M.; Gerson, S.L.; Markowitz, S.D.; Desai, A.B. Inhibition of 15-PGDH Protects Mice from Immune-Mediated Bone Marrow Failure. Biol. Blood Marrow Transplant. 2020, 8, 1552–1556. [Google Scholar] [CrossRef]
- Kishore, A.H.; Liang, H.; Kanchwala, M.; Xing, C.; Ganesh, T.; Akgul, Y.; Posner, B.; Ready, J.M.; Markowitz, S.D.; Word, R.A. Prostaglandin dehydrogenase is a target for successful induction of cervical ripening. Proc. Natl. Acad. Sci. USA 2017, 31, E6427–E6436. [Google Scholar] [CrossRef]
- Rastogi, S.; Willmes, D.M.; Nassiri, M.; Babina, M.; Worm, M. PGE(2) deficiency predisposes to anaphylaxis by causing mast cell hyperresponsiveness. J. Allergy Clin. Immunol. 2020, 6, 1387–1396. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Lim, J.H.; Choi, I.; Lee, E.J. Biocomputational screening of natural compounds targeting 15-hydroxyprostaglandin dehydrogenase to improve skeletal muscle during aging. Mol. Divers. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leiter, E.H. Differential susceptibility of BALB/c sublines to diabetes induction by multi-dose streptozotocin treatment. Curr. Top. Microbiol. Immunol. 1985, 122, 78–85. [Google Scholar] [PubMed]
- Bell, R.C.; Khurana, M.; Ryan, E.A.; Finegood, D.T. Gender differences in the metabolic response to graded numbers of transplanted islets of Langerhans. Endocrinology 1994, 6, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Cortright, R.N.; Collins, H.L.; Chandler, M.P.; Lemon, P.W.; DiCarlo, S.E. Diabetes reduces growth and body composition more in male than in female rats. Physiol. Behav. 1996, 5, 1233–1238. [Google Scholar] [CrossRef]
- Leiter, E.H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: Influence of inbred background, sex, and thymus. Proc. Natl. Acad. Sci. USA 1982, 2, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S. Neural basis for estrous cycle-dependent control of female behaviors. Neurosci. Res. 2022, 176, 1–8. [Google Scholar] [CrossRef]
- Miller, W.L. Disorders in the initial steps of steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt A, 18–37. [Google Scholar] [CrossRef]
- Ullah, I.; Shin, Y.; Kim, Y.; Oh, K.B.; Hwang, S.; Kim, Y.I.; Lee, J.W.; Hur, T.Y.; Lee, S.; Ock, S.A. Effect of sex-specific differences on function of induced hepatocyte-like cells generated from male and female mouse embryonic fibroblasts. Stem Cell Res. Ther. 2021, 1, 79. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liang, M.; Liao, Y.; Ji, Z.; Lin, W.; Pu, X.; Wang, L.; Wang, W. Investigating the Mechanisms of 15-PGDH Inhibitor SW033291 in Improving Type 2 Diabetes Mellitus: Insights from Metabolomics and Transcriptomics. Metabolites 2024, 14, 509. https://doi.org/10.3390/metabo14090509
Huang Y, Liang M, Liao Y, Ji Z, Lin W, Pu X, Wang L, Wang W. Investigating the Mechanisms of 15-PGDH Inhibitor SW033291 in Improving Type 2 Diabetes Mellitus: Insights from Metabolomics and Transcriptomics. Metabolites. 2024; 14(9):509. https://doi.org/10.3390/metabo14090509
Chicago/Turabian StyleHuang, Yuanfeng, Mingjie Liang, Yiwen Liao, Zirui Ji, Wanfen Lin, Xiangjin Pu, Lexun Wang, and Weixuan Wang. 2024. "Investigating the Mechanisms of 15-PGDH Inhibitor SW033291 in Improving Type 2 Diabetes Mellitus: Insights from Metabolomics and Transcriptomics" Metabolites 14, no. 9: 509. https://doi.org/10.3390/metabo14090509
APA StyleHuang, Y., Liang, M., Liao, Y., Ji, Z., Lin, W., Pu, X., Wang, L., & Wang, W. (2024). Investigating the Mechanisms of 15-PGDH Inhibitor SW033291 in Improving Type 2 Diabetes Mellitus: Insights from Metabolomics and Transcriptomics. Metabolites, 14(9), 509. https://doi.org/10.3390/metabo14090509



